Abstract
Smectite clay has great potential for the removal of heavy metal ions from aqueous solution. This work aims to develop inexpensive, highly available, effective metal ion adsorbents from clay minerals as alternatives to existing commercial adsorbents. In particular, natural clay was modified with sulfuric acid to yield excellent adsorbent material. The adsorptive interactions of Cr (VI) ions with natural clay and their acid-activated derivative in aqueous solution are investigated in this study. The adsorption experiments were carried out under batch process with Cr (VI) concentration, pH, time, and temperature as the variables. The adsorption was strongly dependent on pH of the solution. Adsorption was very fast at low coverage, and equilibrium was approached within 35 min. The kinetic data were analyzed using different kinetic models. It was shown that the adsorption of Cr (VI) ions could be described by a pseudo-second-order equation and Elovich model. The experimental data were also analyzed using two- and three-parameter isotherm models of adsorption. Thermodynamic parameters such as ΔG0, ΔH0, and ΔS0 have been evaluated, and it has been found that the sorption process was spontaneous and exothermic. From all our data, we conclude that the treated clay by sulfuric acid investigated in this study showed good potential for Cr (VI) ions removal from aqueous solutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are found in water, air, and soil. The major sources of heavy metals in water and soil are wastewater streams from many industrial processes (Olayinka et al. 2007).
The removal of toxic metal ions from wastewater is an important and widely studied research area. One of the heavy metals that have been a major focus in wastewater treatment is chromium. The increasing use of chromium in many industries has led to large amounts of polluted aqueous effluents which contain high levels of chromium. Due to its solubility, it is highly mobile in soil and aquatic environments and readily penetrates plant and animal epidermis where it irritates tissues (Olayinka et al. 2007; Chauhan and Sankararamakrishnan 2011).
In this respect, because of their toxicity applications, trivalent chromium and hexavalent chromium are two common existing oxidation states of chromium found in the environment. Most of the hexavalent compounds are toxic, carcinogenic, and mutagenic, and it can even cause lung cancer also by El-Sikaily et al. (2007) and Li et al. (2008). Major sources of chromium are effluents from electroplating, metal finishing, chromium mining pigments, leather tanning, wood protection, electrical and electronic equipments, manufactures, and catalysis (Mohan and Pittman 2006). Treatment of the chromium effluents poses a serious problem to ecosystems and cause great public concern. Therefore, it is necessary to eliminate chromium from the environment, and it is also essential that the effluents should be controlled before discharging hexavalent chromium into aquatic environments in order to prevent the deleterious impact of Cr (VI) on the ecosystem and public health (Rengaraj et al. 2001).
For this purpose, researchers in recent years investigated various physicochemical methods for removal of chromium and other heavy metals. The most important of these techniques include chemical precipitation, filtration, ion exchange, reverse osmosis, and membrane systems. However, all these techniques have their inherent advantages and limitations in application. In the last few years, adsorption has been shown to be an alternative method for removing dissolved metal ions from liquid wastes (Bayat 2002). The adsorption method is commonly used and several studies reported on various adsorbents (Olayinka et al. 2007; Chauhan and Sankararamakrishnan 2011; El-Sikaily et al. 2007).
The economic issue and the need for regeneration of used adsorbents forced researchers to investigate other inexpensive and low-cost adsorbents for the removal of chromium (Shen et al. 2012; Sahinkaya et al. 2012; Prigione et al. 2009; Sari et al. 2011). However, the literature is still insufficient to cover this problem, and more work and investigations are needed to deal with other locally available adsorbents to eliminate Cr (VI) from industrial wastewater samples with different compositions and characteristics (Sharma and Bhattacharyya 2004).
Clay minerals are effective adsorbents for the removal of heavy metals, owing to their high specific surface area with unique swelling, intercalation, and ion exchange properties, low cost and ubiquitous presence in most soils. To increase the adsorption capacity, clays are modified in various ways such as treatment by inorganic and organic compounds, acids, and bases (Sharma and Weng 2007). It was reported that heat treatment also enhanced the adsorption capacity of some clays (Lee et al. 2010). Acid or other reagents are used for activation of clays and are due to their high specific surface area, high chemical and mechanical stabilities, and various surface and structural properties. The study of the adsorption of Cr (VI) from aqueous solutions by natural or treated clay minerals was the subject of several investigations (Weng et al. 2008; Fritzen et al. 2006; Maryuk et al. 2005; Benhammou et al. 2007; Huang et al. 2008), but no study to date has considered the adsorption of Cr (VI) onto south Tunisian clay. Therefore, it was the objective of the present work to investigate the amenability of removal of Cr (VI) anionic species by smectite. The effects of various parameters affecting adsorption like contact time, initial metal ion concentration, and pH have been studied, and data have been presented using adsorption kinetic models. Two- and three-parameter isotherm models were used for the description of adsorption data obtained in this study.
Materials and experimental methods
Several factors influence the removal of heavy metals from water by the adsorption process, e.g., type and concentration of ion solution, adsorptive materials, pH, time, and temperature. The experiments were carried out by taking these as the variables and were investigated by varying any one of the process parameters and keeping the other parameters constant.
Materials
In the present work, green clay selected from site of Djebel EL’Aidoudi of “Hamma” area, located in south Tunisia, was used as raw material.
The as-received material was purified by aqueous dispersion and decantation. After a purification process, the clay remained free of quartz and the fraction with particle size less than 40 μm was separated and subjected to activation.
Acid activation
Acid activation was carried out with sulfuric acid H2SO4 in a jacketed glass reactor equipped with a reflux condenser, a thermometer, and a stirrer. At the end of each experiment, the solid content was immediately filtered, washed with water until the washing water was neutral, and dried.
The activated sample was obtained according to the following procedure: 100 g of material clay micro-powder was mixed with an aqueous H2SO4 solution from Scharlau Chemie (acid concentration of 35 %) in a jacketed glass reactor regulated at fixed temperature (96.6 °C) by a thermostatic bath for 3.75 h under mechanical stirring at 200 rpm and then washed by distilled water for many times until pH was achieved. The obtained samples were dried at 60 °C for 24 h.
Batch adsorption studies
Batch experiments, a technique commonly used to obtain data on the removal efficiency of a given adsorbent under static conditions was selected as an appropriate technique in the current study, with various natural and activated clays were conducted to investigate the parametric effects of initial adsorbate concentration on Cr (VI) adsorption. Chromium samples were prepared, a known quantity of potassium dichromate K2Cr2O7 (reagent grade from Merck, Darmstadt, Germany), by dissolving them in double-distilled water and used as a stock solution and diluted to initial concentration (range 10 to 100 mg/l), 50 ml of Cr (VI) solution of known concentration (C 0), and initial pH was taken in a 100-ml screw-cap conical flask with a required 0.05 g amount of adsorbent and was agitated at a speed of 200 rpm in a thermostatic shaker bath at 20 °C for a specified period of contact time. Then, the solution was filtered through a 0.45-μm membrane filter. The initial pH of the solution was adjusted by using either 0.1 N NaOH or 0.1 N HCl.
All reagents used were of analytical grade, and all solutions were prepared in ultrapure water with a minimum resistivity of 18 MΩ cm obtained from a Milli-Q Millipore system (Billerica, MA, USA).
A colorimetric method was used to determine the remaining concentrations of Cr (VI) in the samples. The pink complex formed between 1,5-diphenycarbazide and Cr (VI) was measured at 540 nm using a UV–visible spectrophotometer (Bandegharaei et al. 2010).
All experiments were carried out in triplicate, and the concentrations given are average values.
The filtrate was analyzed for the remaining Cr (VI) concentration. The amount of Cr (VI) adsorbed in (mg/g) at time t was computed by using the following equation:
where q e is the amount of Cr (VI) ions adsorbed on the clay (mg/g), C 0 is the initial Cr (VI) ion concentration in solution (mg/l), C e is the equilibrium Cr (VI) ion concentration in solution (mg/l), V is the volume (l), and m is the amount of clay (g).
Results and discussion
Characterization of the adsorbent
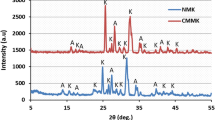
The X-ray diffraction analysis indicated that the mineralogical compositions of clays are mainly composed of smectite, quartz, and smectite-calcite (Fig. 1).
After acid activation, the disappearance of the characteristic peak of the smectite at 9.7 Å was observed (Fig. 1). They recommend that this phenomenon is due to treatment heat at high temperatures. This generates a burst of leaves which in turn changes the structure of the clay mineral (Weng et al. 2008). According to Table 1, the chemical analysis showed that the main constituents of clay are silica, alumina, and iron oxides. After acid activation, percentage of both the exchangeable ions and the octahedral cations decreased while that of silica increased due to its lower solubility in acid solution (Table 1). The acid-activated clay shows slowly increases in specific surface area (Table 1).
Effect of parameters on the adsorption of Cr (VI) onto clay
Various parameters for the effective removal of Cr (VI) from aqueous solutions by using natural smectite clay and their activated form as adsorbent were studied.
Effect of shaking time
The time-dependent behavior of chromium was measured by varying the equilibrium time between the adsorbate and adsorbent in the range of 5 to 200 min. The initial concentration of metal ions was varied from 20 to 40 mg/l. The pH values were kept at 5 and 4.2 for natural and activated clays, respectively, as while the amount of clay added was 1 g/l.
The results of the effects of shaking time and initial Cr (VI) ion concentration on adsorption from solution when using clay are shown in Fig. 2. It is found that the equilibrium is reached quickly only 60 min for the adsorption on natural clay and 30 min for their activated form. The removal of Cr (VI) increases rapidly with time, and then, it continues at a relatively slower rate and reaches saturation in about 15 min. Initially, the removal of Cr (VI) is rapid but it gradually decreases with time until it reaches equilibrium. It seems that at higher concentrations, there occur an initial fast adsorption of the metal ions into the clay, but some of it is lost again before equilibrium sets in. As time passed, the metal ion remained bound to the clay, showing strong metal-clay interaction.
Effect of metal ion concentration
The amount adsorbed is highly dependent on the initial concentration of the chromium ions. This finding is in agreement with recent work by Shukla et al. (Yu et al. 2003), who found the same behavior by studying the adsorption capacity of maple sawdust for the removal of Cr (VI) from aqueous solutions.
The effects of the initial ion concentration on the removal of the Cr (VI) onto the adsorbents (natural and activated smectites) were investigated by changing the initial Cr (VI) concentration in the range of 20 to 40 mg/l at an optimized pH. The results are shown in Fig. 3. The removal of the Cr (VI) ion was found to be dependent on the initial concentration. At lower concentrations, all the Cr (VI) ions present in the solution would interact with the binding sites and facilitate about 96 % metal ion removal. At a higher concentration, more Cr (VI) ions are left unabsorbed in the solution, due to the saturation of the binding sites. This is due to the increase in the number of ions competing for available binding sites in the adsorbent (Dubey and Gopal 2007).
The initial rapid rate of adsorption was maybe due to the availability of the positively charged surface of the adsorbent for anionic Cr (VI) species present in the solution. The later slow adsorption rate was maybe due to the electrostatic hindrance caused by already adsorbed negatively charged adsorbate species and the slow pore diffusion of the ions (Pandey et al. 2010). A higher initial concentration provides an important driving force to overcome all mass transfer resistances of the pollutant between the aqueous and solid phases, thus increases the uptake. Uptake of the Cr (VI) also increased with increasing the initial metal concentration tending to saturation at higher metal concentrations (Malkoc and Nuhoglu 2007).
Effect of pH on metal ion removal
In the adsorption studies, an optimization of the pH value plays a vital role for the adsorption medium. The effect of pH on the adsorption of Cr (VI) ions by natural and activated clays using different pH values ranging from 3 to 9 is illustrated in Fig. 4. The results show that the sorption is strongly pH-dependent. Therefore, the pH 5 and 4.2 were selected for the other entire sorption test in this work. Meanwhile, at higher and lower pH, the capacities of chromium ion adsorption into the two types of adsorbents used in this study show a decline.
In general, a decrease in ion uptake at acidic pH is due to an increase of competition between hydrogen and metal ions for the same adsorption sites. However, an increase in alkalinity enhances metal adsorption rate, due to the predominant presence of hydrated species of heavy metals, changes in surface charge, and the precipitation of the appropriate salt. Therefore, there is an optimum pH in which the competition of hydrogen ions is minimized and metal ion precipitation is avoided, thus enhancing metal adsorption.
In acid solution, the main chromium species are HCrO4 −, Cr2O7 2−, and H2CrO4, so the attraction between these anionic species and positively charged adsorbent surface had been strongly increased (Olayinka et al. 2007).
In an alkaline solution, other negative ions, such as OH−, should compete with the major anion, CrO4 2− ion, for the sorption sites on the adsorbent (Bhattacharyya and Sen Gupta 2006). So, the increase of pH suppresses the hydrolysis of chromium ions which causes a decrease in the adsorption amount. It is clear from Fig. 5 that acid activation increases the number of sites responsible for chromium adsorption and at any pH; the amount of Cr (VI) adsorbed per unit mass of acid-activated clay had a higher adsorption capacity compared to the non-activated clay.
Effect of temperature
To examine the effect of temperature effect on the Cr (VI) ion retention on both natural and activated clays, the same conditions were kept, contact time 2 h and Cr (VI) ion concentration of 40 mg/l, while changing the temperature from 20 to 65 °C. According to Fig. 5, when the temperature increases, the Cr (VI) adsorption capacities decrease, confirming that adsorption is an exothermic phenomenon. The same effect was observed for the activated form of kaolinite and montmorolinite clays (Eloussaief et al. 2011; Akar et al. 2009).
Theoretical basis
Adsorption kinetic models
The kinetics of the adsorption process was studied by carrying out a set of experiments at constant temperature and monitoring the amount adsorbed with time. The sorption kinetic data for Cr (VI) on the various adsorbents studied were analyzed in terms of pseudo-first-order and pseudo-second-order sorption equations, intraparticle diffusion model, and Elovich model.
Pseudo-first-order kinetic model
Pseudo-first-order kinetics using the Lagergren equation is applied (Ozturk and Kavak 2005).
where q e and q t are the values of amount adsorbed per unit mass at equilibrium and at any time t and k 1 is the pseudo-first-order adsorption rate constant. The values of k 1 can be obtained from the slope of the linear plot of Ln (q e − q t ) versus t.
Second-order kinetic model
The pseudo-second-order kinetic model was used to describe the sorption of metal ions (Soliman et al. 2011). It is applied when it is found that lnq e is not equal to the intercept of the first-order plot as obtained from Eq. (2) by using the equation
where k 2 q 2 e is described as the initial adsorption rate at time 0.
The plot of \( \frac{t}{q_t} \) versus t gives a straight line, which allows computation of q e and k 2.
The values of rate constants k 1 and k 2 obtained graphically for both adsorption models are listed in Table 2. The results show that the pseudo-second-order model provided a better approximation to the experimental kinetic data than the pseudo-first-order model (Fig. 6).
Again, correlation coefficients in Table 2 of second-order equation for all concentrations are higher than those of other kinetic models, and its calculated equilibrium sorption capacities fit well the experimental data.
Intraparticle diffusion model
The possibility of intraparticle diffusion was explored by using an intraparticle diffusion model. It is of major concern because it is rate determining step in the liquid adsorption systems. The intraparticle diffusion that varies with square root of time is described as (Karthikeyan et al. 2005)
where C is the constant and k p is the intraparticle diffusion rate constant (mg/g min1/2), q t is the amount adsorbed at a time (mg/g), and t is the time (min). The intraparticle diffusion rate constant was determined from the slope of the linear gradients of the plot q t versus t 1/2. The rate constant of intraparticle diffusion at different concentrations is shown in Table 2. The intraparticle diffusion process is controlled by the diffusion of ions within the adsorbent.
The mechanism of solute transfer to the solid includes diffusion through the fluid film around the adsorbent particle and diffusion through the pores to the internal adsorption sites. Initially, the concentration gradient between the film and the solid surface is large, and hence, the transfer of solute onto the solid surface is faster.
As time increases, intraparticle diffusion becomes predominant. Hence, solute takes more time to transfer from solid surface to internal adsorption sites through the pores (Babu and Gupta 2008).
Elovich model
Elovich equation is one of the most useful models for describing the kinetic adsorption and is also used successfully to describe second-order kinetics, assuming that the actual solid surfaces are energetically heterogeneous, but the equation does not propose any definite mechanism for adsorbate–adsorbent which the linear form of this equation is given as (Ozacar and Sengil 2005)
where α is the initial adsorption rate (mg/g min) and the parameter β is related to the extent of surface coverage and activation energy for chemisorption (g/mg).
Table 2 lists the kinetic constants obtained from the Elovich equation. It will be seen from the data that the values of α and β varied as a function of the initial chromium concentration. Thus, on increasing the initial Cr (VI) concentration from 20 to 40 ppm, the value of α increased from 1.049 to 2.022 (mg/g min) and from 13.25 to 26.408 (mg/g min), and the value of β increased from 0.284 to 0.313 (g/mg) and from 0.344(g/mg) to 0.345(g/mg) for natural and activated clays, respectively. Significant observation is that α increased by ~12 times from natural to activated clays and the other coefficient, β, did not show much variation for all the four adsorbents.
These values indicated rapid uptake of Cr (VI) on the clay surface before coverage becomes appreciable. If the interactions are carried out with a sufficiently large amount of the clay, the surface area available will be very large, giving rise to considerably bigger uptake of the metal ions in a very short time interval. This will enhance the overall rate many times as has been observed by other workers in equivalent situations (Lakshmi and Srinivasan 2004).
Again, in this case, the linear correlation coefficient values obtained from Elovich equation were in the range of 0.88–0.96 for chromium initial concentration of 20–40 mg/l (Fig. 6) for both natural and acid-activated clays and once more demonstrated a high degree of correlation between the experimental data and the theoretical data predicted by the Elovich model.
Kinetic model results
A comparison of calculated and measured results for 20-mg/l initial chromium concentration is shown in Fig. 6. The pseudo-second-order equation provides the best correlation for all of the sorption processes, whereas the Elovich equation also fits the experimental data well. The pseudo-first-order and intraparticle equations do not give a good fit to the experimental data for the sorption of chromium into both natural and acid-activated clays. The kinetics of Cr (VI) adsorption onto smectite and their acid-activated forms, as expected, are not a simple process, and no definite kinetic mechanism could be proposed. The rates are very close to second-order kinetics, but other processes may also be operating simultaneously, in which the number of adsorption sites on the clay surface and the number of Cr (VI) ions in the liquid phase determine the kinetics. Depending on pH, different chromium species that may be held to the clay surface at appropriate ion exchange site suggest any particular mechanism of interaction.
Adsorption isotherm
Adsorption isotherms, which are the presentations of the amount of solute adsorbed per unit of adsorbent, as a function of equilibrium concentration in bulk solution at constant temperature were studied in Fig. 7. If a quantity q e of solute is absorbed by a porous solid adsorbent at constant temperature and the steady state equilibrium concentration, then the function q e describes the adsorption isotherm. It shows the adsorption isotherms for the Cr (VI) adsorption on natural and activated clays. The isotherm rises in the initial stages with higher slope at low C e and q e values. This indicates that, initially, there are numerous readily accessible sites. At higher C e values, a plateau occurs. This confirms the monolayer coverage of Cr (VI) onto natural and activated clay particles. A variety of isotherm equations have been in use, some of which have a theoretical foundation and some being of mere empirical nature.
Equilibrium isotherm modeling
The isotherm equations describe the equilibrium relationship between the amount of the adsorbed metal ions and the remaining concentration in the liquid phase. It gives important information about the main mechanisms involved in the removal of heavy metal.
In this study, the adsorption equilibrium data for Cr (VI) into natural and activated clays were analyzed using program MATLAB 7.9, to fit the two- and three-parameter isotherm models.
The experimental values of q e and C e are initially treated with the models in order to determine the equation parameters, and the isotherms are reconstituted using the determined values.
Langmuir isotherm
Langmuir isotherm model is based on the assumption of monolayer adsorption, assuming that all surface sites are energetically identical and surface itself is homogeneous (Eloussaief et al. 2011). It also assumes that intermolecular forces decrease rapidly with the distance from the adsorption surface.
Langmuir isotherm is expressed as
The above equation can be rearranged to its linear form.
Both q m and k l could be determined from the slope and intercept of the linear plot C e /q e against C e , respectively. C e is the equilibrium concentration of Cr (VI) ion (mg/l), q e is the adsorbed amount of Cr (VI) (mg/g), q m (mg/g) is the maximum adsorption capacity, and k l is the Langmuir constant related to the adsorption energy, especially the adsorption enthalpy (Kundu and Gupta 2006).
Our experimental data were fitted to the Langmuir isotherm to calculate the maximum adsorption capacity of the studied clay samples. The results indicated that higher removal efficiency was achieved by activated clay sample (Table 3). The maximum adsorption capacity (q m ) was 12.5 (mg/g) and 19.23 (mg/g) for natural and activated clays, respectively. This may indicate that the acid treatment of the collected clay sample enhanced its textural properties that would contribute to the higher adsorptive capacities. Furthermore, the high coefficients of determination R 2 further confirmed that our experimental data fit better to the Langmuir model in Fig. 7. This is indicative of the homogenous clay surface as supported by k l values were 0.149 and 0.429 (l mg−1) for natural and activated clays, respectively, at 20 °C.
The Langmuir isotherm can be expressed in terms of the dimensionless constant (R l ) defined as (Aluyor et al. 2009)
where C 0 is the initial metal concentration. The value of R l indicates whether the adsorption process is favorable as follows:
-
R l > 1 unfavorable adsorption
-
R l = 1 linear
-
0 < R l < 1 favorable
-
R l = 0 irreversible.
Therefore, our results indicated that R l ranged between 0 and 1 for the adsorption of Cr (VI) on natural and activated clays, indicating a favorable and high adsorption (Table 5). According to the values of the dimensionless parameter, R l is 0.173 and 0.085 for natural and activated clays that is consistent with the requirement for favorable adsorption. This is in great agreement with the findings regarding to R l values (Babu and Gupta 2008).
Freundlich isotherm
Freundlich isotherm describes multilayer adsorption on energetically heterogeneous surfaces. It is an empirical equation suitable for high and middle range of solute concentration but not for low concentrations. Freundlich isotherm is usually described by the following equation (Babel and Kurniawan 2004):
This equation can be arranged in its linear form.
where k f and n are the Freundlich constants related to the adsorption capacity and intensity of adsorption, respectively. K F and n were determined from the linear plot of lnq e versus lnC e .
Our data showed a good fitting to the Freundlich model in Fig. 7. The high correlation coefficient R 2 > 0.95 and R 2 > 0.97 for natural and activated clays, respectively, may confirm this hypothesis.
The results in Table 3 appeared that the value of 1/n is less than unity, indicating that the Cr (VI) is favorably adsorbed by both natural and activated clays.
Temkin isotherm
The derivation of the Temkin isotherm assumes that due to adsorbate/adsorbent interaction, the heat of adsorption decreases linearly rather than logarithmically, as implied in the Freundlich equation (Kim et al. 2004).
The Temkin isotherm is usually applicable for a heterogeneous liquid and solid interface. It is given in the following:
where b is the adsorption heat (kJ/mol) and A is the equilibrium binding constant (l/g) corresponding to the maximum binding energy. The results presented in Table 3 indicated that a high value of b shows a fast sorption of adsorbate at initial stage. Similarly, a low value of A is related to weak bonding of adsorbate onto the medium. Based on this model, the orders of adsorption heats were 0.861 and 0.404 kJ/mol for activated and natural smectite clays, respectively, showing that the highest sorption of chromium at initial stage was onto natural clay rather than others. On the other hand, the lowest value of A was observed for natural clay rather than activated form, indicating a weak bonding of chromium on natural clay (Fig. 7).
Dubinin–Radushkevich model
This model has been used instead of Langmuir isotherm, since it is more general than the Langmuir model, as its deviations are not based on ideal assumptions such as equipotential of sorption sites, absence of steric hindrances between sorbed and incoming particles, and surface homogeneity on microscopic level (Monika et al. 2009).
The linear form of this isotherm model is represented by this equation (Agrawal et al. 2008).
where q s is the theoretical maximum capacity (mg/g) and it is related to the degree of sorbate sorption by the sorbent surface, β is the D–R model constant (mol2/kJ2), and ε is the Polanyi potential equal to
The plots of Inq e versus ε 2 yielded straight lines and indicate a good fit of the isotherm to the experimental data. The values of q s and β calculated from the intercept slopes of the plots, respectively, are shown in Table 3. According to these results, the highest value of q s was observed for activated clay with sulfuric acid, showing higher sorption capacity of activated adsorbent compared to natural form (Fig. 7).
The mean free energy E (kJ/mol) is a parameter used in predicting the type of adsorption mechanism; it can be calculated using the following relationship:
When the E value is less than 8 kJ/mol, it indicates physical adsorption. When E is between 8 and 16 kJ/mol, it indicates the ion exchange, and when E is between 20 and 40 kJ/mol, it indicates chemisorptions (Marjanovic et al. 2011).
The calculated value of E for this study is below 8 kJ/mol and is an indication of physico-sorption. This constant gives an idea about the mean free energies which were valued as 0.666 and 1.613 kJ/mol for natural clay and activated clay with sulfuric acid, respectively, showing physico-sorption nature of hexavalent chromium on used adsorbents.
Redlich–Peterson isotherm
Redlich–Peterson equation that is an empirical isotherm included three parameters. This equation is widely used as combined elements between Langmuir and Freundlich equations (Prasad and Srivastava 2009). The equation for this model is
where k RP is the Redlich–Peterson constant having unit of (l/g), P e is also a constant (l/mg), and g is an exponent that lies between 0 and 1.
When the value of g is equal to 1, the above equation is reduced to the Langmuir isotherm, while it reduced to a Freundlich isotherm, in case the value of the parameter C g e P e is much bigger than 1. The ratio of k RP/P e indicates the adsorption capacity.
From Table 3, the results show that the k RP/P e has a similar variation to q m . Thus, for each parameter, the values show an increase with increasing pore volume and surface area of activated clay. The higher R 2 values for Redlich–Peterson isotherm model suggest the applicability of this model to represent the equilibrium sorption of Cr (VI) by natural and activated clays (Fig. 7).
Sips model
Sips model predicts a monolayer sorption capacity for high sorbate similar in form to the Freundlich equation, but it has a finite limit when the concentration is sufficiently high (Gunay et al. 2007).
The non-linear Sips isotherm equation can be represented as
where k S is the Sips equilibrium constant (l/mg) and m s is the Sips model exponent.
The values of this isotherm model are presented in Table 3. According to the Sips isotherm model, the maximum sorption capacities were 13.016 and 18.094 mg/g at 20 °C for natural clay and activated clay with sulfuric acid, respectively (Fig. 7).
Khan model
The Khan isotherm model (Padmeh et al. 2006) is given by the following equation:
where q m and b k are the Khan model constants and a k is the Khan model exponent.
According to the results given in Table 3, q m values were 10.835 and 19.69 mg/g for natural smectite and activated clays with sulfuric acid, respectively. The values of b k were 0.179 and 0.572 for natural Tunisian clay and their activated form, respectively.
Equilibrium data were fitted (Fig. 7) onto the entire investigated three-parameter isotherm models very well. However, the highest correlation coefficient was observed with the Sips isotherm isotherm model. Among the tested two-parameter equations, and based on higher determination coefficient, the Dubinin–Radushkevich isotherm model was the best model to describe the adsorption of hexavalent chromium ion on the natural and acid-activated clays. Comparing all the used isotherm models, it seems that three-parameter isotherm model is the best to describe adsorption of hexavalent chromium on the used adsorbents.
Thermodynamic parameters of adsorption
For designing adsorption batch adsorption systems, the designer should be able to understand the following: what changes can be expected to occur and how fast will they take place. The fast of the reaction can be calculated from the knowledge of kinetic studies. But, the changes in reaction that can be expected during the process require the brief idea of thermodynamic parameters. The concept of thermodynamic assumes that in an isolated system where energy cannot be gained or lost, the entropy change is the driving force (Atia 2008). The thermodynamic parameters that must be considered to determine the process are enthalpy of adsorption (ΔH0), free energy change (ΔG0), and entropy change (ΔS0) due to transfer of unit mole of solute from solution onto the solid–liquid interface. The important thermodynamic function ΔH0 is very useful whenever there is a differential change that occurs in the system. Enthalpy is an additive property that its value is additive. The negative value of ΔH0 indicates the exothermic process, and positive value indicates the endothermic process.
The other important thermodynamic parameter is the change in entropy ΔS0. The parameter ΔS0 is used to identify the spontaneity in the adsorption process. The values of ΔH0 and ΔS0 were computed using the equation as follows (Donat 2009):
where
- R :
-
Universal gas constant (8.314 J/mol K)
- T :
-
Absolute solution temperature (K)
- k l :
-
The Langmuir constant that is related with the energy of adsorption.
The value can be calculated from the slope and intercept of plot between lnk l versus 1/T. Another most important thermodynamic parameter involved in the adsorption process is the free energy change and can be calculated using the relation (Wang et al. 2010).
The calculated thermodynamic parameters based on the above functions are listed in Table 4. Negative values of ΔH0 suggest the exothermic nature of the adsorption. However, the negative values obtained for ΔG0 indicated the spontaneous nature of adsorption (Table 4). Moreover, the increase of ΔG0 with temperature indicated that adsorption was unfavorable at higher temperatures. Exothermic adsorption of Cr (VI) ion was also observed on Tunisian clay (Bhattacharyya and Sen Gupta 2006).
Comparison of adsorption capacities of natural and activated clays with other adsorbents
A comparison was made, in term of adsorption capacity (q m ) of our clay with other adsorbents. For a better valorization of our clay, the adsorbent capacity is compared in the same operating condition with different types of adsorbents in this study and in literature for Cr (VI) adsorption. The results show that the retention of Cr (VI) ions onto acid-activated clays was better than their natural forms for all type of clays. The present study shows that natural and activated clays are effective low-cost adsorbents for the removal of Cr (VI) from aqueous solutions. Furthermore, the adsorbents used during our work have higher maximum adsorption capacities than commercial clay and much other types of adsorbents reported in Table 5, which indicated the effectiveness of the Tunisian smectite clay used in the present work.
Conclusions
This study indicates that the natural and activated clays can be used as effective and inexpensive adsorbents for the removal of toxic Cr (VI) ions from aqueous solutions. The pH of the medium is the controlling parameter of this adsorption process. Increases in the initial metal ion concentration and contact time were found to increase the removal of Cr (VI) ions. The adsorption of Cr (VI) onto natural clay followed the pseudo-second-order kinetic and Elovich model. The experimental data fit well with two- and three-parameter isotherm models.
The results of this study indicate that Tunisian smectite clay especially their activated form has the potential to be used as an alternative adsorbent material for the removal of Cr (VI) ions from aqueous solutions owing to its low cost and simple regeneration.
References
Agrawal A, Pal C, Sahu KK (2008) Extractive removal of chromium (VI) from industrial waste solution. J Hazard Mater 159:458–464
Akar ST, Yetimoglu Y, Gedikbey T (2009) Removal of chromium (VI) ions from aqueous solutions by using Turkish montmorillonite clay: effect of activation and modification. Desalination 244:97–108
Aluyor EO, Oboh IO, Obahiagbon KO (2009) Equilibrium sorption isotherm for lead (Pb) ions on hydrogen peroxide modified rice hulls. Int J Phys Sci 4:423–427
Atia AA (2008) Adsorption of chromate and molybdate by cetylpyridinium bentonite. Appl Clay Sci 41:73–84
Babel S, Kurniawan TA (2004) Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemosphere 54:951–967
Babu BV, Gupta S (2008) Adsorption of Cr(VI) using activated neem leaves: kinetic studies. Adsorption 14:85–92
Bandegharaei AH, Hosseini MS, Ghadi M, Zowghi S, Bandegharaei HH (2010) Kinetics, equilibrium and thermodynamic study of Cr(VI) sorption into toluidine blue o-impregnated XAD-7 resin beads and its application for the treatment of wastewaters containing Cr(VI). Chem Eng J 160:190–198
Bayat B (2002) Comparative study of adsorption properties of Turkish fly ashes: the case of nickel(II), copper(II) and zinc(II). J Hazard Mater B95:251–273
Benhammou A, Yaacoubi A, Nibou L, Tanouti B (2007) Chromium(VI) adsorption from aqueous solution onto Moroccan Al-pillared and cationic surfactant stevensite. J Hazard Mater 140:104–109
Bhattacharyya KG, Sen Gupta S (2006) Adsorption of chromium (VI) from water by clays. Ind Eng Chem Res 45:7232–7240
Chauhan D, Sankararamakrishnan N (2011) Modeling and evaluation on removal of hexavalent chromium from aqueous systems using fixed bed column. J Hazard Mater 185:55–62
Donat R (2009) The removal of uranium (VI) from aqueous solutions onto natural sepiolite. J Chem Thermodyn 41:829–835
Dubey SP, Gopal K (2007) Adsorption of chromium (VI) on low cost adsorbents derived from agricultural waste material: a comparative study. J Hazard Mater 145:465–470
Eloussaief M, Kallel N, Yaacoubi A, Benzina M (2011) Mineralogical identification, spectroscopic characterization, and potential environmental use of natural clay materials on chromate removal from aqueous solutions. Chem Eng J 168:1024–1031
El-Sikaily A, El Nemr A, Khaled A, Abdelwehab O (2007) Removal of toxic chromium from wastewater using green alga Ulva lactuca and its activated carbon. J Hazard Mater 148:216–228
Fritzen MB, Souza AJ, Silva TAG, Souza L, Nome RA, Fiedler HD, Nome F (2006) Distribution of hexavalent Cr species across the clay mineral surface–water interface. J Colloid Interface Sci 296:465–471
Gunay A, Arslankaya E, Tosun I (2007) Lead removal from aqueous solution by natural and pretreated clinoptilolite: adsorption equilibrium and kinetics. J Hazard Mater 146:362–371
Huang Y, Ma X, Liang G, Yan Y, Wang S (2008) Adsorption behavior of Cr(VI) on organic-modified rectorite. Chem Eng J 138:187–193
Karthikeyan T, Rajgopal S, Miranda LR (2005) Chromium (VI) adsorption from aqueous solution by Hevea Brasilinesis sawdust activated carbon. J Hazard Mater B124:192–199
Kim Y, Kim C, Choi I, Rengraj S, Yi J (2004) Arsenic removal using mesoporous alumina prepared via a templating method. Environ Sci Technol 38:924–931
Kundu S, Gupta AK (2006) Arsenic adsorption onto iron oxide-coated cement (IOCC): regression analysis of equilibrium data with several isotherm models and their optimization. Chem Eng J 122:93–106
Lakshmi N, Srinivasan K (2004) Lead removal in aqueous medium by agricultural waste-cotton seed (Ceiba Pentandra). Indian J Environ Prot 24:379–384
Lee SM, Kim WG, Laldawngliana C, Tiwari D (2010) Removal behavior of surface modified sand for Cd(II) and Cr(VI) from aqueous solutions. J Chem Eng Data 55:3089–3094
Li H, Li Z, Liu T, Xiao X, Peng Z, Deng L (2008) A novel technology for biosorption and recovery hexavalent chromium in wastewater by bio-functional magnetic beads. Bioresource Technol 99:6271–6279
Malkoc E, Nuhoglu Y (2007) Potential of tea factory waste for chromium (VI) removal from aqueous solutions: thermodynamic and kinetic studies. Sep Purif Technol 54:291–298
Marjanovic V, Lazarevic S, Jankovi-Castvan I, Potkonjak B, Janackovic Ð, Petrovic R (2011) Chromium (VI) removal from aqueous solutions using mercaptosilane functionalized sepiolites. Chem Eng J 166:198–206
Maryuk O, Pikus S, Olszewska E, Majdan M, Skrzypek H, Zięba E (2005) Benzyldimethyloctadecyl ammonium bentonite in chromates adsorption. Mater Lett 59:2015–2017
Mohan D, Pittman CU Jr (2006) Review: activated carbons and low cost adsorbents for remediation of tri-and hexavalent chromium from water. J Hazard Mater 137:762–811
Monika J, Garg V, Kadirvelu K (2009) Chromium (VI) removal from aqueous solution, using sunflower stem waste. J Hazard Mater 162:365–372
Mor S, Ravindra K, Bishnoi NR (2007) Adsorption of chromium from aqueous solution by activated alumina and activated charcoal. Bioresource Technol 98:954–957
Olayinka KO, Alo BI, Adu T (2007) Sorption of heavy metals from electroplating effluents by low cost adsorbents II: use of waste tea, coconut shell and coconut husk. J Appl Sci 7:2307–2313
Owlad M, Aroua MK, Wan Daud WMA (2010) Hexavalent chromium adsorption on impregnated palm shell activated carbon with polyethyleneimine. Bioresource Technol 101:5098–5103
Ozacar M, Sengil IA (2005) A kinetic study of metal complex dye sorption onto pine sawdust. Process Biochem 40:565–572
Ozturk N, Kavak D (2005) Adsorption of boron from aqueous solutions using fly ash: batch and column studies. J Hazard Mater B127:81–88
Padmeh TVN, Vijayaraghavan K, Sekaran G, Velan M (2006) Application of two and three parameter isotherm models: biosorption of acid red onto Azolla microphylla. Bioremediat J 10:37–44
Pandey PK, Sharma SK, Sambi SS (2010) Kinetics and equilibrium study of chromium adsorption on zeolite NaX. Int J Environ Sci Tech 7:395–404
Prasad RK, Srivastava SN (2009) Sorption of distillery spent wash onto fly ash: kinetics and mass transfer studies. Chem Eng J 146(1):90–97
Prigione V, Zerlottin M, Refosco D, Tigini V, Anastasi A, Varese GC (2009) Chromium removal from a real tanning effluent by autochthonous and allochthonous fungi. Bioresource Technol 100:2770–2776
Rengaraj S, Yeon K, Moon S (2001) Removal of chromium from water and wastewater by ion exchange resins. J Hazard Mater 87:273–287
Sahinkaya E, Kilic A, Altun M, Komnitsas K, Lens PNL (2012) Hexavalent chromium reduction in a sulfur reducing packed-bed bioreactor. J Hazard Mater 219–220:253–259
Sari A, Uluozlu OD, Tuzen M (2011) Equilibrium, thermodynamic and kinetic investigations on biosorption of arsenic from aqueous solution by algae (Maugeotia genuflexa) biomass. Chem Eng J 167:155–161
Sharma A, Bhattacharyya KG (2004) Adsorption of chromium (VI) on Azadirachta Indica (neem) leaf powder. Adsorption 10:327–338
Sharma YC, Weng CH (2007) Removal of chromium (VI) from water and wastewater by using riverbed sand: kinetic and equilibrium studies. J Hazard Mater 142:449–454
Shen Y-S, Wang S-L, Tzou Y-M, Yan Y-Y, Kuan W-H (2012) Removal of hexavalent Cr by coconut coir and derived chars—the effect of surface functionality. Bioresource Technol 104:165–172
Soliman EM, Ahmed SA, Fadl AA (2011) Reactivity of sugar cane bagasse as a natural solid phase extractor for selective removal of Fe(III) and heavy-metal ions from natural water samples. Arabian J Chem 4:63–70
Wang XS, Chen LF, Chen KL, Wan WY, Tang YJ (2010) Removal of Cr(VI) with wheat-residue derived black carbon: reaction mechanism and adsorption performance. J Hazard Mater 175:816–822
Weng CH, Sharma YC, Chua SH (2008) Adsorption of Cr(VI) from aqueous solutions by spent activated clay. J Hazard Mater 155:65–75
Yu LJ, Shukla SS, Dorros KL, Shukla A, Margrave JL (2003) Adsorption of chromium from aqueous solutions by maple sawdust. J Hazard Mater 100(1–3):53–63
Acknowledgments
The authors acknowledge the financial support of the Ministerio de Economia y Competitividad-FEDER Project CTQ2012-38635 and Generalitat Valenciana PROMETEO-2014-077.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khalfa, L., Cervera, M.L., Bagane, M. et al. Modeling of equilibrium isotherms and kinetic studies of Cr (VI) adsorption into natural and acid-activated clays. Arab J Geosci 9, 75 (2016). https://doi.org/10.1007/s12517-015-2104-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-015-2104-0