Abstract
The Naozhi Au–Cu deposit is located on the continental margin of Northeast China, forming part of the West Pacific porphyry–epithermal gold–copper metallogenic belt. In this paper, we systematically analyzed the compositions, homogenization temperatures, and salinity of fluid inclusions as well as their noble gas isotopic and Pb isotopic compositions from the deposit. These new data show that (1) five types of fluid inclusions were identified as pure gas inclusions (V-type), pure liquid inclusions (L-type), gas–liquid two-phase inclusions (W-type, as the main fluid inclusions (FIs)), CO2-bearing inclusions (C-type), and daughter-mineral-bearing polyphase inclusions (S-type); (2) W-type FIs in quartz crystals of early, main, and late stage are homogenized at temperatures of 324.7–406.7, 230–338.8, and 154.6–308 °C, with salinities of 2.40–7.01 wt% NaCleq, 1.73–9.47 wt% NaCleq, and 6.29 wt% NaCleq, respectively. S-type FIs in quartz crystals of early stage are homogenized at temperatures of 328.6–400 °C, with salinities of 39.96–46.00 wt% NaCleq; (3) Raman analysis results reveal that the vapor compositions of early ore-forming fluids consisted of CO2 and H2O, with H2O gradually increasing and CO2 being absent at the late mineralization stage; (4) fluid inclusions in pyrite and chalcopyrite have 3He/4He ratios of 0.03–0.104 Ra, 20Ne/22Ne ratios of 9.817–9.960, and 40Ar/36Ar ratios of 324–349. These results indicate that the percentage of radiogenic 40Ar* in fluid inclusions varies from 8.8 to 15.5 %, containing 84.5–91.2 % atmospheric 40Ar; (5) the 206Pb/204Pb, 207Pb/204Pb, and 206Pb/204Pb ratios of sulfides are 18.1822–18.3979, 15.5215–15.5998, and 38.1313–38.3786, respectively. These data combined with stable isotope data and the chronology of diagenesis and metallogenesis enable us suppose that the ore-forming fluids originated from the melting of the lower crust, caused by the subduction of an oceanic slab, whereas the mineralized fluids were exsolved from the late crystallization stage and subsequently contaminated by crustal materials/fluids during ascent, including meteoric water, and the mineral precipitation occurred at a shallow crustal level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epithermal Cu–Ag deposits are an important part of the epithermal–porphyry copper–gold mineralization systems. These epithermal deposits commonly occur at shallower crustal levels than porphyry type deposits, and Au–Ag mineralization and wall rock alteration take place by distinctively chemically different hydrothermal fluids (Hedenquist and Lowenstern 1994; Cooke and Simmons 2000; Mao et al. 2003; Groves and Bierlein 2007; Sillitoe 2008). Since the 1970s, a number of world class super large-scale epithermal gold (copper) deposits have been discovered in the Circum Pacific, Mediterranean–Himalaya orogen, and the Mongolia-Okhotsk Belt (Jiang et al. 2004).
Yanbian area is located near the continental margin of Northeast China, containing porphyry deposit (Nongping) or porphyry-like deposit (Xiaoxinancha), epithalmal low-sulfidation Au deposits (e.g., Ciweigou, Wufeng, and Wuxingshan), and epithermal medium-sulfidation Cu–Au deposits (e.g., Naozhi and Fuyan) (Fig. 1b). These discoveries indicate the high potential of the area to host epithermal gold–copper deposits (Bing et al. 1995; Meng et al. 2001; Han et al. 2013).
The Naozhi Au–Cu deposit, which belongs to the Yanbian ore district, is located in southern Baicaogou town, Yanbian autonomous prefecture, Jilin Province. Since the discovery of Naozhi during the 1970s, scientists have conducted geological, geochemical, and mineralization investigations of the ore deposit. Few systematic fluid inclusion studies have been performed, although various conflicting hypotheses have been advanced to explain its formation, including a volcanic-subvolcanic hydrothermal origin (Li and Yang 1991) a transition type between epithermal and porphyry (Bing et al. 1995) a medium-temperature magmatic hydrothermal origin (Su et al. 2003; Yang et al. 2010) a low-sulfidation epithermal gold origin (Lin 2013; Wu 2013) and a high-sulfidation epithermal gold deposit (Zhao 2007; Wan 2013). In this contribution, on the basis of previous studies, we present a detailed study of the fluid inclusions hosted in quartz at different ore-forming stages, as well as the noble gas isotopic and Pb isotopic compositions in ore minerals from the deposit, for the purpose of tracing possible fluid sources and mineralization processes of the ore-forming fluids.
Geological background and description of the mineral deposit
Geological background
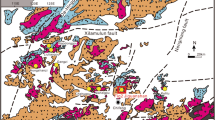
The location of the Naozhi Cu–Au deposit is shown in Fig. 1. Tectonically, it is situated at the eastern end of the Central Asia Orogenic Belt (CAOB) (Tianshan–Mongolia–Hingan), adjacent to the Jiamusi Massif in the north, the North China Plate in the south, and the Khanka Massif in the northeast (Fig. 1a). This region has undergone a number of significant geological events such as the evolution of the ancient Asian Ocean, the Xing’an–Mongolian orogen, Mesozoic subduction of the paleo-Pacific plate, and Cenozoic supercrustal faulting (Peng and Zhao 2001) The exposed strata in the area comprise Paleozoic epimetamorphic rocks, Mesozoic volcano-sedimentary rocks, and Cenozoic volcanic rocks (Meng et al. 2001; Jin 2003). Four stages of intrusive rocks have been identified in the Yanbian area: Middle-Late Permian gabbro and diorite, Late Permian-Early Jurassic granodiorite and monzogranite, Middle-Late Jurassic granite, and Early Cretaceous granitoid complex (Meng et al. 2001; Zhang 2002; Sun et al. 2008a, b). Regional structures include Paleozoic E–W-trending ductile deformation zones and Mesozoic or younger faults that trend NNE–SSW, N–S, and NNW–SSE (Fig. 1b).
Ore deposit geology
The Naozhi Cu–Au deposit is an important member of the eastern Yanbian district. The deposit is located in a NW–SE secondary fault system (Fig. 2a). The deposit is hosted by Lower Jurassic granodiorite, biotite monzonitic granite, and quartz diorite and is overlain by Lower Cretaceous volcanic rocks and subvolcanic intrusions (Zhang 2002; Zhang et al. 2004; Han et al. 2013). Geochronology data for the host rocks and alteration minerals show that the monzonitic granite in the mining area is Early Jurassic in age (Zhang 2002; Han et al. 2013) the main vein rocks associated with the ore body are diorite-porphyry of 130–123.3 Ma in age (Han et al. 2013; Lin 2013) and the Jingouling group formed at 123–115 Ma (Jin 2003). Han et al. (2013) obtained a 40Ar/39Ar plateau age of 128.6 ± 1.4 Ma from secondary sericite, and Meng et al. (2001) obtained a 40Ar/39Ar isochron age of 123.6 ± 2.5 Ma from quartz-hosted fluid inclusions. The above studies indicate that the Naozhi Au–Cu deposit is related to intermediate–basic magmatism and to subvolcanic, shallow magmatism of the Jingouling stage (Early Cretaceous).
The Naozhi ore body consists of ten subparallel bands of gold-bearing polymetallic sulfide-quartz veins with associated alteration haloes, of which the no. 1, 9, 10, and 20 veins are the largest. The ore body is 350–1,020 m long, 0.4–12.3 m wide, and >450 m deep. The ore bodies display parallel along their length and depth (Fig. 2c). The gold grade of these ore bodies reaches 74.5 g/t. Pyrite and chalcopyrite are the dominant ore minerals, together with sphalerite, galena, tetrahedrite, chalcocite, magnetite, and natural gold, electrum, and petzite (Fig. 3). Texturally, the ores show euhedral–subhedral texture and dissolution metasomatism residual texture, as well as banded, brecciated, massive, vein-like, and veinlet-disseminated structures. Wall rock alteration can be divided into three zones on the basis of alteration type and intensity. The inner zone is pervasive marked by k-feldspathization, sericitization, silicification, carbonatization, and pyritization, and it is a gold-bearing high-grade alteration zone; an intermediate quartz-sericite-pyrite (QSP) zone showing outwards a chlorite enrichment and subsequent quartz, sericite and pyrite impoverishment; and an outer chloritized (propylitic) (Fig. 2b).
Hand specimens and micrographs of ore veins in Naozhi Au–Cu deposit. a K-feldspar-altered rocks within the mine shaft. b Pyrite-sericite-quartz vein showing pyrite-sericite-quartz replaces K-feldspar. c, d Pyrite-quartz vein. e Polymetallic sulfide-quartz veins. f Galena veins. g Quartz intergrown with chalcopyrite, galena, and chalcocite. h Quartz intergrown with pyrite, chalcopyrite, and galena. i Including pyrite and a few of chalcopyrite and native gold. Abbreviations: Qz quartz, Py pyrite, Cpy chalcopyrite, CC chalcocite, Gn galena
Based on ore textures, crosscutting relationships, and mineral assemblages, the hydrothermal mineralization process can be divided into four stages (Fig. 4): (1) The K-feldspar-quartz stage commonly shows various degrees of replacement by pyrite-sericite-quartz veins (Fig. 3b). Minerals in these veins include quartz, K-feldspar, albite, pyrite, sericite with minor chlorite, and residual magnetite (Fig. 3a, b). The stage I quartz (Q1) is a pre-ore stage and is not associated with gold mineralization. (2) The quartz-pyrite stage is characterized by veins containing coarse-grained quartz and pyrite, sericite, chlorite, with minor chalcopyrite, and native gold. The stage II quartz (Q2), the most abundant mineral, mainly appears as euhedral-subhedral grains and is milky to gray in color (Fig. 3c, d). (3) The quartz-gold-polymetallic sulfide stage is characterized by the widespread occurrence of pyrite, chalcopyrite, galena, sphalerite, and native gold, with minor tetrahedrite, chalcocite, electrum, and petzite (Fig. 3e–i). Native gold is locally present along the boundaries or in the fissures of pyrite grains (Fig. 3i). The stage III (Q3) is commonly dark-gray (smoky) in color and characterized by fine-grained anhedral aggregates (Fig. 3d–f). (4) The quartz-carbonate stage is marked by the appearance of carbonate and quartz with minor pyrite, electrum, and petzite but no gold. These minerals fill the fissures in early veins or occur in the vugs. Of these stages, Au–Cu mineralization occurred mainly during stages II and III.
Sampling and analytical methods
Sample preparation
The quartz samples for fluid inclusion study were collected from a pyrite-sericite-quartz vein (stage I) at depth 300 m and a pyrite-quartz vein (stage II) at depth 300 m in the no. 9 ore zone and a polysulfide-quartz vein (stage III) at depth 250 m and a galena vein (stage III) at depth 160 m in the no. 10 ore zone. The sulfide samples for the noble gas isotopic and Pb isotopic were also selected from the pyrite-quartz vein and the polysulfide-quartz vein.
Fluid inclusions in quartz
Forty-one doubly polished thin sections (about 0.20 mm thick) were made for fluid inclusion study, and ten of them were selected to do microthermometric measurement and laser Raman spectroscopic detection.
Microthermometry was performed on a LINKAMTHMS-600 heating/freezing stage at Jilin University, China. Thermocouples were calibrated at −56.6, 0.0, and +374.1 °C using synthetic fluid inclusions. Measurement precision was approximately ±0.1 °C on cooling runs and ±2 °C on heating runs. During testing, the following parameters were applied: if the temperature was lower than 30 °C, the heating rate was 1 °C/min; if above 200 °C, the rate was 10 °C min−1; if close to a phase change or around the freezing point, the rate was decreased to <0.2 °C min−1. Salinities of aqueous (NaCl-H2O) fluid inclusions were calculated using the final melting temperatures of ice points (Bodnar 1993). Salinities of halite-bearing fluid inclusions (FIs) were calculated using the dissolution temperatures of the halite daughter mineral (Hall et al. 1988).
To constrain the fluid inclusion compositions, Raman analysis of 35 single mineral inclusions from the ore samples was carried out with a Renishaw RM2000 at Nanjing University, China, using a 514 AR+ laser with a spot size of 1 μm and a spectral counting time of 10 s. Peak values from the full spectra were obtained at intervals of 1 cm.
Isotopic analysis of noble gases from sulfides
Four pyrite samples and two chalcopyrite samples were crushed to <3 mm. To enable noble gas analysis and to avoid analyzing gases from multistage fluid inclusions (Hu et al. 1998; Burnard et al. 1999; Sumino et al. 2001) great care was taken to select about 2 g of each mineral with homogeneous optical properties and free of fractures.
Noble gas analyses were carried out at the Laboratory for Earthquake Chemistry in Tokyo University, Japan. Noble gases were extracted from each sample in a crushing vessel connected to the gas purification line. The cooled and separated noble gases were analyzed with a VG5400 mass spectrometer (MS-III), following Nagao et al. 1996 and Matsuda et al. 2002. Analytical errors are less than 10 % (1σ). Blanks for 4He, 20Ne, 40Ar, 84Kr, and 132Xe gave 1.5–4.5 × 10−11, 0.8–1.9 × 10−12, 3.8–8.4 × 10−10, 1.7–5.3 × 10−14, and 2.2–9.6 × 10−15 cc standard pressure (STP), respectively. The 3He/4He ratio for the helium isotope standard analyzed during the runs was 28.88 ± 0.14 × 10−5 (HESJ) (Schlosser and Winckler 2002).
Lead isotopic analyses of sulfides
Six samples for Pb isotope analyses of sulfides were performed using a Finnegan Element II type, HR–ICP–MS at the State Key Laboratory of Mineralization, Nanjing University, China. After crushing and sieving the ore samples, the single minerals, with purity of more than 98 %, were picked out under the binocular microscope. Sulfide samples were washed with 0.15 mol/L HCl and high purity water. All of these samples were ground to below 74 mm, and decomposed in the Teflon PFA bottle using HCl and HNO3. After complete decomposition, they were evaporated to nearly dry and transformed into 0.15 mol/L HCl and 0.65 mol/L HBr medium, then separated in Bio-RadAG 1 × 8 anion exchange column and leached with 1.0 mol/L HNO3 and heated to dry. Measured Pb isotopic ratios were corrected for instrumental mass fractionation of 0.1 % per atomic mass unit by reference to repeated analyses of the NBS-981 Pb standard. Silicon was the main emitting agent. The prototypes of the Pb isotope ratios were 0.059003 ± 0.000084, 0.91439 ± 0.00017, and 2.16441 ± 0.00097. The blank value for Pb was <1 ng.
Results and discussion
Properties of ore-forming fluid
Types and characteristics of fluid inclusions
FIs were classified petrographically as follows: isolated FIs and randomly distributed groups of FIs hosted in intragranular quartz crystals were interpreted as primary in origin, and those aligned along micro-fractures in transgranular trails were interpreted as secondary (Roedder 1984; Lu et al. 2004).
Based on composition, phases (L-V-S) at room temperatures and phase transformations during heating and cooling, five fluid inclusion types have been identified (Table 1 and Fig. 5): (1) pure gas inclusions (V-type), (2) pure liquid inclusions (L-type), (3) gas–liquid two-phase inclusions (W-type), (4) CO2-bearing inclusions (C-type), and (5) daughter mineral-bearing polyphase inclusions (S-type).
Microphotographs of typical fluid inclusions in Naozhi Au–Cu deposit. a Coexistence of S-type with halite, L-type, and W-type inclusions and b coexistence of V-type, C-type, and W-type inclusions in stage I (quartz from pyrite-sericite-quartz vein). c C-type inclusion composed of liquid CO2 and liquid CO2 and vapor CO2 in stage II (quartz from pyrite-quartz vein). d L-type inclusion composed of liquid H2O and W-type inclusion in stage III (quartz from galena vein). e Necking-down fluid inclusion marked by the red circle in stage II (quartz from pyrite-quartz vein). f W-type inclusions composed of vapor H2O and liquid H2O in stage III (quartz from polysulfide vein). Abbreviations: H halite, \( {L}_{H_2O} \) liquid H2O, \( {L}_{C{O}_2} \) liquid CO2, \( {V}_{H_2O} \) vapor H2O, \( {V}_{C{O}_2} \) vapor CO2
W-type fluid inclusions are the most common FIs in the Naozhi Au–Cu deposit, and they can be further divided into rich-gas inclusions (RV-type) and rich-liquid inclusions (RL-type). The RV-type fluid inclusions are best developed during the early mineralization stage, and the main mineralization stage and the RL-type fluid inclusions occur in Qz of all stages.
Observations under the microscope also recognized necking-down of fluid inclusions in the pyrite-quartz vein (Fig. 5e), suggesting that some of the host quartz and pyrite were locally deformed (Sun et al. 2010). In addition, coexistence of different types of fluid inclusions with very variable liquid/vapor ratios was recognized in the Naozhi samples (Fig. 5a, b), indicating that fluid unmixing might have played an important role during mineralization (Roedder 1984; Li 1988; Lu et al. 2004).
Homogenization temperatures and salinities
The microthermometric dates, summarized in Table 2, were obtained from RV-type, RL-type, and HS-type fluid inclusions of the three hydrothermal stages (Fig. 6). Microthermometric measurements were not possible for some FIs due to their small sizes.
RV, RL, and HS fluid inclusion types have been recognized in the early hydrothermal stage. Homogenization temperatures are mainly between 335.1 and 393.5 °C, 324.7 and 406.7 °C, and 328.6 and 400 °C, respectively. The T m, ice or T m, halite range from −2.7 to −4.4 °C, −1.4 to −3.4 °C, and 328.6 to 400 °C, respectively, corresponding to a salinity of 4.48–7.01 wt% NaCleq, 2.40–5.55 wt% NaCleq, and 39.96–46.00 wt% NaCleq.
Quartz crystals from the main hydrothermal stage contain many RV-type and RL-type FIs. The RV-type FIs yield final T m, ice of −1.7 to −5.6 °C and salinities of 2.89–8.67 wt% NaCleq. These RV-type FIs are homogenized to a liquid phase at temperatures of 270–325.4 °C. The RL-type FIs yielded many T m, ice of −1.0 to −6.2 and three T m, ice of −15.4 to −18 and salinities of 1.73–9.47 wt% NaCleq and 19.13–21.20 wt% NaCleq, respectively. Their homogenization temperatures are mainly between 230 and 338.8 °C.
Only RL-type and few RV-type fluid inclusions were observed in the late-stage quartz. Just one T m, ice of −3.9 and salinity of 6.29 wt% NaCleq was yield in the RL-type FIs. The homogenization temperatures of RL-type and RV-type FIs are 154.6–308 and 195.8–232.2 °C, respectively.
Vapor composition of fluid inclusions
Raman analysis results reveal that the gas compositions of gas–liquid two-phase inclusions of the early mineralization stage (chlorite-bearing brecciated pyrite quartz vein) are predominantly CO2 and H2O (Fig. 7a, b). The gas compositions of gas–liquid two-phase inclusions of the main mineralization stage (chalcopyrite–pyrite quartz vein) are mainly H2O and minor CO2 (Fig. 7c, d), and the gas compositions of gas–liquid two-phase inclusions of the late main mineralization stage (quartz from the galena vein) are mainly H2O (Fig. 7e, f). From these data, it is apparent that the early ore-forming fluids which consisted of CO2 and H2O, with H2O gradually increasing and CO2 being absent at the late mineralization stage, show that the ore-forming fluid was derived from deep magma degassing and volcanic hydrothermal activity. The H-O isotopic characteristics of fluid inclusions in quartz indicate that the δ18OH2O and δDV-SMOW of the early mineralization stage are +5.87 and −94 ‰, respectively, and the δ18OH2O and δDV-SMOW of the main mineralization stage are −4.7 to +4.7 ‰ and −123.8 to −84.6 ‰, respectively (Feng 1994; Huang 1997; Yang et al. 2010; Table 3). typical of magmatic hydrothermal settings. In the plot of δD vs. δ18OH2O(Fig. 8), quartz samples plot near the lower left of the magmatic water field. This may reflect the increasing influence of meteoric water during the evolution of the ore-forming fluid.
Representative laser Raman spectra of fluid inclusions. a, b Vapor phase of W-type inclusions are predominantly CO2 and H2O in the early mineralization stage (quartz from pyrite-sericite-quartz vein). c, d Vapor phase of W-type inclusions dominated by H2O and minor CO2 in the main mineralization stage (quartz from chalcopyrite-pyrite quartz vein). e, f Vapor H2O in W-type inclusion in the late main mineralization stage (quartz from the galena vein)
Noble gas isotopes
Hydrothermal fluids contain noble gases from four ultimate sources (Burnard et al. 1999; Ballentine and Burgess 2002). (1) air-saturated water (i.e., meteoric water, sea water): Air-saturated water has He and Ar isotopic compositions of 3He/4He = 1Ra (where Ra is the 3He/4He value of air, 1.399 × 10−6) and 40Ar/36Ar = 295.5, similar to the atmospheric values because air-saturated water is isotopically in equilibrium with the atmosphere. (2) Mantle-derived fluids: The upper oceanic mantle has 3He/4He ratio of 7–9 Ra, and the subcontinental lithospheric mantle (SCLM) has 3He/4He ratio of 6–8 Ra. Mantle-derived Ar has 40Ar/36Ar ratios >40,000 (Porcelli et al. 1992; Patterson et al. 1994; Dunai and Baur 1995; Reid and Graham 1996; Gautheron and Moreira 2002) (3) He and Ar produced in the crust. Th and K in crust can produce abundant radiogenic and nucleogenic 40Ar and 4He. As a result, fluids reacted with crustal rocks will eventually have He and Ar isotopic compositions similar to that of the crust that has 40Ar/36Ar ratios of 1,000 (Drescher et al. 1998) and 3He/4He ratios of 0.01–0.05 Ra (Stuart et al. 1995).
Diffusion–fractionation effect of noble gas isotopes in fluid inclusions that is caused by physical properties of host minerals has been concerned by geochemists for a long time (Turner and Stuart 1992; Baptiste and Fouquet 1996; Hu et al. 1998; Mao et al. 2003; Sun et al. 2006). In general, physical diffusion of He isotopes occurs most easily. Olivine and sulfides have good sealing ability, and among the sulfides, pyrite has the highest sealing ability and very low diffusion coefficient (Matsuda et al. 2002).
The values of He in fluid inclusions from pyrite and chalcopyrite vary significantly between 272 × 10−9 and 839 × 10−9 cc STP/g (averaging 529 × 10−9 cc STP/g), 20Ne values are 0.512–0.857 × 10−9 cc STP/g (averaging 0.646 × 10−9 cc STP/g), 40Ar values are 187–477 × 10−9 cc STP/g (averaging 308 × 10−9 cc STP/g), 84Kr values are 13.5–38.4 × 10−12 cc STP/g, and 132Xe values are 1.01–4.74 × 10−12 cc STP/g (Table 4). Noble gas contents are shown in Fig. 9. 4He, 20Ne, 40Ar, 84Kr, and 132Xe show normal distributions, indicating that these fluid inclusions have suffered no obvious loss of noble gas during analysis and the chalcopyrite also has well-sealing ability.
Atmospheric He contribution can be determined from the F4He values, which are defined as the 4He/36Ar ratio of the sample relative to the atmospheric 4He/36Ar value of 0.1655. A sample containing 100 % atmospheric He has an F value of unity (Kendrick et al. 2001; Zhai et al. 2006; Zeng et al. 2014). The values of F4He vary from 3,173 to 4,838, which are 3,000 times higher than in the atmosphere and 15,000 times higher than in air-saturated water (0.18–0.28), indicating that He contribution from the atmosphere was negligible, both before and after of the inclusions.
The radiogenic 40Ar* in geologic samples can be calculated using the formula in Table 4 (Schlosser and Winckler 2002). The contents of radiogenic 40Ar* vary from 8.8 to 15.5 %, and the contribution of atmospheric 40Ar is 84.5–91.2 %. The values of He in fluid inclusions show that the radiogenic 40Ar* contents are not mantle-derived but are from crustal sources. Crustal Ar mainly occurs in potassium-bearing micas and K-feldspar (Mcdougall and Harrison 1989). However, as the measured samples are fluid inclusions from sulfides being devoid of K and the time of entrapment is Early Cretaceous (Han et al. 2013) it can be assumed that these values represent those of the fluid at the time of inclusion entrapment.
In terms of isotope ratios, the 3He/4He ratios cover the range 0.033 to 0.104 Ra with an average of 0.061 Ra. These values are far below the He isotopic composition of the upper mantle (6–8 Ra) and the atmosphere (1 Ra) (Allègre et al. 1987; Sarda et al. 1988; Porcelli et al. 1992; Patterson et al. 1994; Dunai and Baur 1995) but close to the composition of fluid in young continental crust (0.01–0.05 Ra) (Stuart et al. 1995). The 20Ne/22Ne and 21Ne/22Ne ratios are in the range of 9.817–9.960 and 0.0290–0.0295, respectively, which is similar to the composition of the atmosphere ([20Ne/22Ne]a = 9.8, [21Ne/22Ne]a = 0.29). The 38Ar/36Ar and 40Ar/36Ar ratios are in the range of 0.18718–0.18761 and 324–349, respectively. The 38Ar/36Ar ratios are consistent with the ratio of air-saturated water (0.188), whereas the 40Ar/36Ar ratios are slightly higher than the ratio of air-saturated water (295.5) and young continental crust fluid and are much lower than the value for the mantle (>40,000). The 40Ar*/4He ratios are 0.018–0.055, far below the ratios of crustal rocks (0.2) and continental lithospheric mantle (0.5) (Ballentine and Burgess 2002). Figure 10a shows 4He contents vs. 3He/4He ratios. All of the compositional points fall in the range of crustal fluid or between the crustal fluid and mantle fluid on the right side of the cutoff line. This indicates that the fluid inclusions from minerals in this deposit have relatively high radiogenic 4He and are dominated by crustal components (Gautheron et al. 2005). On the diagram of 4He vs. 3He/4He (Fig. 10a) and 40Ar*/4He vs. 3He/4He (Fig. 10b), all the compositional points lie in or near the range of crustal fluid, indicating the ore-forming fluids of Naozhi Au–Cu deposit widely hold the properties of young crustal fluid, which is significantly different from the noble gas isotopic components of Xiaoxinancha Au-rich Cu deposit (Sun et al. 2008c).
Plot of a 4He vs. 3He/4He (after Gautheron et al. 2005; Ballentine and Burgess 2002) and b 3He/4He vs. 40Ar/36Ar (after Allègre et al. 1987; O’Nions and Tolstikhin 1994; Langmuir et al. 1978) in fluid inclusions from the Naozhi Au–Cu deposit and Xiaoxinancha Au-rich Cu deposit. Data of Xiaoxinancha Au-rich Cu deposit from Sun et al. (2008c)
Lead isotopic composition of sulfides
The lead isotopic results are given in Table 5. The 206Pb/204Pb, 207Pb/204Pb, and 206Pb/204Pb ratios of three pyrite samples vary from 18.3392 to 18.3667, 15.5323 to 15.5603, and 38.1595 to 38.2508, respectively. The 206Pb/204Pb, 207Pb/204Pb, and 206Pb/204Pb ratios of three chalcopyrite samples vary from 18.1822 to 18.3979, 15.5215 to 15.5998, and 38.1313 to 38.3786, respectively. The lead isotope compositions are similar to those of sulfides in the Xiaoxinancha gold-rich porphyry copper deposit (Men et al. 2011) which show that the data fall in the fields between MORB and a young lower crust source (Fig. 11a), or on the line representing the evolution of young orogenic belts (Fig. 11b). As mentioned above, the deposit has been dated between 130 and 123 Ma (Meng et al. 2001; Han et al. 2013) these values can be assumed to be close to their initial ratios.
Genetic types of deposit
Our results show that polysulfide-quartz vein ores make up the main ore body in the Naozhi Au–Cu deposit. Wall rock alteration includes silicification, sericitization, chloritization, and minor carbonatization and argillization. Mineralization types are mainly pyrite and chalcopyrite, followed by galena, sphalerite, bornite, chalcocite, and natural gold, electrum, and petzite. Mineralization occurred through four stages: a pyrite-sericite-quartz stage, a pyrite-quartz vein stage, a quartz-gold-polymetallic sulfide stage, and a calcite-quartz stage. The homogenization temperatures and salinities of these main mineralization stages were in the range of 230–338.8 °C and 1.73–9.47 wt% NaCleq, respectively; oxidation–reduction reactions (chalcopyrite → bornite + chalcocite) occurred during mineralization. On the basis of the mineralization–alteration features and low fluid inclusion salinity, the deposit is best described as a medium-temperature magmatic hydrothermal deposit or a medium-sulfidation epithermal deposit (Mao et al. 2007; Sillitoe 2008). Likewise, the Naozhi Au–Cu deposit is analogous to the Jinchanggouliang epithermal Au–Cu deposit (122 Ma, Mao et al. 2007) of the Chifeng area and to the Erdaogou epithermal Au–Cu deposit (126 ± 1 Ma, Miao et al. 2003) of western Liaoning in terms of its geological features, fluid inclusion compositions, and formation age (Chen et al. 2005; Miao et al. 2003). This indicates that this stage of mineralization occurred during Early Cretaceous volcanism associated with an extensional system within the Xing’an–Mongolian orogenic belt, a magmatic arc system related to positive subduction of the Pacific Plate underneath the Eurasian Plate, and the ore-forming components were brought to shallow crust by molten magma or supercritical fluid and so on (Sillitoe 1997). The metallogenesis in this area is closely related to high-K calc-alkaline intermediate-acid granitic and porphyritic pyroxene diorite magmatism (Chen et al. 2005; Mao et al. 2007).
Combined with the characteristics of diorite veins closely associated with mineralization ((87Sr/86Sr)I = 0.705024–0.704975, Nd(t) = +2.60 to +2.69, T DM2 = 856–863 Ma, our unpublished data) in the Naozhi deposit, we further conclude that the magma was derived from partial melting of lower continental crust with residual ocean property, produced during the subduction of Pacific plate. Mineralizing fluids exsolved during the late crystallization phase and were strongly contaminated by crustal materials/fluids during ascent, including meteoric water. The mixed ore-forming fluids migrated to the secondary brittle structures in the shear zone and finally precipitated auriferous sulfide-quartz ores because of decline of temperature and pressure of the ore-forming fluids.
Conclusions
Through systematic research of ore geology, microscopic feature, components and rare gases, isotopic components of fluid inclusion, and lead isotopic components of sulfides, we can make further conclusions as follows:
-
1.
The Naozhi deposit is a predominantly sulfide-quartz vein Au–Cu gold deposit, formed through four stages: a K-feldspar stage, a pyrite-quartz vein stage, a quartz-gold-polymetallic sulfide stage, and a calcite-quartz vein stage. Fluid inclusions are mainly gas–liquid two-phase inclusions (W), followed by pure gas inclusions (V), pure liquid inclusions (L), CO2-bearing inclusions (C), and rare daughter mineral-bearing polyphase inclusions (S).
-
2.
The initial ore-forming fluid of Naozhi Cu–Au gold deposit is CO2-H2O-NaCl ore-bearing fluid with medium–high temperature and low salinity. The mineralizing fluid is not homogeneous from the early stage to the late stage and occurs boiling during the early stage and main ore-forming stage, which result in precipitating of ore-forming elements.
-
3.
Combined with stable isotope data and the chronology of diagenesis and metallogenesis, our new data suggest that the ore-forming fluids originated from melting of the lower crust, produced during the subduction of an oceanic slab. Mineralizing fluids exsolved during the late crystallization phase and were strongly contaminated by crustal materials/fluids during ascent, including meteoric water. Mineral precipitation occurred at a shallow crustal level.
References
Allègre CJ, Staudacher T, Sarda P (1987) Rare gas systematics: formation of the atmosphere, evolution and structure of the Earth’s mantle. Earth Planet Sci Lett 81:127–150. doi:10.1016/0012-821X(87)90151-8
Ballentine CJ, Burgess R (2002) Tracing fluid origen, tansport and interaction in the crust. Rev Mineral Geochem 47:539–614. doi:10.2138/rmg.2002.47.13
Baptiste PJ, Fouquet Y (1996) Abundance and isotopic composition of helium in hydrothermal sulfides from the East Pacific Rise at 13°N. Geochim Cosmochim Acta 60:87–93. doi:10.1016/0016-7037(95)00357-6
Bing ZY, Zhang HT, Wang LS, Chen RY (1995) The multiple metallogenic model of the porphy-epithermal copper-gold deposits, in Eastern Jilin and Heilongjiang. Mineral Deposits 14:174–183 (in Chinese with English abstract)
Bodnar RJ (1993) Revised equation and table for determining the freezing point depression of H2O-NaCl solutions. Geochim Cosmochim Acta 57:683–684. doi:10.1016/0016-7037(93)90378-A
Burnard PG, Hu RZ, Turner G, Bi XW (1999) Mantle, crustal and atmospheric noble gases in Ailaoshan Gold deposits, Yunnan Province, China. Geochim Cosmochim Acta 63:1595–1604. doi:10.1016/S0016-7037(99)00108-8
Chai P, Sun JG, Xing SW, Men LJ, Han JL (2015) Early Cretaceous arc magmatism and high-sulphidation epithermal porphyry Cu–Au mineralization in Yanbian area, Northeast China: the Duhuangling example. Int Geol Rev 57:1267–1293. doi:10.1080/00206814.2014.960013
Chen JQ, Sun JG, Piao SC, Zhao JK, Zhai YF (2005) Genesis and significance of dark dikes in the Jinchanggouliang mine area, Inner Mongolia: evidences from geochemistry of the major and trace elements. J Jilin Univ (Earth Sci Ed) 35:707–713 (in Chinese with English abstract)
Cheng MH, Xue XY, Li WL (2009) Geologic characteristics of volcanic-type gold deposit in Wangqing County, Naozhi. Jilin Geol 28:25–27 (in Chinese with English abstract)
Cooke DR, Simmons SF (2000) Characteristics and genesis of epithermal gold deposits. Rev Econ Geol 13:221–244
Drescher J, Kirsten T, Schäfer K (1998) The rare gas inventory of the continental crust, recovered by the KTB Continental Deep Drilling Project. Earth Planet Sci Lett 154:247–263. doi:10.1016/S0012-821X(97)00185-4
Dunai TJ, Baur H (1995) Helium, neon and argon systematics of the European subcontinental mantle: implications for its geochemical evolution. Geochim Cosmochim Acta 59:2767–2783. doi:10.1016/0016-7037(95)00172-V
Feng SZ (1994) Geological characteristics and metallogenic model of Naozhi gold deposit, in Wangqing. Gold Geol 39:23–26 (in Chinese)
Gautheron C, Moreira M (2002) Helium signature of the subcontinental lithospheric mantle. Earth Planet Sci Lett 199:39–47. doi:10.1016/S0012-821X(02)00563-0
Gautheron C, Moreira M, Allègre C (2005) He, Ne and Ar composition of the European lithospheric mantle. Chem Geol 217:97–112. doi:10.1016/j.chemgeo.2004.12.009
Groves DI, Bierlein FP (2007) Geodynamic settings of mineral deposit systems. J Geol Soc Lond 164:19–30. doi:10.1144/0016-76492006-065
Hall DL, Sterner SM, Bodnar R (1988) Freezing point depression of NaCl-KCl-H2O solutions. Econ Geol 83:197–202. doi:10.2113/gsecongeo.83.1.197
Han SJ, Sun JG, Bai LA, Xing SW, Chai P, Zhang Y, Yang F, Meng LJ, Li YX (2013) Geology and ages of porphyry and medium- to high-sulphidation epithermal gold deposits of the continental margin of Northeast China. Int Geol Rev 55:287–310. doi:10.1080/00206814.2012.695472
Hedenquist JW, Lowenstern JB (1994) The role of magmas in the formation of hydrothermal ore deposits. Nature 370:519–527. doi:10.1038/370519a0
Hu RZ, Burnard PG, Turner G, Bi XW (1998) Helium and argon isotope systematics in fluid inclusions of Machangqing copper deposit in west Yunnan province, China. Chem Geol 146:55–63. doi:10.1016/S0009-2541(98)00003-5
Huang GC (1997) Dicussion on genetic relation between the Naozhi gold deposit and the Mesozoic volcanic rock series in Naozhi, Jilin province. Miner Resour Geol 11:32–38 (in Chinese with English abstract)
Jiang SH, Nie FJ, Zhang Y, Hu P (2004) The latest advances in the research of epithermal deposits. Earth Science Front 11:401–411 (in Chinese with English abstract)
Jin K (2003) Petrology and geochemistry of Mesozoic volcanic rocks in Yanbian area: constraints on transformation of tectonic system and composition of lithosphere. Ph.D. thesis, Jilin University (in Chinese with English abstract)
Kendrick MA, Burgess R, Pattrick RAD, Turner G (2001) Fluid inclusion noble gas and halogen evidence on the origin of Cu-porphyry mineralizing fluids. Geochim Cosmochim Acta 65:2651–2668
Langmuir CH, Vocke RD, Hanson GN, Hart SR (1978) A general mixing equation with applications to Icelandic basalts. Earth Planet Sci Lett 37:380–392. doi:10.1016/0012-821X(78)90053-5
Li ZL (1988) Experimental geochemistry. Geological Publishing House, Beijing, pp 1–326 (in Chinese)
Li HN, Yang GB (1991) Geochemical characteristics of the volcanic gold deposits and mineralization in Yanbian Prefecture, Jilin Province. Gold 12:6–12 (in Chinese with English abstract)
Lin BL (2013) Study on the mineralization tectonic setting and genesis of Naozhi gold deposit, eastern Jilin Province. Masters thesis, Jilin University (in Chinese with English abstract)
Lu HZ, Fan HR, Ni P, Ou GX, Shen K, Zhang WH (2004) Fluid inclusions. Science Press, Beijing, pp 1–487 (in Chinese with English abstract)
Mao JW, Li YQ, Goldfarb R, He Y, Zaw K (2003) Fluid inclusion and noble gas studies of the Dongping gold deposit, Hebei province, China: a mantle connection for mineralization? Econ Geol 98:517–534. doi:10.2113/98.3.517
Mao JW, Li XF, White NC, Zhao CS, Zhang ZH, Wang YT, Hu HB (2007) Types, characteristics, and geodynamic settings of Mesozoic epithermal gold deposits in eastern China. Resour Geol 57:435–454. doi:10.1111/j.1751-3928.2007.00033.x
Matsuda J, Matsumoto T, Sumino H, Nagao K, Yamamoto J, Miura Y, Kaneoka I, Takahata N, Sano Y (2002) The 3He/4He ratio of the new internal He Standard of Japan (HESJ). Geochem J 36:191–195. doi:10.2343/geochemj.36.191
Mcdougall I, Harrison TM (1989) Geochronology and thermochronology by the 40Ar-39Ar method. University Press, Oxford
Men LJ, Sun JG, Zhang ZJ, Li YX, Xing SW, Cui PL (2011) An isotopic (Sr, Nd and Pb) tracer study on the Xiaoxinancha gold-rich copper deposit in Yanbian, China: implication for the geodynamic model of diagenesis and metallogenesis. Acta Geol Sin-Engl 85:175–188. doi:10.1111/j.1755-6724.2011.00388.x
Meng QL, Zhou YC, Chai SL (2001) The porphy-epithermal veins copper-gold deposits, in Eastern Jilin and Heilongjiang. Jilin Science and Technology Press, Changchun, pp 1–162 (in Chinese with English abstract)
Miao LC, Fan WM, Zhai MG, Qiu YM, McNaughton NJ, Groves DI (2003) Zircon SHRIMP U-Pb geochronology of the granitoid intrusions from Jinchanggouliang-Erdaogou gold orefield and its significance. Acta Petrol Sin 19:71–80
Nagao K, Ogata N, Miura YN, Yamaguchi K (1996) Ar isotope analysis for K-Ar dating using two modified-VG5400 mass spectrometers-I: isotope dilution method. J Mass Spectrom Soc Jpn 44:39–61. doi:10.5702/massspec.44.39
O’Nions RK, Tolstikhin IN (1994) Behaviour and residence times of lithophile and rare gas tracers in the upper mantle. Earth Planet Sci Lett 124:131–138. doi:10.1016/0012-821X(94)00070-0
Patterson DB, Honda M, McDougall I (1994) Noble gases in mafic phenocrysts and xenoliths from New Zealand. Geochim Cosmochim Acta 58:4411–4427. doi:10.1016/0016-7037(94)90344-1
Peng YJ, Zhao CB (2001) The evolution of the paleo Jihei orogenic belt and accretion of the continental crust. Jilin Geol 20:1–9 (in Chinese with English abstract)
Porcelli DR, O’Nions RK, Galer SJG, Cohen AS, Mattey DP (1992) Isotopic relationships of volatile and lithophile trace elements in continental ultramafic xenoliths. Contrib Mineral Petrol 110:528–538. doi:10.1007/BF00344086
Reid MR, Graham DW (1996) Resolving lithospheric and sub-lithospheric contributions to helium isotope variations in basalts from the southwestern US. Earth Planet Sci Lett 144:213–222. doi:10.1016/0012-821X(96)00166-5
Roedder E (1984) Fluid inclusions. Rev Mineral 12:1–644
Sarda P, Staudacher T, Allègre CJ (1988) Neon isotopes in submarine basalts. Earth Planet Sci Lett 91:73–88. doi:10.1016/0012-821X(88)90152-5
Schlosser P, Winckler G (2002) Noble gases in ocean water and sediments. Rev Mineral Geochem 47:701–730. doi:10.2138/rmg.2002.47.15
Sillitoe RH (1997) Characteristics and controls of the largest porphyry copper-gold and epithermal gold deposits in the circum-Pacific region. Aust J Earth Sci 44:373–388. doi:10.1080/08120099708728318
Sillitoe RH (2008) Special paper: major gold deposits and belts of the North and South American Cordillera: distribution, tectonomagmatic settings, and metallogenic considerations. Econ Geol 103:663–687. doi:10.2113/gsecongeo.103.4.663
Stuart FM, Burnard PG, Taylor RP, Turner G (1995) Resolving mantle and crustal contributions to ancient hydrothermal fluids: He–Ar isotopes in fluid inclusions from Dae Hwa W–Mo mineralisation, South Korea. Geochim Cosmochim Acta 59:4663–4673. doi:10.1016/0016-7037(95)00300-2
Su FX, Wang XY, Jia WG (2003) Study on typomorphic characteristics of pyrite in the Naozhi Gold Deposit, Jilin. Gold 24:13–16 (in Chinese with English abstract)
Sumino H, Nagao K, Notsu K (2001) Highly sensitive and precise measurement of helium isotopes using a mass spectrometer with double collector system. J Mass Spectrom Soc Jpn 49:61–68. doi:10.5702/massspec.49.61
Sun XM, Xu L, Zhai W, Tang Q, Liang YH, Liang JL, Zhang ZM, Shen K (2006) Noble gases isotopic compositions of fluid inclusions in quartz veins and crystals collected from CCSD and Donghai HP-UHP metamorphic rocks. Acta Petrol Sin 22:1999–2008
Sun JG, Chen L, Zhao JK, Men LJ, Feng W, Chen D, Liang SN (2008a) SHRIMP U-Pb dating of zircons from Late Yanshanian granitic complex in Xiaoxinancha gold-rich copper orefield of Yanbian and its geological implications. Mineral Deposits 27:319–328 (in Chinese with English abstract)
Sun JG, Men LJ, Zhao JK, Chen L, Liang SN, Chen D, Feng W (2008b) Zircon chronology of melanocratic dykes in the district of the Xiaoxinancha Au-rich Cu deposit in Yanbian and its geological implication. Acta Geol Sin-Engl 82:517–527
Sun JG, Zhao JK, Chen JQ, Nagao K, Sumino H, Shen K, Men LJ, Chen L (2008c) Ore-forming mechanism for the Xiaoxinancha Au-rich Cu deposit in Yanbian, Jilin Province, China: evidence from noble gas isotope geochemistry of fluid inclusions in minerals. Sci China Ser D 51:216–228
Sun XM, Wei HX, Zhai W, Shi GY, Liang YH, Mo RW, Han MX, Zhang XG (2010) Ore-forming fluid geochemistry and metallogenic mechanism of Bangbu large-scale orogenic gold deposit in southern Tibet, China. Acta Petrol Sin 26:1672–1684
Taylor HP (1997) Oxygen and hydrogen isotope relationships in hydrothermal mineral deposits. In: Barnes HL (ed) Geochemistry of hydrothermal ore deposits, 3rd edn. Wiley, New York, pp 229–302
Turner G, Stuart FM (1992) Helium/heat ratios and deposition temperatures of sulphides from the ocean floor. Nature 357:581–583. doi:10.1038/357581a0
Wan F (2013) Characteristics, genesis and prospecting criteria of the Mesozoic epithermal gold deposits in Yanbian area. Masters thesis, Jilin University (in Chinese with English abstract)
Wu TT (2013) Geological characteristics and metallogenic regularity of gold deposits from Naozhi to Duhuangling in Wangqing County, Jinlin Province. Masters thesis, Jilin University (in Chinese with English abstract)
Wu FY, Sun DY, Ge WC, Zhang YB, Grant ML, Wilde SA, Jahn BM (2011) Geochronology of the Phanerozoic granitoids in northeastern China. J Asian Earth Sci 41:1–30
Yang SS, Qing M, Hao TS, Wang KY, Wan D, Piao XH, Bian HY (2010) Geological characteristics and genesis of Naozhi gold deposit, in Yanbian area, Jilin Province. Global Geol 29:607–613 (in Chinese with English abstract)
Zartman RE, Doe BR (1981) Plumbotectonics—the model. Tectonophysics 75:135–162. doi:10.1016/0040-1951(81)90213-4
Zeng QD, Wang ZC, He HY, Wang YB, Zhang S, Liu JM (2014) Multiple isotope composition (S, Pb, H, O, He, and Ar) and genetic implications for gold deposits in the Jiapigou gold belt, Northeast China. Miner Deposita 49:145–164. doi:10.1007/s00126-013-0475-2
Zhai W, Sun XM, He XP, Su LW, Wu YL (2006) Noble gas isotopic geochemistry of Axi gold deposit, and its metallogenic implications, Xinjiang, China. Acta Petrol Sin 22:1–7
Zhang YB (2002) The isotopic geochronoligic frame of granitic magmatism in Yanbian area. Ph.D. thesis, Jilin University (in Chinese with English abstract)
Zhang Y (2010) Gold deposit geological characteristics and exploration potential of the Miantian-Naozhi region. Sci Technol West Chin 9:4–7 (in Chinese with English abstract)
Zhang YB, Wu FY, Wilde S, Zhai MG, Lu XP, Sun DY (2004) Zircon U-Pb ages and tectonic implications of ‘Early Paleozoic’ granitoids at Yanbian, Jilin Province, northeast China. Island Arc 13:484–505. doi:10.1111/j.1440-1738.2004.00442.x
Zhao HG (2007) Study on the metallogenesis and models in Mesozoic epithermal gold deposits in Yanbian, Jilin Province. Ph.D. thesis, Jilin University (in Chinese with English abstract)
Acknowledgments
The authors thank Prof. I. Kaneoka, Drs. A. Shmizu and N. Ebisawa (Laboratory for Earthquake Chemistry, Graduate School of Science, University of Tokyo) and Prof. Lai Yong (Peking University) for their support during the fluid inclusion noble gas experiments, and the staff of the State Key Laboratory of Mineralization, Nanjing University and the Geological Fluid Laboratory, Jilin University for help with measuring the temperature and composition of fluid inclusions. In particular, we thank two reviewers for their critical reviews and suggestions that improved the manuscript. This work was funded by the National Natural Science Foundation of China (Grant Nos. 40772052 and 41172072), the major project for the National Natural Science Foundation of China (Grant No. 41390444), the Project for Scientific and Technology Development (Grant No. 20100450), the Chinese Geological Survey Programme (Grant No. [2010]26-06), and the Geological Survey of Heilongjiang Province (Grant No. 3R1101604422).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gu, AL., Men, Lj., Sun, JG. et al. Origin and evolution of ore fluids in the late Mesozoic Naozhi epithermal Au–Cu deposit, Yanbian area, Northeast China: evidence from fluid inclusion and isotopic geochemistry. Arab J Geosci 9, 117 (2016). https://doi.org/10.1007/s12517-015-2030-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-015-2030-1