Abstract
In this study, the distribution of the chemical constituents (major, minor, and trace elements) is determined in different localities in Egypt’s western desert oases, i.e., Bahariya, Farafra, Bahrean, Siwa, El-Gara, and Al-Jaghbub oases. The Nubian Sandstone Aquifer (NSA) is shared between all oases; however, the Limestone Aquifer (LA) is shared only between the western oases, i.e., Bahrean, Siwa, El-Gara, and Al-Jaghbub. The LA is usually located above NSA in Egypt’s western desert. The results indicate notable difference in chemical characteristics between the LA and the NSA waters. Under furrow surface irrigation systems that are commonly used in the Bahariya and Farafra oases, negligible potential for harmful effects on soils and plants is anticipated. However, in case of using drip or sprinkler irrigation systems, the iron and manganese identified in groundwater may precipitate, causing blockages to occur. The LA waters salinity is found to be higher than NSA and above acceptable level for irrigation. Besides, salinity, chloride, and water sodicity make this water unsuitable for irrigation. Durov and piper diagrams reveal that the majority of investigated waters were calcium–magnesium sulfate water type corresponding the Bahariya and Farafra, and sodium, chloride, and sulfate water type corresponding the Siwa, Bahrean, El-Gara, and Al-Jaghbub waters. The saturation indices for minerals indicated that most studied LA waters were undersaturated for halite; however, the NSA waters were saturated with respect to aragonite, calcite, and dolomite.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many people believe that the water will be the oil of the twenty-first century. Successful water management will be the key to future economic growth and social wealth in both developed and developing countries (Aly et al. 2014a). Egypt and other countries in the region cannot afford to turn a blind eye to the shortage of water and the shortsighted misuse of stored underground water supplies. The limitation of the water resources in Egypt coupled with the continuous population growth invokes a pressure on the region resources especially water. To cope with water resources limitation, it is inevitable to adopt a national policy for using brackish groundwater and recycling wastewater (Aly 2007). Unluckily, Egypt’s groundwater quality has deteriorated dramatically in recent years (Aly and Benaabidate 2010). A good understanding of the hydrochemical processes that govern groundwater quality is required for the sustainable management of the groundwater resources (El-Sayed et al. 2012). The chemical composition of groundwater is determined by cation exchanges with the surrounding geological layers, dissolution and precipitation of minerals, and evaporation and oxidation-reduction reactions. Understanding these complicated hydrogeochemical processes will help to get an insight in the contribution of rock-water interactions that influence groundwater quality (Aly et al. 2014b). These geochemical processes are responsible for the spatial and temporal variations of the groundwater’s chemistry (Kumar et al. 2006). In addition, geochemical patterns (major ion and trace element content) of groundwater largely determine its suitability for drinking, domestic, or agricultural purposes (Kaitantzian et al. 2013; Adhikary et al. 2012; Elkrail and Obied 2013). The Egyptian western desert corresponds to the eastern part of a very large sedimentary basin (about 1,800,000 km2) which underlies extensive areas of Egypt, Libya, Sudan, and Chad. This basin is filled with a thick succession of Sandstone and shales of Palozoic to Mesozoic age and is known as the Nubian Sandstone complex which constitutes the Nubian Sandstone multi-layer Aquifer System (Trac 1984). In Egypt, the Nubian Sandstone Aquifer (NSA) occupies an area of about 850,000 km2, including the area known as Wadi El Gedid (New Valley) at Kharga, Dakhla, Farafra, and Bahariya (Karanjac 1995). The groundwater resources of the NSA in the western desert of Egypt have attracted the interest of investigators since the end of the nineteenth century. Methodical studies of the aquifer in the Kharga and Dakhla oases date from 1956 when the Government of Egypt initiated the New Valley Development Project. Since that date, a series of exploratory and production well-drilling programs have been undertaken, and around 500 wells have been completed to various depths in the Kharga and Dakhla oases, and also the two northern oases of Fafafra and Bahariya, as well as their environs (Trac 1984). Furthermore, the number of wells had exceeded 1200 in Siwa Oasis (Aly et al. 2008). The continuous agricultural expansion, urban development, and the increased demands on Egypt western desert groundwater supplies cause the more groundwater abstraction and lead to the dangers of overexploitation and deterioration of the groundwater quality (Aly et al. 2008). Furthermore, Aly and Benaabidate (2010) concluded that the groundwater depletion was observable in Egypt groundwater, and if this depletion continues unabated, the agricultural land depended on groundwater for irrigation may not be able to survive.
In 1956, major agricultural development programs were started at Kharga and Dakhla Oases in the western desert, where a large number of deep wells were drilled. A similar development program was begun in Libya’s Kufra Oasis in 1972. At the Kharga and Dakhla projects, water originally flowed under artesian pressure, with an annual discharge rate of about 200 million cubic meters (MCM). In 1956, with the expansion of facilities, piezometric head levels started declining by as much as 1 m/year. This caused the production from individual wells to decrease or, in the case of some shallow wells, requiring the drilling of new deep wells in order to maintain overall production. The declining head has also caused at least half of the deep wells in Kharge Oasis to stop flowing freely; consequently, these artesian wells have been transformed to pumping wells (Ahmed 1977).
In the present study, the objectives are (a) to record and compare the present quality status of the groundwater for irrigation purposes in the Bahariya and Farafra oases, (b) to classify the hydro-chemical characterization of studied waters, and (c) to aid the management and future development of groundwater resources in Egypt western desert.
Materials and methods
Description of the study sites
Bahariya oasis
The Bahariya depression is a natural excavation in the central part of the Egyptian Western Desert, some 130 km west of El-Minia governorate in the Nile valley and about 360 km S–W of Cairo. It situated essentially between 27° 48′ and 28° 30′ N and 28° 29′ and 29° 08′ E. It comprises an area of approximately 2250 km2.
Bahariya Oasis faces a sever constraint in the availability of good agricultural land. This is further aggravated by the scarcity of irrigation water and suitable soils under the aridic conditions of the area (Rasheed et al. 2008).
Farafra oasis
The Farafra Oasis is one of the small oasis located in Western Egypt, near latitude 27.06° N and longitude 27.97° E. It is located in the Western Desert of Egypt, approximately mid-way between Dakhla and Bahariya. Farafra has an estimated 5000 inhabitants (2002) living within its single village and is mostly inhabited by the local Bedouins. Also located near Farafra are the hot springs at Bir Setta and the El-Mufid lakes. A main geographic attraction of Farafra is its White Desert (known as Sahara el Beyda, with the word sahara meaning a desert). The White Desert of Egypt is located 45 km (30 miles) north of Farafra. The desert has a white, cream color and has massive chalk rock formations that have been created as a result of occasional sandstorms in the area.
Siwa oasis
The Siwa Oasis is considered one of the seven major important depressions in the Western desert of Egypt. It is about 306 km southwest Marsa Matrouh and 60 km east of Libyan border and about 400 km west of Bahariya oasis. The total area of the oasis is about 1100 km, about 5 % of this area are now under cultivation, while the remaining area is mainly saline, rocky, or occupied by salty lakes. The main activities of the population are agriculture together with some small industries such as olive oil extraction, olive pickling, date drying, and the production of carpets and kilims. The only source of water in the Siwa Oasis is groundwater, as rainfall is almost nil. The water salinity of shallow wells ranges between 1600 and 8000 ppm, while the salinity of deep artesian wells ranges between 300 and 400 ppm (Aly 2001). The Siwa Oasis represents a fragile oasis-like ecosystem, lies in a depression averaging some 20 m below sea level and the extensive irrigation made it vulnerable to environmentally induced water-associated land productivity problems. Such problems are aggravated by poor natural resource management (NRM) practices. In Siwa ample evidences of soil salinity, fertility depletion and decline of soil productivity were evident. In brief, the Siwa ecosystem suffers from serious land and water degradation problems that threaten its very existence and thus need urgent restoration interventions (Aly 2007).
El-Gara oasis
The Gara Oasis is the one of the smallest oasis located in Western Egypt, close to west part of Qattara Depression, approximately mid-way between Marsa Matrouh and Siwa Oasis. El-Gara has an estimated only 300 inhabitants living within its single village and is mostly inhabited by the local Bedouins. Also, El-Gara includes hot springs and wells. The Gara oasis lies in a depression averaging some 40 m below sea level.
Sampling sites and dates
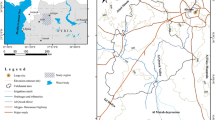
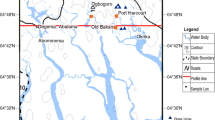
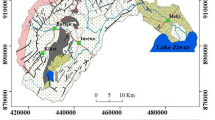
In this study, water samples were collected from 39 different locations that cover five oases of Egypt western desert and one oasis of Libyan Desert, in attempt to capture the spatial variations in the water resources quality of the western desert groundwater (Fig. 1). The coordinates of the studied wells were obtained using a custom made Real-Time Differential GPS, which uses a radio signal to correct the GPS signal in real time and provides an accuracy of approximately 5 m. Garmin 12XL GPS (Garmin Corporation 1997) was used to locate the artesian wells, using either the geographic latitude or the UTM northing–easting coordinates systems (Fig. 1).
The collected samples were stored in ice boxes and transported to the laboratory for chemical analysis. For heavy metals determination, water samples were filtered if necessary and preserved by acidity with concentrated nitric acid to pH < 2 (Klute 1986).
Water quality measurements
The water samples were analyzed according to the following procedures:
-
Water reaction (pH): The pH of the water was determined using a pH meter (pH meter–CG 817).
-
The total dissolved solids were measured by using electrical conductivity meter (E.C) in dS/m at 25 °C (Test kit Model 1500_20 Cole and Parmer).
-
Calcium and magnesium were determined by versenate titration method (EDTA) according to Klute (1986).
-
Sodium and potassium were determined by using flame photometer.
-
Carbonates and bicarbonates were determined by titration with 0.005 N sulfuric acid using phenolphthalein as indicator for the former and methyl orange as indicator for the latter according to Klute (1986).
-
Chlorides were determined by titration with 0.005 N silver nitrate solution and potassium chromate as indicator according to Klute (1986).
-
Boron was determined calorimetrically by using carmine according to U.S. Salinity Laboratory staff (1954).
-
Heavy metals, iron, zinc, manganese, copper, and cadmium were measured by flame atomic absorption spectrometry, A Perkin Elmer AA model (6083).
The suitability of water resources in Baharyia and Farafra oases for irrigation was evaluated by FAO method (Ayers and Westcot 1985).
Ion balance errors
The correctness of the chemical analysis was verified by calculating ion balance errors; furthermore, standard solutions and blanks were commonly run to check for possible errors in the analytical procedures. The level of error in the data was calculated using the following formula (Appelo and Postma 1996):
An error of up to ±3 % is tolerable, while every water sample with a calculated error outside this range should be measured again. Approximately 90 % of the measured water samples were within this range. This means that the resultant data quality is sufficient for chemical modeling and/or for drawing simple conclusions about water quality.
Treatment of analytical data
The chemical analysis data of the water samples were plotted on the Piper, and Durov diagrams using Geochemistry Software Aq.QA, version AQC10664 (Rockware AqQA Software 2011) for the identification of water types.
Geochemical modeling
Interactions between water and the surrounding rocks and soil are considered to be the main processes controlling the observed chemical characteristics of the water. The deviation of water from equilibrium with respect to dissolved minerals is quantitatively described by the saturation index (SI). The SI of a mineral is obtained from the following formula:
where IAP is the ion activity product of the dissociated chemical species in solution and k t is the equilibrium solubility product of the chemical involved (Alexakis 2011).
The hydrogeochemical equilibrium model, Phreeqc model (Parkhurst and Appelo, 2003), was used to calculate the SI of the untreated groundwater with respect to the main mineral phases.
Finally, the Excel and Sigma plot programs were used for data statistics calculation.
Results and discussion
NSAS in the western desert
The major ions chemistry of groundwater samples of Bahariya and Farafra statistically analyzed and the results summarized by minimum, maximum, mean,…etc. (Tables 1, 2, 3, and 4). Suitability of the groundwater for irrigation purpose was discussed based on the basic criteria of FAO (1985). The data showed that there are generally no considerable spatial differences between water qualities obtained from different locations and depths within the two Oases. In general, water obtained from Bahariya and Farafra oases was the same in its quality. It was also observed that the water salinity as one primary indicator for water quality for irrigation was in the acceptable level for irrigation in all places (Tables 1 and 2). The average water salinity (TDS) in Bahariya groundwater was 220.4 mg/L and ranged between 128 and 448 mg/L. On the other hand, the average water salinity in Farafra studied wells was 157.9 mg/L and ranged, as Bahariya, between 128 and 448 mg/L. In general, the studied Bahariya and Farafra groundwater salinity, chloride, and sodium concentrations were within acceptable limit for irrigation (Tables 1 and 2). The permissible limit of the TDS, Cl, and Na according to FAO (1985) is 500 mg/L, 9.0 meq/L, and 10 meq/L, respectively. Specific toxic elements such as boron were in the acceptable safe limit in most wells (<0.7 mg/L); however, only one well water sample no. 6, Bir Kasa 1, in Bahariya and two wells no. 14 and 15, Ain Kadra and Bir white desert, in Farafra have boron concentrations more than 0.7 mg/L and less than 3 mg/L, this mean that these three wells have slight to moderate degree of restriction on agriculture use according to FAO (1985) (Table 3). The trace elements levels, iron, copper, and zinc, generally were within the safe and acceptable levels for surface irrigation except Ain El-Sera (3) which contain iron concentration more than 5 mg/L, this mean that this well’s water may contribute to the loss of availability of essential phosphorus and molybdenum. On the other hand, the copper and zinc concentration in all wells samples was lower than recommended maximum concentration of the Zn and Cu in irrigation water. Most of Bahariya and Farafra groundwater manganese concentration may be toxic to a number of crops, but usually only in acid soils (Table 3) (AL-Farraj et al. 2013). The recommended maximum FAO’s iron, copper, zinc, and manganese concentrations on irrigation water are 5.0, 0.2, 2.0, and 0.2, respectively. The pH of Bahariya and Farafra groundwaters was within the normal range (6.5–8.4) except Bir Kasa 1 and Bir Kasa 2 (no. 1 and 6) which were slightly alkaline. In case of using drip or sprinkler irrigation systems, the water suitability of Bahariya and Farafra groundwater might be different, due to the expected chemical and physical blocking processes that might occur through the irrigation nets by the heavy precipitated materials of iron and manganese (Aly et al. 2011). Overhead sprinkling may result in unsightly deposits on plants, equipment, and buildings. A water sample from western lake in Baharyia oasis was also tested hydrochemically and found contain high salt content (EC = 63.1 dS/m). Furthermore, it contains high concentration of boron (59.9 mg/L). The lake’s trace elements, i.e., iron, manganese, copper, and zinc, were found within the safe concentration. This is due to the aeration and oxidation process of trace elements in the lake’s surface area is expected as follow (AL-Farraj et al. 2013):
Comparison the hydrochemical features of studied oases groundwater
As shown in Tables 1 and 2, and Figs. 2 and 3, the dominant cations in Bahariya and Farafra oases groundwaters were magnesium then calcium, this mean that there is a mineralization and dolomitization in the water–rock interaction in the groundwater of these two areas. According to Appelo and Postma (1994) in an aquifer containing dolomite and calcite, it might be expected that the groundwater be close to equilibrium for both minerals as follow:
On the other hand, the dominant cations in the Bahrean, Siwa, El-Gara, and Al-Jaghbub oases were sodium and then magnesium for both LA and NSA. The dominant anions in Bahariya and Farafra groundwater and LA were chloride then bicarbonate, while the dominant anions in the other deep western desert groundwater (NSA), i.e., Siwa and El-Gara, were bicarbonate then chloride (Figs. 2 and 3). The dominant ions in the LA waters of Siwa, Bahrean, El-Gara, and Al-Jaghbub oases were sodium and chloride. This mean that there is water–halite interaction during upward fresh water flows from the NSA to the fractured carbonate zones through the fault planes. From the previous discussion, we can conclude that there are considerable similarities between the hydrochemical characteristics of Bahariya and Farafra oases. There is also some similarity between water salinity of these two oases and the NSA groundwaters of the western oases. In contrast, the LA groundwaters of the western oases exhibited marked difference from Farafra and Bahariya NSA groundwaters (Tables 1, 2, and 4; and Figs. 2 and 3).
The copper and zinc concentrations were low in general; however, the iron concentration was the highest trace elements in the NSA groundwater in all studied oases flowed by manganese this is due to the Ferruginous mineralization in the rock–water interface may takes place (Table 3).
The heavy metals and boron data for Bahariya and Farafra oases were also presented in box plots graph (Fig. 4a, b); the box plots graph data as a box representing statistical values. The boundary of the box closest to zero indicates the 25th percentile, a line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile. Whiskers above and below the box indicate the 90th and 10th percentiles. Figure 4 showed that the iron is highest heavy metals concentration; however, the copper was lowest concentration.
Generally, the trace elements in the shallow LA of the western oasis were lower than the deep NSA. The iron concentration in Bahariya and Farafra was higher than the other western oases. Boron concentration in all LA of western desert oases was generally in acceptable limit except the sample of Bir Ghagboub (no. 39) which has boron concentration higher than the acceptable limit (11.2 mg/L).
Hydrochemical aspects
The chemical data of the treated groundwater samples were plotted on a Piper trilinear (Piper 1944) (Fig. 5). The piper diagrams provide a convenient method to classify water types collected from different groundwater resources, based on the ionic composition of different water samples (Al-Omran et al. 2012; Semerjian 2011; Baba et al. 2008; Al-Ahmadi 2013). The main water types have been identified on the basis of the major ion concentrations as in Aly and Benaabidate (2010), Aly et al. (2013), and Loni et al. (2014). This diagram reveals that there are different types of mineral water in Egypt western desert groundwater. Three main mineral water types have been identified on the basis of the major ion concentrations as in Aly and Benaabidate (2010) and Baba et al. (2008). The first is rich in a calcium–magnesium sulfate water type which corresponds to the Bahariya and Farafra water samples. The second is rich in sodium, chloride, and sulfate water type corresponding to Siwa, Monkar Eltalh, Bahrean, El-Gara, and Ghagboub shallow groundwater samples. The third is rich in sodium bicarbonate water type corresponding to Siwa, Monkar Eltalh, Bahrean, and El-Gara deep groundwater samples (Fig. 5). In the study area of the Bahariya and Farafra, the type of water that predominates is Mg/CaSO4, which is mostly due to the geology of the area which comprises gypsum, anhydride, and dolomite; however, in the study area of the Siwa, Monkar Eltalh, Bahrean, El-Gara, and Ghagboub shallow groundwater samples, the type of water that predominates is NaCl, which is mainly due to the geology of the area which comprises halite.
The major cation and anion concentrations of the samples collected from groundwaters in the region were plotted on Durov's diagram (Fig. 6). Durov’s diagram helps the interpretation of the evolutionary trends and the hydrochemical processes occurring in the groundwater system and can indicate mixing of different water types, ion exchange and reverse ion exchange processes. The result shows that the majority of the Bahariya and Farafra NSA groundwater samples fall in field 4 and 5, the zone of high-water salinity. The samples belonging to field 4 suggest the presence of SO4 −2 and Ca2+ as dominant type of water and indicating gypsum-bearing sedimentary aquifer and the groundwater affected by oxidation of pyrite and other sulfide minerals. However, field 5 of Durov’s diagram indicating mixing processes of two or more different facies might be occurring. The LA groundwaters are located in field 7 and 8. The field 7 suggests the presence of Cl− and Ca2+ as dominant type of water and indicating the invasion of fresh water by saline water, and field 8 suggests the presence of Cl− and Mg2+, Na+, and Ca2+ as dominant type of water indicating mixing of fresh and saline water. In high salinity water environment, the process of reverse ion exchange may create CaCl2 waters due to removal of Na+ from solution by exchanging Ca2+ from clay fraction in aquifer. Alternatively, CaCl2 type waters could also be a result of mixing process between fresh water with more saline older water (Adams et al. 2001). Gibbs’s diagrams, representing the ratios of Na+ + K+: (Na+ + Ca2+ + Mg2+) and Cl−: (Cl− + HCO3 −) as a function of TDS, are widely employed to assess the functional sources of dissolved chemical constituents, such as precipitation-dominance, rock-dominance, and evaporation-dominance (Gibbs 1970). The chemical data of groundwater sample points of the studied area were plotted in Gibbs’s diagrams (Fig. 7). The distribution of sample points suggests that the chemical weathering of rock-forming minerals and evaporation are influencing the studied groundwater quality. Evaporation increases salinity by increasing Na+ and Cl− with relation to increase of TDS. The rock domain suggests that rock–water interaction is the major source of dissolved ions over the Bahariya and Farafra NSA groundwater chemistry. The rock–water interaction process includes the chemical weathering of rocks, dissolution–precipitation of secondary carbonates, and ion exchange between water and clay minerals. The evaporation greatly increases the concentrations of ions formed by chemical weathering, leading to higher salinity. The moving of groundwater sampling points in the Gibbs field towards the evaporation domain in limestone aquifer suggests an increase of Na+ and Cl− ions and consequent higher TDS due to mixing of different water types, ion exchange and reverse ion exchange processes, and irrigation-return flows (Subba Rao 2006; Kumar et al. 2014).
Geochemical modeling
The saturation index (SI) is the form most commonly used for groundwater. Water is in equilibrium with a mineral when the SI of this mineral is equal to zero. It is undersaturated if this index is below zero and it is oversaturated when the SI is above zero. However, the inaccuracy on the pH measurements due to measuring devices, the variation of this parameter when the water flow toward surface, and the error that could occur during chemical analysis, result in an inaccuracy in the calculation of the saturation index. Therefore, it is recommended to consider that the saturation is obtained in a wider area such that −1 < SI < +1 (Daoud 1995; Nasher et al. 2013). The use of the SI showed that almost all studied waters in western oases LA groundwaters were undersaturated with respect to the halite. Therefore, there is a possibility for further Na+ and Cl− concentration increase in the studied water samples due to the dissolution of halite (Al-Omran et al. 2012; Alexakis, 2011; Aly et al., 2011) (Fig. 8). However, in Bahariya and Farafra oases, the use of the SI showed that almost all studied NSA groundwaters are saturated with respect to aragonite, calcite, and dolomite (Fig. 8). The dissolution of gypsum has a considerable role in the saturation state for calcite and dolomite where saturation state indicates the direction of the process; thus, precipitation of calcite and dolomite and dissolution of gypsum are expected (Appelo and Postma, 1996; Jalali, 2006).
Fractured carbonate aquifer in the western desert
This study suggests that the fractured carbonates aquifer in the western oases, i.e., Bahrean, Siwa, El-Gara, and Al-Jaghbub is the only exploited water in these oases. The EC of the upper 250 m fractured carbonate aquifer ranges between 3.4 and 10.5 dS/m. The reason of the high salinity of these shallow wells (LA) is the water–rock (halite) interaction during the deep fresh water flows upward from the NSA to the fractured carbonate zones through the fault planes (Fig. 9) (Elhossary 1999).
Conclusions
Groundwater, a major source of water supply in western desert of Egypt, is facing severe quantity and quality problems. Water scarcity combined with the typically arid climate and the excessive use of soils for agriculture causes severe water quality problems. In general, water obtained from Bahariya and Farafra oases was the same in its quality. It was observed that the water salinity was in the acceptable level for irrigation in all water samples of the two oases; however, the shallow groundwater samples of the other oases were considered high in its salinity. The pH and bicarbonate values were in safe and normal range in all studied western desert wells. Most studied waters were within the acceptable levels for boron and trace elements, and the iron concentration was considered the highest heavy metals concentration on all studied groundwaters. The dominant ions in Bahariya and Farafra oases groundwaters were magnesium, calcium, and chloride; however, the dominant ions in Siwa, Bahrean, El-Gara, and Al-Jaghbub oases were sodium, magnesium, and chloride. In the study area of the Bahariya and Farafra, the type of water that predominates is Mg/CaSO4, which is mostly due to the geology of the area which comprises gypsum, anhydride, and dolomite; however, in the study area of the Siwa, Monkar Eltalh, Bahrean, El-Gara, and Ghagboub, the type of water that predominates is NaCl, which is mainly due to the geology of the area which comprises halite. The use of the SI showed that almost all studied waters in western oases LA groundwater were undersaturated with respect to the halite. However, in Bahariya and Farafra NSA groundwater, the use of the SI showed that almost all studied waters are saturated with respect to aragonite, calcite, and dolomite.
References
Adams S, Titus R, Pietersenb K, Tredouxc G, Harris C (2001) Hydrochemical characteristics of aquifers near Sutherland in the Western Karoo. S Afr J Hydrol 241:91–103
Adhikary PP, Dash CJ, Chandrasekharan H, Rajput TBS, Dubey SK (2012) Evaluation of groundwater quality for irrigation and drinking using GIS and geostatistics in a peri-urban area of Delhi, India. Arab J Geosci 5:1423–1434
Ahmed MU (1977) Digital computer model for designing well. Fields in the Libyan sahara, Proc. of IntI. Conf. on Computer Applications in Developing Countries, Vol. 1 & 2, sponsored by Asian Inst. Tech, 13 Bangkok
Al-Ahmadi ME (2013) Groundwater quality assessment in Wadi Fayd, Western Saudi Arabia. Arab J Geosci 6:247–258
Alexakis D (2011) Assessment of water quality in the Messolonghi-Etoliko and Neochorio region (West Greece) using hydrochemical and statistical analysis methods. Environ Monit Assess 182:397–413
AL-Farraj AS, Al- Sewailem MS, Aly AA, Al – Wabel MI, El-Maghraby SE (2013) Assessment and heavy metals behaviors of industrial waste water on Riyadh City, Saudi Arabia. Proc Int Acad Ecol Environ Sci 3(3):266–277
Al-Omran MA, El-Maghraby SE, Aly AA, Al-Wabel IM, Al-Asmari ZA, Nadeem ME (2012) Quality assessment of various bottled waters marketed in Saudi Arabia. Environ Monit Assess 185:6397–6406
Aly AA (2001) Spatiotemporal monitoring and assessment of water resources in Siwa Oasis. M.Sc. Thesis, Faculty of Agriculture, Alexandria University, Egypt
Aly AA (2007) A Holistic Ecosystem Approach for Sustainable Management of Land and Water Resources in Siwa Oasis. PhD Thesis, Faculty of Agriculture, Alexandria University, Egypt
Aly AA, Benaabidate L (2010) Salinity of water resources in the Siwa Oasis: Monitoring and diagnosis. Water-Rock Interaction - Proceedings of the 13th International Conference on Water-Rock Interaction, WRI-13. Pp. 363–365
Aly AA, Gaber HM, Kishk FM (2008) Long term change detection and assessment of groundwater quality in Siwa Oasis. Egypt available water resources and future constrain conference, Hold on the Geography and GIS Dept., Faculty of Arts, Alexandria University, Alexandria, Egypt. PP. 19
Aly AA, Abbas AA, Benaabidate L (2011) Hydrochemistry and Quality of Groundwater Resources in Egypt: Case Study of the Egyptian Southern Oases. In Water Security in the Mediterranean Region (pp. 239–254). Springer, Netherlands
Aly AA, Alomran MA, Alwabel MA, Almahaini M, Alamari A (2013) Hydrochemical and quality of water resources in Saudi Arabia groundwater: a comparative study of Riyadh and Al-Ahsa Regions. Proc Int Acad Ecol Environ Sci 3(1):42–51
Aly AA, Al-Omran AM, Alharbya MM (2014a) (b) The water quality index and hydrochemical characterization of groundwater resources in Hafar Albatin, Saudi Arabia. Arab J Geosci. doi:10.1007/s12517-014-1463-2
Aly AA, Gaber HM, Kishk FM, Al-Omran MA (2014b) Long-term detection and hydrochemistry of groundwater resources in Siwa Oasis, Egypt. J Saudi Soc Agric Sci. doi:10.1016/j.jssas.2014.04.003
Appelo CA, Postma D (1994) geochemistry, groundwaer, and pollution. A.A. Balkema, Rotterdam, Brookfield
Appelo CAJ, Postma D (1996) Geochemistry, groundwater and pollution. A.A, Balkema, Rotterdam
Ayers RS, Westcot DW (1985) Water quality for agriculture. Irrigation and drainage paper 29 (rev.1). FAO, Rome, Italy
Baba A, Erees FS, Hicsonmez U, Cam S, Ozdilek HG (2008) an assessment of the quality of various bottled mineral water marketed in Turkey. Environ Monit Assess 139:277–285
Daoud D (1995) Caractérisation géochimique et isotopique des eaux souterraines et estimation du taux d’évaporation dans le bassin de Chott Chergui (zone semi-aride) Algérie. Thèse univ. Paris sud. Centre d’Orsay
Elhossary MF (1999) Evaluation and management of groundwater resources in Siwa area with emphasis on the Nubia Sandstone aquifer. PhD Thesis, Faculty of Science, Ain Shams University, Egypt
Elkrail AB, Obied BA (2013) Hydrochemical characterization and groundwater quality in Delta Tokar alluvial plain, Red Sea coast—Sudan. Arab J Geosci 6:3133–3138
El-Sayed MH, Abo El-Fadl MM, Shawky HA (2012) Impact of hydrochemical processes on groundwater quality, Wadi Feiran, South Sinai, Egypt. Aust J Basic Appl Sci 6(3):638–654
FAO (1985) Water quality for agriculture. Irrigation and drainage paper 29 (rev.1). FAO, Rome, Italy
Garmin Co (1997) Owner's manual and references. Olathe, KS, USA
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 17:1088–1090
Jalali M (2006) Chemical characteristics of groundwater in parts of mountainous region, Alvand, Hamadan. Iran Environ Geol 51:433–446
Kaitantzian A, Kelepertzis E, Kelepertsis A (2013) Evaluation of the Sources of Contamination in the Suburban Area of Koropi–Markopoulo, Athens, Greece. Bull Environ Contam Toxicol 91:23–28
Karanjac J (1995) Regional Programme for the Development & Utilization of the Nubian Sandstone Aquifer. CEDARE and IFAD, Cairo, Egypt: 7–20 September 1995 and Atlanta, USA: 21–24 September 1995
Klute A (1986) Methods of soil analysis. Part 1. 2nd (ed.) Agron Monor. 9. ASA and SSSA, Madison, WI
Kumar M, Ramanathan AL, Rao MS, Kumar B (2006) Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi, India. J Environ Geol 50:1025–1039
Kumar KS, Bharani R, Magesh NS, Godson PS, Chandrasekar N (2014) Hydrogeochemistry and groundwater quality appraisal of part of south Chennai coastal aquifers, Tamil Nadu, India using WQI and fuzzy logic method. Appl Water Sci. doi:10.1007/s13201-013-0148-4
Loni OA, Zaidi FK, Alhumimidi MS, Alharbi OA, Hussein MT, Dafalla M, AlYousef KA, Kassem OMK (2014) Evaluation of groundwater quality in an evaporation dominant arid environment; a case study from Al Asyah area in Saudi Arabia. Arab J Geosci. doi:10.1007/s12517-014-1623-4
Nasher G, Al-Sayyaghi A, Al-Matary A (2013) Identification and evaluation of the hydrogeochemical processes of the lower part of Wadi Siham catchment area, Tihama plain, Yemen. Arab J Geosci 6:2131–2146
Parkhurst DL, Appelo CAJ (2003) PHREEQC—a computer program for speciation, batch reactions, one dimensional transport and inverse geochemical calculations. US Geological Survey, Reston
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analyses. Trans Am Geophys Union 25:914–928
Rasheed MA, Gad A, Zoelitz-Moeller R, Darwish KM (2008) Geographic mapping and analysis using GIS of Study Areas in Bahariya Oasis, Egypt. J Agric Environ Sci 4(1):125–130
Rockware AqQA Software (2011) Version AQC10664. http://www.rockware.com
Semerjian LA (2011) Quality assessment of various bottled waters marketed in lebanon. Environ Monit Assess 172:275–285
Subba RN (2006) Seasonal variation of groundwater quality in a part of Guntur District, Andhra Pradesh, India. Environ Geol 49:413–429
Trac NQ (1984) Nubian sandstone aquifer modeling and groundwater resources planning for new valley. United Nations Educational, Scientific and Cultural Organization (UNESCO). Technical Report PP/1981-1983/2/7.3/03
U.S. salinity laboratory staff (1954) Diagnosis and improvement of saline and alkali soil. U.S. Dept. Agric. Hand book No. 60, 1609
Acknowledgments
The authors wish to thank King Saud University, Deanship of Scientific Research, College of Food, and Agriculture Science, Research Center for supporting the research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aly, A.A. Hydrochemical characteristics of Egypt western desert oases groundwater. Arab J Geosci 8, 7551–7564 (2015). https://doi.org/10.1007/s12517-014-1680-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12517-014-1680-8