Abstract
The Nile River is the major source of water and soils in Egypt. Silt is the main soil component of it. The Nile is one of the international rivers; it passes through ten countries in Africa and is divided into two branches, Rosetta and Damietta at the end of the River. A hydrochemical facies study of the Rosetta branch was done. The geochemical processes which control the water quality and its suitability for drinking, fishing, and irrigation purposes were evaluated. Thus, the hydro-environmental status is studied. Fifteen water samples were collected for determining the physical and chemical parameters. The results show that several parameters are above the desired limits. High concentrations of total dissolved solids (TDS), electrical conductivity, total alkalinity, ammonia (NH3), electrical conductivity (EC), biological oxygen demand (BOD), chloride (Cl−), and sulfate (SO4 2−) were found. While a reduction in dissolved oxygen (DO) in the drains, especially Tala and El Rahawy drains, were noticed. In addition, residual sodium carbonate (RSC), sodium adsorption ratio (SAR), sodium percent (Na %), and magnesium hazard (MH) were measured. The results show that the salinity values for the selected samples minimized their utilization for irrigation. The results indicate that the main geochemical process in the study area is cation exchange and evaporation which control the main ion distribution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water resources in Egypt
Nile River is the main source of the water in Egypt; it covers more than 95% of the Egyptians’ freshwater demands. Seventy-eight million people live along the Nile in an area about 4% of the total area of Egypt (Abdel-Wahaab and Mohy 2011). The Nile travels along with 950 km in Egypt starting from south of Egypt at Aswan (Abdel-Dayem 2000). It ends at Cairo in the north, where it divides into two branches, Rosetta and Damietta branches. The Nile receives about 45 BCM per year of water (Allam and Gamal 2007). Egypt is classified as an arid area due to the lack of rainfall which does not exceed 1.0 BCM/year (Abdin and Gaafar 2009). The annual flood water is about 1.0 BCM. This flash flood supports the groundwater storage by 0.82 BCM every year (Abdel-Dayem 2000).
Sources of the pollutants
The industrial areas in Egypt suffer from many environmental problems, such as industrial wastes. Industrial wastes are disposed into the Nile River. In addition, the discharges of domestic and agricultural wastes are thrown also into the river without any pre-treatment (Wahaab and Mohamed 2004). This causes water quality degradation. In addition, the leaching of pesticides and fertilizers affects the water quality, too. One hundred twenty-eight drains discharge their effluents directly into the Nile, 72 drains from agricultural areas, and 56 drains of industrial type (El-Bouraie et al. 2011). Furthermore, a huge amount of untreated domestic sewage is disposed directly into the Nile River. Only about 24% of Egyptians have sanitation services, and about 5% of their homes are connected to the national sewerage system (Abdel-Shafy and Aly 2002). A great number of factories discharge their wastes directly to the Nile or into agricultural drains linked to the River. Wastewater plants release about 555 MCM of poorly treated water per year, and this water is directly disposed into the Nile River (312 MCM in the mainstream, 118 MCM in canals, 74 MCM in drains, and 51 MCM in lakes) (Abdel-Shafy and Aly 2002).
Water problem in Egypt
Egypt depends principally on conventional water resources. Egypt also suffers from a decrease in the water amounts coming from Ethiopia due to the Elnahda dam. Its capital city, Cairo, is the largest city in the Middle East; it has about 20 million with exponential population growth (Wagdy 2009). Due to the rapid growth of the population and the increase in industrial demand, the per capita of the available water is continuously decreasing. This causes a decline in the per capita of water from 1950 with 2200 to 700 m3 in 2013 (Wagdy 2009). Nowadays, a lot of the Egyptians suffer from lack of water, especially in rural regions. The agriculture demand consumes about 80% of the water supplies while the domestic and industrial demands consume about 10 and 9.5%, respectively (CEDRAE 2011). Surface water in Egypt is exposed to contamination, causing harm to human and aquatic ecosystem. In addition, 80% of untreated industrial sewage of factories is discharged into the river (ADB 2009). The Nile River water quality considerably affects the Egyptians health. This is because the Nile River is still considered as the main source of freshwater in the country (Abdel-Wahaab and Mohy 2011). The increased water contamination causes a rapid increase in severe diseases such as hepatitis, kidney failure, and congenital heart disease (Bottoms 2014). Egypt is the first country suffering from hepatitis C virus, and about 20% of the infected people are infected from drinking water. Bottoms (2014) shows that 5.5% of the total deaths in Egypt are caused by the polluted drinking water. The water for agricultural purposes is pumped out directly from the river without any treatment, while the drinking water is poorly treated in treatment plants.
Rosetta branch
The Rosetta branch covers 239 km in the northwestern border of Egypt. Its depth ranges from 2 to 4 m, and its width averages 180 m (Negm et al. 2011). It is used for drinking, fishing, and irrigation purposes. The daily flow of the Rosetta branches averages 21,500,000 m3/day (Negm et al. 2011). The drains receive domestic, industrial, and agricultural wastes. These wastes are disposed directly into the Rosetta branch without any treatments. It was reported that more than 900 MCM of wastes from the Greater Cairo area of agriculture, domestic, and industrial wastes discharges monthly in the Rosetta branch (El-Barbary et al. 2008). Abdo (2002) showed that the El-Rahawy drain is considered as the major contamination source along the Rosetta branch. It was shown that the El Rahawy drain is highly polluted by organic and inorganic contaminants (El-Bouraie et al. 2011; Ezzat et al. 2012). This pollution damages aquatic life (Raju et al. 2009; Singh et al. 2007; Belkhiri et al. 2010).
This work investigates the classification of potential contaminants and evaluates the suitability of water for drinking and fishing. Hydrochemistry of the Rosetta water and the sources responsible for its hydrochemistry changes are assessed too. This study is different from other previous ones in recognizing the geochemical processes controlling the water quality. In addition, it determines the hydrochemistry facies of the studied water. In addition, the irrigation function (sodium adsorption ratio (SAR), residual sodium carbonate (RSC), sodium percent (Na%), and magnesium hazard (MH)) is calculated to identify hydro-environmental status.
Material and methods
Sampling
Study area
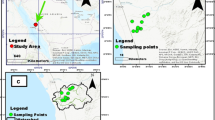
The study area extends 120 km along the Rosetta branch (Fig. 1). Fifteen stations were selected (Table 1), starting from upstream of the El-Rahawy drain to downstream of the Tala drain, including five drains (EL-Rahawy, Sabal, Zawiet El-Bahr, El-Tahreer, and Tala). Three stations at each drain (upstream, outfall, downstream) were selected.
Physicochemical analyses
Water samples were collected in winter 2014. The samples were collected in triplicate from the study area in polyethylene bottles (Ramizankhani et al. 2008a, b) by water samplers. The bottles were rinsed primarily with distilled water to reduce the possibility of any pollution. The samples were kept in ice boxes and transported directly to the laboratory. The samples were preserved for physicochemical parameters. The pH, temperature, electrical conductivity (EC), total dissolved solids (TDS), and dissolved oxygen (DO) were determined in the field in situ by a multi-probe system model Hydrolab Surveyor.
Physical and chemical analyses were done according to standard methods of APHA 2012 (Al-Sabahi et al. 2007). The concentrations of sodium (Na), potassium (K), calcium (Ca), and magnesium (Mg) ions were measured by atomic absorption spectroscopy and flame photometry (Perkin-Elmer Corporation). While the concentrations of bicarbonate (HCO3) and chloride (Cl) were estimated by titration. On the other hand, sulfate (SO4) was determined by colorimetric and turbid metric methods.
An Aquachem program was used for hydrochemistry plotting and irrigation assessment parameters.
Results and discussions
Physical and chemical analysis
To evaluate the suitability of water for different purposes, the physicochemical parameters were determined (Table 2). The pH values of the investigated samples vary from 7 to 8. The samples are neutral to slightly alkaline. The relatively lower value of pH at the station (II) of the El Rahawy drain outlet of 7 ± 0.06 may be due to lower actions of phytoplankton and fungal bacterial activities. EC of the studied water collected from the area ranges from 377 to 1625 μmoh/cm. The EC and TDS values are greater than the permissible limit in some sites. Generally, conductivity is correlated with the concentrations of total salt content and chlorosity. HCO3 − is the prevailing ion in the Rosetta branch, and it ranges from 152.3 to 426.6 mg/l. HCO3 − is the main anion in the samples due to the great amount of organic matter accessible to bacterial decomposition via increasing effluents (Toufeek and Korium 2008). SO4 2− concentrations range from 31.5 to 322.6, while Cl− concentrations range from 22.9 to 214.6 mg/l. High chloride levels in drains could be related to the effluent of sulfur compound (Abdel-Satar and Elewa 2001). It may be also attributed to the drought period. In addition, it may be related to the agricultural runoff which discharges into drains after the washing of some reclaimed lands, i.e., Ca2+ values show variation from 34.3 to 74.5 mg/l (Cerling et al. 1989). Furthermore, Na+ is a widespread cation, which ranges from 32.1 to 187.4 mg/l. The drains have higher concentrations of SO4 2− and Cl− than other samples, while CO3 − concentrations are not detected in all samples. Mg2+ values show a variation from 7.2 to 28.9 mg/l whereas K+ concentrations range from 7.5 to 30.5 mg/l. It is found that the order of predominance of anions of the Rosetta branch is HCO3 − > SO4 2− > Cl− > CO3 − while the cations show Na+ > Ca2+ > Mg2+ > K+ arrangement.
The relatively high concentrations of SO4 2− and Cl− in the investigated drains predict that potassium and sodium came from a mix of different contaminated sources of water (Karmegam et al. 2011). The higher concentrations of Na+ may be due to the chemical fertilizer. Na+ has higher concentrations than Ca2+; this could be related to the ion exchange process Ca2+ values increased due to [Ca(H2SO4)2+ Ca (HPO4)] fertlizers. The lower concentrations of K+ are due to high adsorbtion of K+ than Na+ (Goher 1998). Also, it could be attributed to take of K+ from surface water by organisms. Naik et al. 2009 found in the Koyna River that most of the water samples are controlled by alkaline earths (Ca2+, Mg2+) and weak acids (HCO3 −, CO3 2−). This result emphasizes on the suggestion that the running surface waters (i.e., rivers) are characterized by their high Ca2+ and Mg 2+ concentrations.
Table 3 shows a positive correlation between Na+ and Cl−, Na+ and SO4 2−, and K+ and SO4 2− representing that these ions may be derived from common sources. The positive correlation of the ions Na+, K+, SO4 2−, Cl−, and K+ suggests good indication of the influence of agricultural fertilizers in this region (Subba Rao et al. 2012).
Hydrochemical facies
The Piper trilinear diagram (Piper 1944) is a technique applied for the description of hydro-chemical progress and reorganization of the dominant processes.
Five hydrochemical facies are shown in the Piper trilinear diagram (Piper 1944). They have been recognized as Na-Ca-Mg-HCO3-Cl, the major facies (52%), Na-Ca-Mg-HCO3-SO4-Cl (24%), Ca-Na-Mg-HCO3-SO4 (12%), Ca-Mg-Na-HCO3 (6%), and Na-Ca-Cl-HCO3 (6%), depending on major ion chemistry. The resulted diagram is shown in Fig. 2.
The type of water demonstrates the dispersion of the different anion (HCO3 −, Cl−, and SO4 2−) and different cation (Na2+, Ca2+, and Mg2+) contents. The diagram indicates that water is a mix of different sources or evolution pathways under various complex physicochemical processes (Karmegam et al. 2011). Figure 2 shows that the predominant type of water is bicarbonate alkaline, with higher alkaline contents and no tendency to a regime of dominant water types.
TDS are an important factor for wastewater quality evaluation and effluents prior to discharge. The water samples have relatively high TDS content; it varies from 242 to 1040 mg/l. Also, the comparatively higher electric conductivity (1625 mg/l) refers to the higher dissolved load of drains from agricultural and industrial effluents. The lower concentrations of K+ may be interrupted to its resistance to depletion. The agricultural activities are responsible for that much more than the potassium fertilizers (Sharifi and Safari Sinegani 2012).
Geochemical processes
To estimate the change in water geochemistry, it is necessary to know and reorganize the hydrochemical processes related to it. Normally, the Langelier-Ludwig diagram is utilized to recognize water deterioration of the study area (Fig. 3). According to Langelier-Ludwig, the source of contamination may be indicated by the interruption of biologic influence problems. This comes after the discharge of untreated domestic and agricultural wastes directly into the water (Hem 1992).
Langelier-Ludwig diagram (Fig. 3), it shows that sulfate and chloride concentrations rise in the El rahawy and Tala drains. On the other hand, the increase in potassium and sodium concentrations results from mixing with different contaminated sources of water (Abdel-Satar and Elewa 2001). The contaminant concentrations indicate that the contaminant levels were generally controlled by the land use type of the area. It is intensive in some regions of the drains due to the change in water quality of the non-point sources of contamination.
Evaporation
A diagram of Na against Cl− and the Na/Cl ratio against EC (Fig. 4a, b) demonstrates that evaporation is the main process that controls the water chemistry. Na+ and Cl− fluctuated sharply while the unchanged ratio of Na/Cl (Fig. 4a) suggests that the mineral precipitation is due to the evaporation process. Also, this indicates that Na+ and Cl− are derived from the same source (Singh et al. 2007). Figure 4b shows the Na/Cl ratio against EC, and it has an almost linear relationship with very little inclination. This suggests that the high concentration of Na+ especially in the drains not only resulted from the evaporation process but also is due to the effect of the land drainage and seepage from agriculture wastes. Sodium sources are NaNO3 fertilizers, beside the human activities and domestic wastes (Reddy and Kumar 2010).
The surface water is contaminated by agricultural and industrial sewage. It is considered to be unsuitable for drinking and fishing especially the El Rahawy drain. Thus, this water requires a specific treatment.
Cation-exchange reaction
Distribution of ions in the water is controlled by the most significant geochemical reactions which is cation exchange reactions. The excess of Ca2+ related to Na+ can be recognized as cation exchange reaction. Figure 5a shows the decrease in Ca2+ cations compared to Na+ cations. This indicates that the cation exchange reaction takes place in the water (Singh et al. 2005). Also, the relation between Na+ and Cl− can be recognized as cation exchange reaction, which is one source of increasing Na+. Low concentrations of both Na+ comparable to Cl+ or excess concentrations of both Na+ and Cl− is an indication of the cation exchange process (Rajmohan and Elango 2004). Usually, when cation exchange reaction releases an excess of Na+, it will be balanced by SO4 2− and alkalinity but not by Cl−. In the same way, when Na+ is precipitated, an overload of Cl− is balanced by Mg2+ and Ca2+, but not by Na+.
By calculating the chloro-alkaline index (CAI) (Doneen 1964), it can be shown that most of the water samples have a negative index. This means that in most of the water samples, cation-anion exchange reaction occurs. Other positive value indices refer to the replacement of K+ and Na+ with Ca2+ and Mg2+ in the sediments, representing the base exchange reaction (Aghazadeh and Mogaddam 2011). Also, the diagram of Ca2+ + Mg2+ vs HCO3− + SO4 2− (Fig. 5b) shows that the results shift to the right side because of the excess of SO4 2− + HCO3 − and the data located under the equiline indicating the dominance of ion exchange phenomena (Fisher and Mullican 1997). The diagram of Na+ + K+ vs Cl− (Fig. 5c) shows that all samples located over the equiline have an extra source of K+ and Na+ from weathering silicate minerals. Usually, Cl− favors to connect with Na+ and K− more than Ca2+ and Mg2+. This suggests that Cl− may be used in forming sodium and potassium chloride, besides formation of sodium and potassium bicarbonate and sulfate (Tirumalesh et al. 2010). A high concentration of Na+ compared to Cl− proposes that Cl− dissolution occurs by the evaporation process.
Water quality assessments
To determine the water quality, assessments of its suitability for drinking, fishing, and irrigation uses should be evaluated (Dar et al. 2011)
Suitability for drinking uses
Most of the water samples in the study area are not suitable for drinking purposes. TDS should be less than 500 mg/l to be suitable for drinking water (WHO 2011); more than 1000 mg/l is unsafe. All the analysed samples from outfall drains’ TDS are between 500 and 1000 mg/l except the Tala outfall drain which is more than 1000 mg/l.
On the other hand, high concentrations of Cl− and HCO3 − have harmful effects on humans (Sharifi and Safari Sinegani 2012). The results show high HCO3 − values for all outfall drains, but at the other measure stations (upstream, downstream), it is in the permissible limit. The values of SO4 2− are under the permissible limit (100 ml/l) except at the outfall drains. At low alkalinity, sulfate can cause corrosion of metals. Also, sulfate can be changed to hydrogen sulfide in anaerobic conditions, which is the reason of odor corrosion of the sewer (Shuval et al. 1986). The data show high values of NH3 ranging from 1.5 to 22.6 mg/l. Generally, there is an obvious increase in TDS, EC, and NH3 for the studied drains to be considered as drinking water (WHO 2011).
Suitability for fishing uses
Dissolved oxygen (DO) is considered to be an important parameter for evaluating the degree of pollution in water. Also, DO is essential for estimating the metabolism of aerobic organisms and it affects organic decomposition, too. At low oxygen concentrations, the chemicals increase the susceptibility of fish to toxicity. Oxygen content has an obvious effect on the dissolution of inorganic nutrients. The conditions of the environment, whether it is anaerobic or aerobic, can be detected by oxygen contents. Dissolved oxygen in the investigated samples, which is less than 0.5 mg/l, is a great problem for aquatic organisms (Sabre and Abdel-Satar 2001). In the studied drains, all except the El Tahreer drain are below 5 mg/l, which produces anoxic condition in the drains especially in the El Rahawy drain. The low DO of the El-Rahawy drain may be related to the high concentrations of organic contaminants and nutrients which consume the dissolved oxygen through oxidation processes (Howari and Banat 2002). The DO of the Nile River is much lower than the standard limits for the drain waters; the DO varies from 0.5 to 7.4 mg/l.
Ammonia value is more than 1 mg/l. It can be considered as a sign of organic pollution. If ammonia is higher than 2.5 mg/l, then the water will be toxic to aquatic organisms (Ghallab 2000). Also, ammonia is a vital compound for animal production. The results show high values of NH3 ranging from 1.5 to 22.6 mg/l which exceed the permissible limits of 0.5 mg/l. Higher concentrations of NH3 are an indication of organic pollution. It may be attributed to an increase in the denitrification process by bacteria in the water under anaerobic conditions of low oxygen levels (Korium and Toufeek 2008). Generally, high concentrations of NH3 and BOD, beside a depletion of DO in drains, have a bad effect on fish especially in the El Rahawy drain. These factors result in that there are no possibilities of fishing in all drains, and this will be decreased in the downstream area.
Suitability for irrigation uses
To evaluate the appropriateness of the Rosetta branch for irrigation uses, parameters like sodium percent, SAR, and residual sodium carbonate (RSC) were determined (Table 4).
Sodium percent
The sodium values and electric conductivity are very significant parameters. There are always commensurable substances of dissolved solid in the water used for irrigation. These solids are very important for plant growth, besides their effect on soil such as aeration and permeability (Arveti et al. 2011). Salinization is the unfavorable side effect of irrigation. The saline soil is formed when the salt concentrations are increasing, especially sodium ions. Na % has bad influences on crop growth and yield. It can damage the soil, especially with a bad drainage. Na % can be calculated using the following formula (Todd 1980).
Na % should not exceed more than 60% for the irrigation utilizations (Sadashivai et al. 2008). The results range from 23.3 to 60.1%. The high value of Na % causes the weakness of soil permeability and deflocculation (Brindha and Elango 2012). The concentration of Na+ in the outfall is always greater than in the downstream area. This is due to low water level at winter season in the outfall, while at the downstream area the levels of water increase causing an increase in leaching of fertilizer residues into the aquatic ecosystem of the branch through drains. Also, the high concentrations of Na % in drains may be due to high salinity of atmospheric water precipitation (Abdo et al. 2010). This reduces their utilization for irrigation purposes.
Sodium adsorption ratio
The resulted values of sodium for irrigation water, which is known as the sodium adsorption ratio (SAR), were divided into four groups C-1, C-2, C-3, and C4 (USSL 1954). The relative and absolute concentrations of sodium were determined. SAR is calculated using the following formula:
Soil sodium adsorption depends on SAR of water; high sodium values and low calcium value destroy the soil (Hem 1992).
Irrigation waters are divided into four groups depending on the sodium adsorption ratio (SAR) as S-1 (<10), S-2 (10–18), S-3 (18–26), and S-4 (>26). The results of SAR in the water samples range from 1.1 to 4.7. Salinity categorization was utilized to the samples. The diagram (Fig. 6) (Richards 1954) shows that about half of the water samples of the Rosetta branch have medium salinity with low Na ion exchange (C2-S1). The other half of the samples have high salinity with low Na ion exchange (C3-S1). This means that the water samples of drains in the Rosetta branch are classified as of poor category, and they are harmful to be used for irrigation.
Residual sodium carbonate
Residual sodium carbonate (RSC) is an amount of carbonate and bicarbonate in excess of alkaline earth which has affected the convenience of water for irrigation uses (Ragunath 1987). Its formula is illustrated bellow in Eq. 4 (Ragunath 1987). Ca2+ and Mg2+ can be participated when their values are less than the values of bicarbonate and carbonate (Todd and Mays 2005). This leads to an increase in the sodium concentrations as sodium carbonate. An excess concentration of RSC causes a rise in the sodium sorption on soil (Eaton 1950).
In the studied samples, RSC values range from −1.2 to 1.14 meq/l. Irrigation water which has RSC values greater than 5 meq/l will damage the plants. Water with RSC values above 2.5 meq/l is not appropriate for irrigation uses. All the RSC samples are below 2.5 meq/l (Dehayer et al. 1997).
Thakur et al. (2016) proved that groundwater in Punjab, India, is suitable for irrigation in all types of soil through the results of geochemical analysis of groundwater. They conclude that due to some chemical indices such as Na %, sodium absorption ratio (SAR), and residual sodium carbonate (RSC) parallel with the abundance ions of HCO3 − > Cl− > SO4 2− > NO3 − and Mg2+ > Ca2+ > Na+ > K+, the analyzed water is fit for irrigation purposes.
Magnesium hazard
Magnesium hazard (MH) values were also calculated by Szabolcs and Darab’s (1964) formula:
An MH value more than 50 is unsafe and unsuitable for irrigation water proposes (Joshi et al. 2009). In the study area, all the samples that contain magnesium hazard (MH) are less than 50. The results range from 30.4 to 48.5.
Conclusions
There are serious problems of contamination found in the Rosetta branch. This is due to the increase in waste discharge along drains into the Rosetta branch. More attention must be given to reduce the contamination from these drains because of their effects which become significant. Extreme Nile discharge within the Rosetta branch and severely contaminated irrigation drains could relieve the problem of salinity concentration. Therefore, constant monitoring of the water quality of Nile is required to notice any change in the quality and reduce outbreaks of health disorders. Also, it will decrease the harmful influences on the aquatic ecosystem especially after decreasing the water level due to the Ethiopia dam. The surface water is contaminated by agricultural and industrial sewage, and it is considered to be unsuitable for drinking and fishing especially the El Rahawy drain. The Rosetta branch is dominated by HCO3 − anions. Hydrochemical reaction modeling shows that the cation exchange process and evaporation processes are the domain processes that determine the water chemistry in the study area. The water types of the drains resulted from different contamination sources under various physicochemical processes.
References
Abdel-Dayem S (2000) Drainage experiences in arid and semi-arid regions, principal drainage specialist. the World Bank, Washington, DC
Abdel-Satar AM, Elewa AA (2001) Water quality and environmental assessments of the River Nile at Rosetta Branch. The Second International Conference and Exhibition for Life and Environment, Alexandria 136-164.
Abdel-Shafy I, Aly O (2002) Wastewater management in Egypt. In: Zaidi MK (ed) Wastewater reuse–risk assessment, decision-making and environmental security. Springer, Cairo, pp 375–382
Abdel-Wahaab R, Mohy E (2011) Wastewater reuse in Egypt: opportunities and challenges, pp. 22–24, Water Resources and Irrigation (MWRI), Dubai.
Abdin A, Gaafar I (2009) Rational water use in Egypt, Technological Perspectives for Rational Use of Water Resources in the Mediterranean Region
Abdo H (2002) Environmental studies on Rosetta branch and some chemical applications at the area extend from El Kanater El Khyria to Kafr ElZyat City. Ph. D Thesis, Fac. of Sci. Ain Shams Univ.,Cairo, Egyp
Abdo H, Sabae Z, Haroon M, Refaat M, Mohammed S (2010) Physico-chemical characteristics, microbial assessment and antibiotic susceptibility of pathogenic bacteria of Ismailia Canal water, River Nile, Egypt. J of Amer Sci 6(5):234–250
African Development Bank (ADF) (2009). Egypt private sector country profile accessed 15 May 2012 http://www.afdb.org/fileadmin/uploads/afdb/Documents/Project-andOperations/Brochure%20Egypt%20Anglais.pdf
Aghazadeh N, Mogaddam A (2011) Investigation of hydrochemical characteristics of groundwater in the Harzandat aquifer, northwest of Iran;. Environ Monit Asses 176(1–4):183–195
Allam N, Gamal I (2007) Water resources in Egypt: future challeges and opportunities. Water Int 32(2):205–218
Al-Sabahi E, Rahim A, Zuhairi W, Al-Nozaily F, Alshaebi F (2007) The characteristics of leachate and groundwater pollution at municipal solid waste landfill of Ibb City, Yemen. Am J Environ Sci 5(3):256–266
Arveti N, Sarma M, Aitkenhead-Peterson J, Sunil K (2011) Fluoride incidence in groundwater: a case study from Talupula. Andhra Pradesh, India, Environ Monit Asses 172:427–443
Belkhiri L, Boudoukha A, Mouni L (2010) Groundwater quality and its suitability for drinking and agricultural use in Ain Azel plain, Algeria. J Geogr Reg Plan 3(6):151–157
Bottoms I (2014) Water pollution in Egypt: causes and concerns, Egyptian Center for Economic and Social Rights. Accessed 13 July 2014. http://ecesr.org/en/wp-content/uploads/2014/03/ECESR-Water-Polllution-En.pdf
Brindha K, Elango L (2012) Impact of tanning industries on groundwater quality near a metropolitan city in India. Water Resour Manag 26(6):1747–1761
Center for Environment and Development for the Arab Region and Europe (CEDARE) (2011). Water use efficiency and economic approach, national study Egy. http://www.planbleu.org/publications/etudes_efficience_eau/National_ report_ Water_EG.pdf pt
Cerling TE, Pedrson BL, Damm KLV (1989) Sodiumcalcium ion exchange in the weathering of shales: implication fror global weathering budgets. Geology 17:552–554
Dar I, Sankar K, Shafi T, Dar M (2011) Investigation of groundwater quality in hardrock terrain using geoinformation system. Environ Monit Assess 176(1–4):575–595
Dehayer R, Diatloff N, Gordon I (1997) Irrigation water quality salinity and soil structure stability, Water facts, ISSN 1327–5364
Doneen D (1964) Notes on water quality in agriculture. Water Science and Engineering, University of California, Davis, CA
Eaton FM (1950) Significance of carbonates in irrigated waters. Soil Sci 69:127–128
El-Barbary AA, El-Bouraie MM, Yehia MM (2008) Evaluation of organochlorine pesticides (OCPs) in surface water and bed sediment samples from the River Nile at Rosetta branch. J Appl Sci Res 4:1985–1993
El-Bouraie MM, El-Barbary AA, Yehia MM (2011) Determination of organochlorine pesticide (OCPs) in shallow observation wells from ElRahawy contaminated area, Egypt. Enviro Rese Engin and Manag 3(57):28–38
Ezzat SM, Mahdy HM, Abo-state MA, Abd El-Shakour EH, El-Bahnasawy MA (2012) Water quality assessment of River Nile at Rosetta branch. Impact of drains discharge. Middle-East J Sci Res 12(4):413–423
Fisher R, Mullican F (1997) Hydrochemical evolution of sodium sulphate and sodium chloride groundwater beneath the northern Chihuahuan desert, Trans-Pecos, Rexas. USA Hydrogeol Jour 10:455–474
Ghallab M H (2000) Some physical and chemical changes on the River Nile downstream of Delta barrage at El-Rahawy drain. M.Sc. Thesis. Fac. Sci. Ain Shams Univ., Egypt
Goher M (1998) Factors affecting the precipitation and dissolution of some chemical elements in River Nile at Damietta branch, M. Sc. Thesis, Fac. Of Sci. Menofiya Univ. Egypt
Hem J (1992) Study and interpretation of the chemical characteristics of natural waters, 3rd edn US Geol. Survey, water supply paper 2254, scientific pub, Jodhpur
Howari F, Banat K (2002) Hydrochemical characteristics of Jordan and Yarmouk River waters: effect of natural and human activities. Journal of Hydrology and Hydromechanics 50(1):50–61
Joshi DM, Kumar A, Agrawal N (2009) Assessment of the irrigation water quality of River Ganga in Haridwar District India. J Chem 2(2):285–292
Karmegam U, Chidambaram S, Prasanna M, Sasidhar P, Manikandan S, Johnsonbabu G, Dheivanayaki V, Paramaguru P, Manivannan R, Srinivasamoorthy K, Anandhan P (2011) A study on the mixing proportion in groundwater samples by using Piper diagram and Phreeqc model. The Chinese Journal of Geochemistry 30:490–495
Korium M, Toufeek M (2008) Studies of some physico-chemical characteristics of old Aswan Dam reservoir and River Nile water at Aswan. EgyptJ Aqua Res 34:149–167.3
Naik PK, Awasthi AK, Anand A, Behera PN (2009) Hydrogeochemistry of the Koyna River basin, India. Environmental Earth Sciences 59(3):613–629
Negm A, Tarek M, Mohamed N, Wessam Y (2011) Impact of future discharges on Damietta branch morphology, Fifteenth International Water Technology Conference, IWTC Alexandria, Egypt
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analysis. USGS Groundwater Note, No. 12
Ragunath H (1987) Groundwater, 2nd, edn edn. Wiley Eastern Ltd., New Delhi, p 563
Rajmohan N, Elango L (2004) Identification and evolution of hydrogeochemical processes in an area of the Palar and Cheyyar River Basin. Southern India, Environ Geol 46:47–61
Raju NJ, Ram P, Dey S (2009) Groundwater quality in the Lower Varuna River Basin, Varanasi District, Uttar Pradesh. J Geol Soc India 73:178–192
Ramizankhani R, Shareef M, Sadatipour M, Abdulahzadeh R (2008a) A mathematical model to predict nickel concentration in Karaj River sediments Iran. J Environ. Sci. Eng 5(2):91–94
Ramizankhani R, Shareef M, Sadatipour M, Abdulahzadeh R (2008b) A mathematical model to predict nickel concentration in Karaj River sediments Iran. J Environ Sci Eng 5(2):91–94
Reddy A, Kumar K (2010) Identification of the hydrogeochemical processes in groundwater using major ion chemistry: a case study of Penna–Chitravathi river basins in southern India. Environ Monit assess 170:365–382
Richards L (1954) Diagnosis and improvement of saline and alkali soils, U S Dept Agri Hand Book, No. 60
Sabre S, Abdel-Satar A (2001) Chemical and bacteriological studies on El-Salam Canal. Egypt J Egypt Acad Soc Environ Develop 2:173–179
Sadashivai C, Ramakrishnaiah C, Ranganna G (2008) Hydrochemical analysis and evaluation of groundwater quality in Tumkur Taluk, Karnataka State, India. Intl J Environ Research and Publichealth 5(3):158–164
Sharifi Z, Safari Sinegani A (2012) Arsenic and other irrigation water quality indicators of groundwater in an agricultural area of Qorveh Plain, Kurdistan, Iran. Am Eurasian J Agric Environ Sci 12(4):548–555
Shuval H, Adin A, Fiatal B, Raawitz E, Yekuterl P (1986) Wastewater irrigation in developing countries. Health effects and technological solutions, 52nd edn. World Bank Technical Paper, Washington DC
Singh A, Mondal G, Singh S, Singh P, Singh T, Tewary B (2005) Hydrochemistry of reservoirs of Damodar River basin, India: weathering processes and water quality assessment. Environ Geol 8:1014–1028
Singh A, Monda G, Singh S, Singh P, Singh T, Tewary SA (2007) Aquatic geochemistry of Dhanbad District, Coal City of India: source evaluation and quality assessment. Jour Geol Soc India 69:1088–1102
Subba Rao N, Surya Rao P, Venktram Reddy G, Nagamani M, Vidyasagar G, Satyanarayana N (2012) Chemical characteristics of groundwater and 3 assessment of groundwater quality in Varaha River Basin, Visakhapatnam District, Andhra Pradesh, India. Environ Monit Assess 184:5189–5214
Szabolcs I, Darab C (1964) The influence of irrigation water of high sodium carbonate content of soils. In: Proceedings of 8th international congress of ISSS, Transaction II, pp 803–81
Thakur T, Rishi MS, Naik PK et al (2016) Elucidating hydrochemical properties of groundwater for drinking and agriculture in parts of Punjab, India. Environ Earth Sci 75:467
Tirumalesh K, Shivanna K, Sriraman A, Tyagi A (2010) Assessment of quality and geochemical processes occurring in groundwaters near central air conditioning plant site in Trombay, Maharashtra, India. Environ Monit Assess 163(1–4):171–184
Todd D (1980) Groundwater hydrology, 2nd edn. Wiley, New York, p 535
Todd D, Mays L (2005) Groundwater hydrology, 3rd edn. Wiley, Hoboken, NJ, p 656
Toufeek M, Korium M (2008) Nutrient salts in Aswan Dam reservoir and River Nile water at Aswan Egypt. Egyptian J of aquat Resear 34:218–236
USSL (1954) Diagnesis and improvement of saline and alkali soils. USDA Hand book 60:147pp
Wagdy A (2009) Progress in water resources management: Egypt, Proceedings of the 1st Technical Meeting of Muslim Water Researchers Cooperation (MUWAREC), Malaysia
Wahaab R, Mohamed I (2004) Water quality assessment of the River Nile system: an overview. Biomed Environ Sci 17:87–100
WHO (2011) Guidelines for drinking water quality. World Health Organisation, Geneva. http://www.who.int/water_sanitation_health/publications/2011/dwq_chapters/en/ date of access : 12.4.2016
Acknowledgements
The authors are very thankful to TU Darmstadt University, Germany, for all facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We confirm that this work is original and the manuscript has not been submitted to another journal. The manuscript has not been published previously (partly or in full). No data have been fabricated or manipulated. No data, text, or theories by others are presented.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Elhaddad, E., Al-Zyoud, S. The quality assessment of pollution of Rosetta branch, Nile River, Egypt. Arab J Geosci 10, 97 (2017). https://doi.org/10.1007/s12517-017-2870-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-017-2870-y