Abstract
Lack of nutrients is a common challenge for enteric pathogens, like Shigella, when discharged in waters and soils by infected persons. These bacteria must evolve protective mechanisms to survive nutrient changing conditions. Many studies have demonstrated that bacterial starvation can affect cell morphology and surface properties such as lipopolysaccharide content. This study focuses on the morphological and physiological alterations of Shigella flexneri, a human pathogen, under nutrient starvation during 30 days. Our results showed that S. flexneri can survive under nutrient deficiency during 4 weeks. However, starved cells revealed several morphological changes indicating decrease of the size and change of the cell shape as observed by transmission electron microscopy. Furthermore, the outer membrane proteins and lipopolysaccharide profiles of starved cells revealed the disappearance, the appearance, and changes of the protein band levels. The survival and physiological adaptation of S. flexneri under nutrient limitation during 30 days may increase the risk of human and animal infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacteria are in several ways affected by the environment, given their vital interaction with the vast surroundings. Bacteria’s survival rests on a dynamic equilibrium characterized by a cycle of exchange with the environment. Bacteria are sensitive to the changes in the natural habitats, where a biotic or abiotic disturbance may be a stress factor inducing a divergence from this equilibrium (Weilharter et al. 2011; Batool et al. 2014). These disturbances stand behind the bacteria developing some mechanisms of resistance to enable an adaptation to stress and ensure its survival (Booth 2002). This adaptation can manifest itself in various ways leading to a wide range of morphological, physiological, cellular, and biochemical changes (Gustavs et al. 2009; Batool et al. 2014).
Being the first line of defense, the membrane structure is the first target of the deterioration of the physiological cells state in a stress situation (Beal et al. 2008), which explains their involvement in stress response. For the bacteria to adapt, the structure of their outer membrane undergoes numerous changes.
When they are discharged in waters and soils by infected persons, enteric pathogens are commonly challenged by the lack of nutrients. Some Enterobacteriaceae have developed morphological differentiation, resulting in reduced cellular forms adapted to harsh environmental conditions. Overall, during the transition from exponential growth phase and the stationary phase, the cells become spherical due to changes in the cell envelope.
Shigella, a Gram-negative, enteroinvasive bacterium belonging to the family of Enterobacteriacae, is the causative agent of shigellosis or “bacillary dysentery,” a disease which is a major public health problem in both developing and industrialized countries (Phalipon and Sansonetti 2007). The annual incidence of shigellosis throughout the world is estimated at 164.7 million cases of which more than million of those infected die, with 69% of all deaths attributable to shigellosis involving children less than 5 years old (Kotloff et al. 1999; Mani et al. 2016). The disease is highly contagious due to its low infectious dose as 10 to 100 viable bacteria (Phalipon and Sansonetti 2007). Shigella is dispersed in the environment such as soil and wastewater. So, this pathogen is facing many potentially stressful environments during its life cycle as it is exposed to the outside environment (Nicolas et al. 2007).
In our study, we aimed to study the adaptation of Shigella flexneri to starvation conditions by evaluating the morphological changes, outer membrane proteins, and lipopolysaccharide profiles.
Material and methods
Bacterial strain and survival study
S. flexneri ATCC 12022 was used in this work. For the experiments, the cells were grown at 37 °C in tryptic soy broth (TSB) for 24 h, and then they were washed three times using autoclaved saline solution (0.9% NaCl) and suspended in 10 ml of autoclaved NaCl (0.9%). To prepare the laboratory microcosm, an Erlenmeyer flask containing 500 ml of NaCl (0.9%) was autoclaved and inoculated with S. flexneri. The microcosms were incubated under constant shaking (horizontal shaker) at 25 °C. The survival of cells was monitored weekly during 1 month by CFU determination. Triplicate determinations were made in all cases.
Determination of morphological changes
In order to visualize the putative morphological changes in the starved strains, Shigella cells were examined by transmission electron microscope (TEM). For these experiments, cells were inoculated in 2 ml of nutrient broth and 0.4 ml of formaldehyde and then washed three times with 4 ml of 0.9% NaCl and centrifuged (3000×g/20 min). One drop from the final solution was deposed on the grid plus one drop of uranyl acid (Beveridge et al. 2007).
Realization of semi-thin sections
To achieve the semi-thin sections, 15 ml of culture was prefixed with 15 ml of glutaraldehyde (GA) for 1 h at room temperature and washed three times with 0.1 M sodium cacodylate buffer at 3000×g/20 min. The pellet was fixed with 2 ml of GA (1%) for 3 h at 4 °C. Then, the bacterial suspension was washed three times and centrifuged at 3000×g/20 min and resuspended in 0.5 ml of sodium cacodylate and 0.5 ml osmium tetroxide OSO4 (2%) for 1 h at room temperature. After washing with the cacodylate buffer and centrifugation at 3000×g/20 min, 1 ml of uranyl acetate (1%) was added and incubated for 1 h. Then, the suspension was washed first by cacodylate and second by distilled water and centrifuged at 3000×g/20 min. The residues obtained were placed at 47–50 °C with a few drops of distilled water and agar. The mixture obtained was poured on a slide, and after cooling, it was cut into small cubes which were placed in 1 ml of uranyl acetate (1%). These cubes were afterwards passed into progressive ethanol baths at increasing percentages (30, 50, 70, 90, and 100%) during 25 min for each one (Belguith et al. 2009). Then, they were placed in propylene oxide for 15 min to be delivered in propylene oxide plus epon resin at different volumes (2 vol/1 vol, 1 vol/1 vol, 1 vol/2 vol) for 15 min each one and finally in only epon during 18 h. The cubes were placed in blocks containing epon and placed at 80 °C for 2 days. The semi-thin sections of 0.5 μm, obtained using an ultramicrotome, were filed on grids and contrasted for 2 h in uranyl acetate and 5 min in the lead citrate to be examined by electron microscopy transmission.
Outer membrane proteins (OMPs) assay
S. flexneri OMPs profiles were determined before and after cell incubation in the laboratory microcosms prepared according the protocol described by Ferreiros et al. (1990). To prepare the inocula for the microcosms, Shigella was grown overnight in tryptic soy broth (TSB). Cell samples were taken at time 0 and after 1, 2, 3, and 4 weeks. For each sample, 50 ml was collected at the appropriate time. Briefly, cells were harvested by centrifugation at 10,000×g/4 °C during 10 min. The cell pellets were resuspended in 6 ml of acetate tampon (acetic acid 0.2 M; sodium acetate 0.2 M). The tubes were then incubated during 2 h at 45 °C under continuous shaking. The content was poured to a new tube using a syringe with 21 Ga needles promoting the membranes breaking into small vesicles. The new tubes were centrifuged at 10,000×g/4 °C during 10 min. Supernatant was collected and centrifuged at 50,000×g/4 °C during 36 min using ultracentrifuge Beckman Coulter (Rotor Type 90TI). The resulting pellet was transparent and was resuspended in 50 μl of distilled water. The concentration of OMPs in the final preparation was determined using the Bradford kit (Thermo Scientific, NanoDrop 2000). The OMPs were separated on 12% SDS gel and stained by Brilliant Blue G-Colloidal Concentrate 2025 (Sigma) (Neuhoff et al. 1988).
Lipopolysaccharide (LPS) extraction and purification
LPS was extracted by hot phenol-water method as described by Rezania et al. (2011). In brief, bacterial suspension was centrifuged at 10,000×g during 5 min. First, the pellets were washed with phosphate buffer solution (PBS) (pH = 7.2, 0.5 M) containing CaCl2 (0.15 mM) and MgCl2 (0.5 mM) then washed by PBS only. Pellets were then resuspended in 1 ml of PBS and sonicated for 2 min on ice. In order to eliminate contaminating protein and nucleic acids, treatments with proteinase K (100 μg/ml), DNase (20 μg/ml), and RNase (40 μg/ml) were performed prior the extraction step.
At the next step, an equal volume of hot (65–70 °C) phenol (90%) was added to the mixtures followed by vigorous shaking at 65–70 °C (15 min). Suspensions were then cooled on ice, transferred to 1.5 ml polypropylene tubes and centrifuged at 8500×g (15 min). Suspensions were transferred to 15-ml conical centrifuge tubes, and phenol phases were reextracted in 300 μl of distilled water. Sodium acetate (0.5 M) and ethanol (95%) were added, and samples were incubated at −20 °C overnight in order to precipitate LPS. Tubes were then centrifuged at 8000×g/4 °C for 10 min, and pellets were resuspended in 1 ml of distilled water. Extensive dialysis against double distilled water at 4 °C was carried out until the residual phenol in the aqueous phases was totally eliminated. Final purified LPS product was lyophilized and stored at 4 °C.
The purified LPS components were separated by electrophoresis on 12% SDS gel and stained with ProteoSilverTM Silver Stain Kit (Gharahdaghi et al. 1999).
Results and discussion
Shigella flexneri survival under starvation
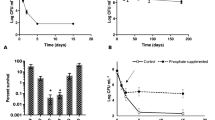
Relative changes in counts of S. flexneri during the period of starvation are shown in Fig. 1. From the first week of starvation until the fourth one, the number of viable cells of S. flexneri decreased slightly. Therefore, the result showed that Shigella was able to adapt and survive in nutritional deficiency conditions during a month of incubation in physiological water microcosms. Similar results were reported by Ellafi et al. (2011) for different strains of Shigella but with an important decrease of the number of viable cells since the first days of starvation. Besides, Bogosian et al. (1998) noted that the number of viable cells of some enteric bacteria decreased slowly during long period of starvation. Similar results were obtained for Klebsiella pneumonia which showed a decrease in its viability (Bossolan et al. 2005). However, the viability of Vibrio anguillarum decreased within 72 to 168 h of the nutritional deficiency (Nelson et al. 1997), while the number of viable cells of Vibrio sp. was approximately the same during 5 weeks of starvation (Amy et al. 1983).
During starvation, some bacterial species lose their cultivability but remain viable (Fakruddin et al. 2013). This phenomenon is known as viable but nonculturable (VNC): it is a survival strategy in response to adverse environmental conditions (Su et al. 2015). The VNC state has been observed in several bacterial species, and it seems that there are different molecular mechanisms trailing this phenomenon. It is characterized by low metabolic activities and some distinctive morphological changes of bacteria in the stationary phase (Li et al. 2014; Su et al. 2015; Zhao et al. 2016).
Cell morphology of starved Shigella flexneri
These morphological alterations were noted in this study, when the cells of S. flexneri have passed from the exponential phase to starvation. We observed considerable changes in apparent structure of the cells and roughness of the outer surface. Vegetative cells of S. flexneri have a bacillary form up to 2–3 μm in length with a very clear cell wall and an external membrane surrounding an expended cytoplasm (Fig. 2a), whereas the starved cells decreased in length up to 0.5–1 μm and transformed to a coccoid form with a condensed cytoplasm. The starved cells of S. flexneri turned into small spheroids via fragmentation and continuous size reduction (Fig. 2b).
The coccoid form of Shigella was also observed after incubation in seawater (Ellafi et al. 2011). Similarly, K. pneumoniae cells showed a reduction in size and a change of bacillary form to the coccoid or coccobacillus form (Bossolan et al. 2005). Further, the reduction in size was also observed in Salmonella enterica and S. typhimurium (Spector 1990; Huisman et al. 1996). In addition, after starvation, V. cholerae cells had serious changes of their cell morphology from curved bacilli cells to spheroid ones (Krebs and Taylor 2011). This coccoid form was described for the first time when the cells were in VNC state (Carroll et al. 2001; Chaiyanan et al. 2001, 2007; Johnston and Brown 2002). The most notable differences for the cells of Vibrio (Listonella) anguillarum were the reduction in the diameter by 15% and the appearance of rounded cells after 168 h of nutrient deprivation (Nelson et al. 1997). Furthermore, the conversion of the cell morphology was observed in many other pathogenic bacteria like the uropathogenic Escherichia coli (UPEC), which was organized into coccoid cells tucking most of epithelial cells of the urinary tract (Justice et al. 2004). Other studies of starved E. coli have shown that these coccoid cells can divide and exist individually or grouped (Corbin et al. 2002). This response is similar to our result for S. flexneri.
The observation of semi-thin sections of S. flexneri showed a mosaic of cells which were microcells, rounded cells, and elongated ones (Fig. 3). In addition, the result revealed that the cell envelope of the majority of stressed cells has been altered, the external and internal membranes were separated, and the cytoplasm was disorganized. Further, we observed some form of vesicles that appeared on the surface of the outer membrane. These vesicles reflect the breakdown of membrane integrity (Fig. 4).
It was demonstrated that starved cells presented numerous mutations. These mutations lead to the coexistence of many subpopulations allowing the increasing of genetic diversity (Reeve et al. 1984; Finkel and Kolter 1999). Further, the ultrastructure of starved bacterial strains presented many morphological changes (Stretton et al. 1997; Mizunoe et al. 2000). Nelson et al. (1997) reported also that Vibrio anguillarum starved cells became rounded containing less coloration at the inner material and less integrity. They were elongated in curved or helical filaments. Similarly, some cells of Vibrio VP190 contained less inner material. They had some membrane vesicles over a portion of the outer membrane and have lost the membrane integrity. The lysis of the cell envelope and the inhomogeneous arrangement of the cytoplasm were also noted for the Salmonella strains after exposure to aqueous garlic extract (Belguith et al. 2009).
Effect of starvation on the outer membrane proteins
Many bacteria exhibit the expression of coordinated protective mechanisms to overcome multiple physical stresses. It is thought that the acquisition of cross protection during starvation is accomplished by the expression of starvation-stress proteins (Givskov et al. 1994a).
In our study, the starvation altered the Shigella OMPs. These alterations were illustrated by the disappearance, the appearance of proteins bands, and the changes of the protein band concentrations (Fig. 5). At the initial phase of starvation (T1) and at the end of the process (T4), the cells preserved some of their initial OMPs and expressed new proteins (with low level of expression) which disappeared during the T2 and T3 phases. Our results showed also that during starvation, the cells did not synthesize any protein with molecular mass up to 52 KDa and kept the concentrated band corresponding to the molecular weight between 38 and 52 KDa. Similar results were obtained for starved E. coli (Walczak et al. 2012).
Lipopolysaccharide profiles of starved cells
Polyacrylamide gel is known to be affected by the number and size of repeating oligosaccharide units in long-chain LPS, such that bands in the profile represent progressively larger concatemers of the repeating oligosaccharide units (Wu et al. 2006). The starvation process showed a huge increase of bands with high expression level (Fig. 6). The LPS profiles of the S. flexneri showed rapid migrating bands before and during starvation with less concentrated bands in the beginning of the process. Previous studies have shown alteration of the lipid A content during starvation of marine heterotrophic bacteria (Malmcrona-Friberg et al. 1986). Also, Walczak et al. (2012) mentioned that the outer membrane LPS sugar contents did not display significant changes during starvation in E. coli K12.
Conclusion
Our results showed that S. flexneri is capable to survive lengthy periods under nutrient deprivation. Phenotypic and physiological plasticity of S. flexneri under starvation provides advantage to survive several stresses and results in public health risks.
References
Amy PS, Pauling C, Morita RY (1983) Starvation-survival processes of a marine Vibrio. Appl Environ Microbiol 45:1041–1048
Batool R, Yrjälä K, Hasnain S (2014) Impact of environmental stress on biochemical parameters of bacteria reducing chromium. Braz J Microbiol 45:573–583
Béal C, Marin M, Fontaine E, Fonseca F, Obert JP (2008) Production et conservation des ferments lactiques et probiotiques. In: Corrieu G, Luquet FM (eds) Bactéries lactiques, de la génétique aux ferments. Tec et Doc Lavoisier, Paris, pp 661–785
Belguith H, Kthiri F, Ben Ammar A, Jaafoura H, Ben Hamida J, Landoulsi A (2009) Morphological and biochemical changes of Salmonella hadar exposed to aqueous garlic extract. Int J Morphol 27:705–713
Beveridge TJ, Lawrence JR, Murray RGE (2007) Sampling and staining for light microscopy. In: Reddy CA, Beveridge TJ, Breznak JA, Marzluf GA, Schmidt TM, Snyder LR (eds) Methods for general and molecular microbiology, 3rd edn. ASM Press, Washington, DC, pp 19–33
Bogosian G, Morris PJL, O'Neil JP (1998) A mixed culture recovery method indicates that enteric bacteria do not enter the viable but nonculturable state. Appl Environ Microbiol 57:875–878
Booth IR (2002) Stress and the single cell: intrapopulation diversity is a mechanism to ensure survival upon exposure to stress. Inter J Food Microbiol 78:19–30
Bossolan NRS, Godinho MJL, Volpon AGT (2005) Growth and starvation of a strain of Klebsiella pneumoniae isolated from a Brazilian oil formation. World J Microbiol Biotechnol 21:1471–1475
Carroll JW, Mateescu MC, Chava K, Colwell RR, Bej AK (2001) Response and tolerance of toxigenic Vibro cholerae O1 to cold temperatures. A Van Leeuw J Microbiol 79:377–384
Chaiyanan S, Chaiyanan S, Huq A, Maugel T, Colwell RR (2001) Viability of the nonculturable Vibrio cholerae O1 and O139. Syst Appl Microbiol 24:331–341
Chaiyanan S, Chaiyanan S, Grim C, Maugel T, Huq A, Colwell RR (2007) Ultrastructure of coccoid viable but non-culturable Vibrio cholerae. Environ Microbiol 9:393–402
Corbin BD, Yu XC, Margolin W (2002) Exploring intracellular space: function of the Min system in round-shaped Escherichia coli. EMBO J 21:1998–2008
Ellafi A, Ben Abdallah F, Lagha R, Bakhrouf A (2011) Outer membrane proteins and morphological alterations of Shigella spp. under starvation in seawater. Afr J Microbiol Res 5:904–909
Fakruddin M, Bin Mannan KS, Andrews S (2013) Viable but nonculturable bacteria: food safety and public health perspective. ISRN Microbiol. doi:10.1155/2013/703813
Ferreirós CM, Criado MT, Del Río MC, Pintor M (1990) Analysis of the expression of outer-membrane proteins in Neisseria meningitidis in iron-replete and iron-deficient media. FEMS Microbiol Lett 71:49–54
Finkel SE, Kolter R (1999) Evolution of microbial diversity during prolonged starvation. Proc Natl Acad Sci U S A 96:4023–4027
Gharahdaghi F, Weinberg CR, Meagher DA, Imai BS, Mische SM (1999) Mass spectrometric identification of proteins from silver-stained polyacrylamide gel: a method for the removal of silver ions to enhance sensitivity. Electrophoresis 20:601–605
Givskov M, Eberl L, Molin S (1994a) Responses to nutrient starvation in Pseudomonas putida KT2442: two dimensional electrophoretic analyses of starvation- and stress-induced proteins. J Bacteriol 176:4816–4824
Gustavs L, Eggert A, Michalik D, Karsten U (2009) Physiological and biochemical responses of green microalgae from different habitats to osmotic and matric stress. Protoplasma 243:3–14
Huisman GW, Siegele DA, Zambrano MM, Kolter R (1996) Morphological and physiological changes during stationary phase. In: Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (eds) Escherchia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, pp 1672–1682
Johnston MD, Brown MH (2002) An investigation into the changed physiological state of Vibrio bacteria as a survival mechanism in response to cold temperatures and studies on their sensitivity to heating and freezing. J Appl Microbiol 92:1066–1077
Justice SS, Hung C, Theriot JA, Fletcher DA, Anderson GG, Footer MJ, Hultgren SJ (2004) Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc Natl Acad Sci U S A 101:1333–1338
Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, Adak GK, Levine MM (1999) Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ 77:651–666
Krebs SJ, Taylor RK (2011) Nutrient-dependent, rapid transition of Vibrio cholerae to coccoid morphology and expression of the toxin co-regulated pilus in this form. Microbiology 157:2942–2953
Li L, Mendis N, Trigui H, Oliver JD, Faucher SP (2014) The importance of the viable but non-culturable state in human bacterial pathogens. Front Microbiol. doi:10.3389/fmicb.00258
Malmcrona-Friberg K, Tunlid A, Marden P, Kjelleberg S, Odham G (1986) Chemical changes in cell envelope and poly-p-hydroxybutyrate during short term starvation of marine bacterial isolates. Arch Microbiol 144:340–345
Mani S, Wierzba T, Walker RI (2016) Status of vaccine research and development for Shigella. Vaccine 34:2887–2894
Mizunoe Y, Wai SN, Ishikawa T, Takade A, Yoshida S (2000) Resuscitation of viable but nonculturable cells of Vibrio parahaemolyticus induced at low temperature under starvation. FEMS Microbiol Lett 186:115–120
Nelson DR, Sadlowski Y, Eguchi M, Kjelleberg S (1997) The starvation-stress response of Vibrio (Listonella) anguillarum. Microbiol 143:2305–2312
Neuhoff V, Arold N, Taube D, Ehrhardt W (1988) Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis 9:255–262
Nicolas X, Granier H, Le Guen P (2007) Shigellose ou dysenterie bacillaire. Presse Med 36:1606–1618
Phalipon A, Sansonetti J (2007) Shigella’s ways of manipulating the host intestinal innate and adaptive immune system: a tool box for survival? Immunol Cell Biol 85:119–129
Reeve CA, Bockman AT, Matin A (1984) Role of protein degradation in the survival of carbon-starved Escherichia coli and Salmonella typhimurium. J Bacteriol 157:758–763
Rezania S, Amirmozaffari N, Tabarraei B, Jeddi-Tehrani M, Zarei O, Alizadeh R, Masjedian F, Zarnani AH (2011) Extraction, purification and characterization of lipopolysaccharide from Escherichia coli and Salmonella typhi. Avicenna J Med Biotechnol 3:3–9
Spector MP (1990) Gene expression in response to multiple nutrient-starvation conditions in Salmonella typhimurium. FEMS Microbiol Ecol 74:175–184
Stretton S, Danon SJ, Kjelleberg S, Goodman AE (1997) Changes in cell morphology and motility in the marine Vibrio sp. strain S14 during conditions of starvation and recovery. FEMS Microbiol Lett 146:23–29
Su X, Sun F, Hasmi MZ, Guo L, Ding L, Chen C (2015) Identification, characterization and molecular analysis of the viable but nonculturable Rhodococcus biphenylivorans. Sci Rep. doi:10.1038/srep18590
Walczak JJ, Wang L, Bardy SL, Feriancikova L, Li J, Shangping X (2012) The effects of starvation on the transport of Escherichia coli in saturated porous media are dependent on pH and ionic strength. Nat Inst Health 90:129–136
Weilharter A, Mitter B, Shin MV, Chain PSG, Nowak J, Sessitsch A (2011) Complete genome sequence of the plant growthpromoting endophyte Burkholderia phytofirmans strain PsJN. J Bacteriol 193:3383–3384
Wu T, McCandlish AC, Gronenberg LS, Chng SS, Silhavy TJ, Kahne D (2006) Identification of a protein complex that assembles lipopolysaccharide in the outer membrane of Escherichia coli. Natl Acad Sci USA 103:11754–11759
Zhao F, Wang Y, An H, Hao Y, Hu X, Liao X (2016) New insights into the formation of viable but nonculturable Escherichia coli O157:H7 induced by high-pressure CO2. MBio. doi:10.1128/mBio.00961-16
Acknowledgements
We would like to thank all the team of electron microscopy of Murcia University for their help.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Georesources and Environmental Management
Rights and permissions
About this article
Cite this article
Chourabi, K., Torrella, F., Kloula, S. et al. Adaptation of Shigella flexneri to starvation: morphology, outer membrane proteins and lipopolysaccharide changes. Arab J Geosci 10, 274 (2017). https://doi.org/10.1007/s12517-017-3064-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-017-3064-3