Abstract
Estuaries are both chemically and physically dynamic ecosystems that, due to their location at the river-sea interface, act as buffer zones between the continent and sea. Metals may be partly dissolved in the water column or partly adsorbed on suspended matters during estuarine mixing. The metal concentration in the surface waters can significantly be reduced by adsorption through suspended matters and flocculation process during estuarine mixing. In the present study, the flocculation and adsorption of Cu, Ni, Mn, Zn, and Pb through estuarine mixing of Karganrud River with Caspian Sea water at eight different salinity regimes are studied. The flocculation trend of Mn (94.8 %) > Zn (60.04 %) > Pb (36.63 %) > Cu (30.32 %) > Ni (14.84 %) is indicated as the nonconservative behavior for Mn, Zn, Pb, and Cu, while Ni had relatively shown a conservative behavior during estuarine mixing. The maximum adsorption capacity of heavy metals (in terms of milligram of metals per kilogram of suspended matters) by suspended matters is as follows: Cu (13.68 mg/kg) > Zn (10.41 mg/kg) > Ni (6.58 mg/kg) > Mn (5.96 mg/kg) > Pb (0.146 mg/kg). Also, it is found that the percentage of desorption of metals from suspended matters during estuarine mixing occurs in the order of Pb (22.6 %) > Zn (7.6 %) > Ni (3.62 %) > Mn (3.6 %) > Cu (2.8 %). Cluster analysis (CA) indicates that adsorption of Zn, Cu, and Mn is governed by NO3. Flocculation process of Ni, Zn, and, to a lower extent, Cu is mainly controlled by NO3. In addition, NaClO played an effective role in removal of Mn through a flocculation process. According to the chemical sequential extraction, high concentrations of Cu, Ni, Mn, and Zn are found in carbonate and sulfide fractions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nutrients and environmental pollutants such as heavy metals are drained by rivers into the saltwater-like oceans, seas, and lakes through estuaries (Karbassi et al. 2010). Estuaries are some of the most productive aquatic ecosystems in the world by preserving the coastal biota (Currier and Small 2005; Dobson and Frid 1998; Karbassi et al. 2008). Many animal species rely on estuaries for food and as a place to nest and breed. It should be noted that higher concentrations of heavy metals have a toxic effect on living organisms and humans (Emam and Saad-Eldin 2013). Estuaries are both chemically and physically dynamic ecosystems that, due to their location at the river-sea interface, act as buffer zones between the continent and sea. Thus, it is essential to study in detail the overall geochemical cycle of trace elements during mixing of freshwater with saltwater in estuaries. A wide range of physicochemical processes occur during mixing of river water and sea water through estuaries in response to strong and intensive gradients in a number of parameters such as ionic strength, relative concentration of major elements, pH, and redox condition (Venkatramanan et al. 2012; Krishnakumar et al. 2013). It is essential to find out the estuarine process to predict the geochemical behavior of each individual element and its potential effect on different organisms as well as the important role of these processes in the chemical mass balance between rivers and seas (Boyle et al. 1977; Karbassi et al. 2008; Mackenzie and Garrels 1966; Sholkovitz et al. 1978). Metals may be partly dissolved in the water column or partly adsorbed on suspended matters and bed sediments as well, during mixing of freshwater from rivers and seawater (Hartnett and Berry 2012). Suspended matters are also supplied to estuaries from rivers and from different sources such as erosion of older deposits in the estuary and nearshore sea, local runoff, waste disposal, organic production, and from the atmosphere (Eisma 1986). Suspended particles in aquatic systems play an important role on controlling the reactivity, transport, and biological effects of substances. Also, it is a crucial link for chemical constituents among the water column, bed sediment, and food chain (Turner and Millward 1994). El Kashouty and El Sabbagh (2011) reported higher concentrations of Cu, Sr, Zn, Mn, Fe, and Al in finer sediments rather than that in coarser sediments, and also, sequential extraction recorded a very good immobilization of the heavy metals by the organic matter-bound fraction and is followed by the carbonate-exchangeable-bound fraction. It is generally known that, during estuarine mixing, the partitioning of metallic species between the solution and suspended particles is governed by two important, contractive, and nonbiological mechanisms (Comans and Van Dijk 1988; Samarghandi et al. 2007; Sholkovitz 1976). These mechanisms are desorption of metals from resuspension of riverine particle matter and metal removal through flocculation of humic and fulvic acid–metal complexes (Li et al. 1984; Sholkovitz 1976). Portions of dissolved metals in contact with the particulate phase including sediment and suspended matters are adsorbed on the particle matters because of the density of electrons on the surface of the particles or possible chemical reactions (Chapman 1992; Forstner and Wittmann GT 1981; Saeedi et al. 2003). The adsorption process of dissolved metals on the sediments and suspended particles during estuarine mixing can significantly change the chemical and physical forms of the metals and reduce the potential adverse effect of heavy metals to organisms (Dojlido and Best 1993; Saeedi et al. 2003). Ben Garali et al. (2010) showed clearly the important role of sediments in the pollutants’ geochemical cycle. Little information is available on the recognition of adsorption of dissolved metals during estuarine mixing of rivers with the Caspian Sea water (Saeedi et al. 2003). During the past years, considerable investigations have been carried out to fully understand more the physicochemical behavior of inorganic constituents, especially trace elements, across the freshwater–seawater mixing zone. Based on many studies, some controlling mechanisms such as colloidal stability, surface properties, humic acid, salinity, pH, EC, dissolved oxygen, Eh (Biati et al. 2010a; Eckert and Sholkovitz 1976; Hunter 1983; Karbassi et al. 2007; Saeedi et al. 2003; Zhiqing et al. 1987), and dissolved organic carbon (DOC) (Biati et al. 2010b) on the flocculation process of dissolved metals during estuarine mixing are recognized. The flocculation process of heavy metals through estuarine mixing of riverine water with saltwater from the sea particularly occurs in the upper part of the estuary where lower salinity regimes are found (Chenar et al. 2012). In wetlands, the flocculation process of dissolved elements is controlled by pH, turbulence, concentration of suspended matters, ionic strength, and high algal content (Matagi et al. 1998). Redox processes are the major concern for mobilization of heavy metals during estuarine mixing where the oxygen content varies in addition to the salinity regime variation (Biati and Karbassi 2010; Gerringa et al. 2001). In anoxic conditions, precipitation of sulfides of heavy metals such as zinc (Zn) and cadmium (Cd) may occur (Biati and Karbassi 2010; Malakootin et al. 2009). During estuarine mixing of freshwater and saltwater from seas, some metals like Zn and Cd are released into the water as a result of an oxidation process (Biati and Karbassi 2010; Duinker and Nolting 1976; Sholkovitz 1976). Karbassi et al. (2008) suggested that more studies should be carried out to find out about the possibility of formation of NaClO and its possible role in metal flocculation in the estuarine zone where Na and Cl are present in the plentiful. Also, due to the lack of adequate information about the controlling mechanism on the flocculation process of metals during the mixing of riverine freshwater with brackish lake water such as the Caspian Sea water, more investigations are required (Karbassi and Nadjafpour 1996; Saeedi et al. 2003). There are many rivers flowing into the Caspian Sea via its southern coast through the northern part of Iran that are very important ecologically, regarding the Caspian Sea ecosystem as the largest lake in the world (e.g., Sefidrud, Chaloos, Haraz, Babol, Talar, Tadjan, Aras, and Karganrud) (Saeedi et al. 2003). Many of these rivers act as mean of transport agents for the disposal of industrial, agricultural, and urban wastes (Saeedi et al. 2003). Thus, it is important to study the geochemical cycle of heavy metals in this region. Caspian Sea lies below sea level between the Caucasus Mountains and northern Iran, and its salinity varies from 4 ppt in the northern parts to approximately 13 ppt in the southern parts. The Karganrud River has a length of 42.5 km with an average annual discharge of about 252 × 106 m3/year. The catchment area of the river is about 615.4 km2 with an average precipitation of 1,150 mm. In the present study, flocculation, adsorption, and desorption processes of heavy metals (Mn, Zn, Cu, Pb, and N) during estuarine mixing of Karganrud River water with Caspian Sea water in relation to the parameters such as pH, salinity, DOC, NO3, and NaClO are investigated. Furthermore, to determine the proportions of metals in different forms, a four-step chemical sequential extraction was done for each aquarium during which the adsorption process is observed.
Materials and methods
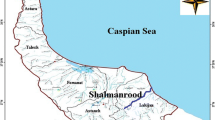
River water and suspended matters were collected in a prelabeled and precleaned 25-L polyethylene bucket from the surface of Karganrud River at a point (ca. 16-km upstream) where no saline water can surely penetrate the freshwater on 29 Feb. 2013. The location of freshwater sample was at longitude of 48°54′50.5836″ and latitude of 37°48′44.9856″. On the same day, freshwater was filtered through 0.45-μm Millipore AP and HA filters. Also, a suspended matter sample was dried at 50 °C for 29 h (Karbassi et al. 2007). Approximately, 1 L of filtered freshwater was acidified with concentrated nitric acid (HNO3) to a pH of 1.8 and kept in polyethylene bottles in a refrigerator prior to the analysis of dissolved trace metals. The rest of the filtered freshwater sample was also stored in the refrigerator. It should be noted that 5 gr of riverine suspended matters was used for metal analysis. Similarly, on the same day, the saline water sample from Caspian Sea was collected approximately 20-km away from the shore where no seawater was diluted by the river water (salinity = 0.21 ‰). The location of the seawater sample was at longitude of 49°2′16.767″ and latitude of 37°51′50.9328″. Figure 1 shows the locations of both Caspian Sea water and freshwater samples. To study the flocculation process of metals and adsorption process of metals, two series of laboratory experiments were done individually. The flocculation process was conducted by adding an appropriate volume of filtered seawater to the constant volume of filtered river water at room temperature (ca. 25 °C) in eight proportions yielding salinity of 0.5–3 ‰. Also, to recognize the effect of NaClO on flocculation of metals and due to the instability of this compound, the increasing amounts of NaClO were added to each aquarium in a laboratory condition. The eight mixtures were kept for 24 h with occasional stirring. The resulting flocculants were collected through a 2.5-cm diameter Millipore membrane filter (type HA, pore size of 0.45 μm). Millipore filters were digested using 5-mL concentrated HNO3 overnight. After 24 h, 50 mL of the mixed samples from each aquarium was taken to measure the physicochemical parameters such as DOC, NO3, salinity, and pH of aliquots. Finally, the concentration of metals (Cu, Zn, Ni, Pb, and Mn) was determined using inductively coupled plasma atomic emission spectroscopy (ICP-AES). Also, to determine the capacity of adsorption and desorption of metals, the constant volume of freshwater sample was mixed with the seawater sample to obtain a series of mixtures with various salinity regimes (0.5–3 ‰).
It should be pointed out that 5 gr of suspended particle matters (SPMs) is added to each aquarium. The eight mixtures were kept for 24 h with occasional stirring. Also, the physicochemical parameters (DOC, NO3, salinity, and pH) of each aquarium were measured prior to the metal analysis. Particulate samples were collected on 0.45-μm Whatman filters, and the concentration of Cu, Ni, Pb, Zn, and Mn was determined using ICP-AES. The ICP was calibrated using the dilution of single concentrated standards purchased from SPEXCerPrep Company. Procedural blanks and duplicates were run alongside the samples in a similar way. The accuracy of the analysis was about 5 % for all elements in the dissolved, flocculant, and particulate phases. In addition, chemical partition studies were done for any aquarium during which the adsorption process is observed. Chemical partition studies in this investigation were conducted in four sequential steps. Extractants used in each extraction step and extraction phases of SPM in the sequential extraction procedure (Morillo et al. 2002) are shown in Table 1. Furthermore, a five-phase, residual or inert fraction (fraction 5) was determined as the difference between the total metal concentration and the sum of the concentrations in the four previous phases. Of the existing clustering techniques, the weighted pair group (WPG) method used in this study.
Results and discussion
Tables 2 and 3 show the base metal (Cu, Ni, Pb, Cu, and Mn) concentration found in flocculants and SPMs at various salinity regimes as well as other physicochemical characteristics (pH, DOC, and NO3). However, it should be noticed that during natural estuarine mixing, flocculation and adsorption processes may not occur as shown in Tables 2 and 3. In fact, some of the dissolved metals, at the first stages of mixing of fresh river water with the saline water from the sea, may ooze out of the freshwater in the form of flocculants and adsorbed by the particulate matters. Thus, at the later stages of mixing (i.e., higher salinities), fresh water is impoverished in base metal contents, and fewer flocculates are formed. The values presented in Tables 4 and 5 are actually derived from Tables 2 and 3 by subtracting the concentration of metals in flocculates and SPMs at each salinity regime from the sum of the previous steps. Thus, we do not discuss the data of Tables 2 and 3 as they are just indicative of the laboratory conditions. According to Table 4, the maximal removal of Cu and Zn occurs between the salinities of 0.5 and 1.5 ‰. About 36 % of Ni is removed at the first step of the mixing experiment (salinity = 0.21–0.5 ‰). Nickel shows the minimum flocculation tendency among all studied metals, and generally, its flocculation rate does not exceed 14.84 % of the total dissolved concentration at various salinity regimes. It can be noticed that Mn undergoes maximum flocculation during mixing experiments. As is presented in Table 5, the maximal adsorption capacity of all studied metals, except for Pb, occurs between the salinities of 0.5 and 1.5 ‰. The flocculation rate and maximal adsorption capacity of metals by suspended matters during estuarine mixing are in the following orders, respectively: Mn (94.8 %) > Zn (60.04 %) > Pb (36.63 %) > Cu (30.32 %) > Ni (14.84 %) and Cu (13.68 mg/kg) > Zn (10.41 mg/kg) > Ni (6.58 mg/kg) > Mn (5.96 mg/kg) > Pb (0.146 mg/kg).
In the present study, Cu shows maximum adsorption capacity between all studied metals. As documented in Tables 4 and 5, the concentration of NO3 decreases with an increase in salinity in the area of study. The concentration of total DOC in the fresh river water was about 1.92 mg/L that increased to 22.34 mg/L at a salinity of 3 ‰. Such an increase is an indication of a marine origin in the estuarine zone. It is broadly confirmed that DOC represents a dynamic component in the interaction between the geosphere, biosphere, and hydrosphere. Based on an investigation on the estuarine mixing in Beaulieu Estuary, England, the DOC conservation behavior is reported (Moore et al. 1979). A linear decrease in DOC during estuarine mixing over the salinity range of 0.21–9.55 ‰ is reported by Karbassi et al. (2008). According to Table 1, in the present study, a consistent linear DOC increase with an increase in salinity value is an indication of nonterrigenous DOC. Zhimang et al. (2005) studied the effect of NaClO for the removal of Fe from water. Cluster analysis (CA) of dissolved element concentration along with pH, salinity, DOC, NO3, and NaClO in Karganrud River water during estuarine mixing was applied in this study. Figures 4 and 5 show dendrograms of CA during the flocculation process and adsorption of metals by SPMs of various salinity regimes (0.5 to 3 ‰). The percentages of metal concentrations desorbed from suspended matters are presented in Fig. 2 and the values of adsorbed metals by SPMs in Fig. 3. According to the reports, from the rivers flowing into the southern Caspian Sea, Pb and Ni have a minimum flocculation rate in comparison with Cu, Zn, and Mn (Karbassi et al. 2008). In this study, Pb has the maximum percentile of desorption rate from suspended particulate matters. Based on Fig. 5, due to a high similarity coefficient between Ni, Zn, and NO3, it can be inferred that flocculation rates of Ni and Zn are controlled by NO3. CA (Fig. 4) shows that Mn, salinity, DOC, and NaClO are joined together with a high similarity coefficient indicating that flocculation of Mn is governed by NaClO, salinity, and DOC. In the present study, pH does not play any role on the flocculation and adsorption processes of the studied metals (Figs. 4 and 5). Also, based on a cluster in Fig. 5, the adsorption rate of Mn, Zn, and Cu is significantly joined to NO3 with a high similarity coefficient. Thus, it can be inferred that adsorption rate of Mn, Zn, and Cu is controlled by NO3. The results of the four-step sequential chemical partitioning are presented in Tables 6, 7, 8, and 9. A high concentration of Cu, Ni, Mn, and Zn is found in carbonate and sulfide ions. Approximately 25 % of the total heavy metal (Cu, Ni, Zn, and Mn) contents were in the form of sulfide ions. It is widely accepted that heavy metals in carbonate, sulfide, and organic compounds are more toxic due to the higher bioavailability and thus are more critical from an ecological risk assessment standpoint. Although metals were found at various concentrations in the anthropogenic fraction, the loosely bonded ions are the most potentially bioavailable. Results indicated that Mn, Cu, Ni, and Zn might pose an environmental risk. Also, it should be noted that the sum of loose, sulfide, and organic fractions is grouped as the anthropogenic fraction.
Conclusion
In this study, flocculation, adsorption, and desorption processes of Cu, Zn, Ni, Pb, and Mn during the mixing of Karganrud River water with Caspian Sea water at salinity ranges from 0.5 to 3 ppt were investigated. The highest percentage of flocculation is observed for Mn. Also, Pb showed desorption behavior from suspended particulate matter during estuarine mixing. The maximum adsorption capacity belongs to Cu. Among the studied physicochemical parameters of mixing samples, DOC shows a linearity-increasing behavior toward salinity. The flocculation process of Zn, Cu, and, to lower extent, Ni is controlled by NO3. Statically, pH does not play any role in the flocculation process in Karganrud Estuary. Also, the adsorption process of Zn, Cu, and Mn is governed by NO3. Desorption rates of the studied metals from SPMs in the Karganrud Estuary are in the following order: Pb (22.6 %) > Zn (7.6 %) > Ni (3.62 %) > Mn (3.6 %) > Cu (2.8 %). About 97 % of the adsorption process of Cu by SPMs occurs between the salinity of 0.5 and 1.2 ‰. Wide ranges of the investigation about the variation in the rate of flocculation of trace elements throughout the year have been carried out in a specific river or from river to river. In all such investigations, the salinity of seawater has been almost constant. Therefore, other constituents of seawater along salinity that are variable throughout the year should have been considered in a metal flocculation process. In this study, the effect of NaClO on the flocculation process of Zn, Mn, Ni, Cu, and Pb is investigated. Also, based on the CA, the flocculation process of Mn is mainly controlled by NaClO. According to the chemical partitioning study, it should be noted that about 63 % of the concentration of adsorbed Cu is found in carbonate fractions. Generally, the highest percent of metal contents is found in sulfide and carbonate compounds. The flocculation and adsorption rate of the studied metals showed that overall colloidal metal pollution loads can significantly be reduced by various percentiles at different salinity regimes. According to the mean annual discharge of the Karganrud River (252 × 106 m3/year), the annual discharge of dissolved Cu, Mn, Ni, Zn, and Pb into the Caspian Sea would be reduced from 11.09, 9.07, 9.57, 32, and 5.54 to 7.72, 0.47, 8.15, 12.8, and 3.57 ton/year, respectively. This not only states the importance of these processes in natural self-purification of estuarine ecosystems but also shows the ecological importance of the estuarine process. Future investigations should focus on the role of seawater in the treatment of trace metals during industrial wastewater purification.
References
Ben Garali A, Ouakad M, Gueddari M (2010) Contamination of superficial sediments by heavy metals and iron in the Bizerte lagoon, northern Tunisia. Arab J Geosci 3:295–306
Biati A, Karbassi AR (2010) Comparison of controlling mechanisms of flocculation processes in estuaries. Int J Environ Sci Technol 7(4):731–736
Biati A, Karbassi AR, Hassani AH, Monavari SM, Moattar F (2010a) Role of metal species in flocculation rate during estuarine mixing. Int J Environ Sci Technol 7(2):327–336
Biati A, Moattar F, Karbassi AR, Hassani AH (2010b) Role of saline water in removal of heavy elements from industrial wastewaters. Int J Environ Res 4(1):177–182
Boyle EA, Edmond JM, Sholkovitz ER (1977) The mechanism of Fe removal in estuaries. Geochim Cosmochim Acta 41(9):1313–1324
Chapman D (1992) Water quality assessment. Chapman & Hall, London
Chenar SS, Karbassi AR, Zaker NH, Ghazban F (2012) Electroflocculation of metals during estuarine mixing (Caspian Sea). J Coast Res 29(4):847–854
Comans RN, Van Dijk CPJ (1988) Role of complexation processes in cadmium mobilization during estuarine mixing. Nature 336(6195):151–154
Currier DR, Small KJ (2005) Macrobenthic community responses to long-term environmental change in an east Australian sub-tropical estuary. Estuar Coast Shelf Sci 63(1–2):315–331
Dobson M, Frid C (1998) Ecology of aquatic systems. Longman, London
Dojlido JR, Best GA (1993) Chemistry of water and water pollution. Ellis Horwood, New York
Duinker JC, Nolting RF (1976) Distribution model for particular trace metals in Rhine estuary, Southern Bight and Dutch Wadden Sea. Neth J Sea Res 10:71–102
Eckert JM, Sholkovitz ER (1976) The flocculation of Fe, Al and humates from river water by electrolytes. Geochim Cosmochim Acta 40(7):847–856
Eisma D (1986) Flocculation and de-flocculation of suspended matter in estuaries. Neth J Sea Res 20(2–3):183–199
El Kashouty M, El Sabbagh A (2011) Distribution and immobilization of heavy metals in Pliocene aquifer sediments in Wadi El Natrun depression, Western Desert. Arab J Geosci 4(5–6):1019–1039
Emam A, Saad-Eldin M (2013) Distribution and environmental geochemistry of some heavy metals in stream sediments of Wadi Allaqi, South Eastern Desert of Egypt. Arab J Geosci 6(5):1325–1332
Forstner U, Wittmann GT W (1981) Metal pollution in the aquatic environment. Springer, Berlin
Gerringa LJA, de Baar HJW, Nolthing RF, Paucot H (2001) The influence of salinity on the solubility of Zn and Cd sulfides in the Scheldt estuary. Neth J Sea Res 46(3–4):201–211
Hartnett M, Berry A (2012) Numerical modelling of the transport and transformation of trace metals in a highly dynamic estuarine environment. Adv Eng Softw 44:170–179
Hunter KA (1983) On the estuarine mixing of dissolved substances in relation to colloidal stability and surface properties. Geochim Cosmochim Acta 47:467–473
Karbassi AR, Nadjafpour SH (1996) Flocculation of dissolved Pb, Cu, Zn, and Mn during estuarine mixing of river water with the Caspian Sea. Environ Pollut 93:257–260
Karbassi AR, Nouri J, Ayaz GO (2007) Flocculation of trace metals during mixing of Talar River water with Caspian Seawater. Int J Environ Res 1(1):66–73
Karbassi AR, Nouri J, Nabi Bidhendi GHR, Ayaz GO (2008) Behavior of Cu, Zn, Pb, Ni and Mn during mixing of freshwater with the Caspian Seawater. Desalination 229(1–3):118–124
Karbassi AR, Nabi Bidhendi GHR, Saeedi M, Rastegari A (2010) Metals removal during estuarine mixing of Arvand River water with the Persian Gulf water. Cent Eur J Geosci 2(4):531–536
Krishnakumar P, Lakshumanan C, Jonathan M.P, Sundararajan M, Navarrete-Lopez M (2013) Trace metal in beach sediments of Velanganni Coast, South India: application of autoclave leach method. Arab J Geosci 1–11
Li YH, Burkhardt L, Teroka H (1984) Desorption and coagulation of trace elements during estuarine mixing. Geochim Cosmochim Acta 48(10):1879–1884
Mackenzie FT, Garrels RM (1966) Chemical mass balance between rivers and oceans. Am J Sci 264:507–525
Malakootian M, Nouri J, Hossaini H (2009) Removal of heavy metals from paint industries wastewater using Leca as an available adsorbent. Int J Environ Sci Technol 6(2):183–190
Matagi SV, Swai D, Mugabe R (1998) A review of heavy metal removal mechanisms in wetlands. Afr J Trop Hydrobiol Fish 8:25–23
Moore RM, Burton JD, Williams PJB, Young ML (1979) The behavior of dissolved organic material, Fe and Mn in estuarine mixing. Geochim Cosmochim Acta 43:919–926
Morillo J, Usero J, Gracia I (2002) Partitioning of metals in sediments from the Odiel River (Spain). Environ Int 28:263–271
Saeedi M, Karbassi AR, Mehrdadi N (2003) Flocculation of dissolved Mn, Zn, Ni and Cu during the estuarine mixing of Tadjan River water with Caspian Sea water. Int J Environ Stud 60:567–576
Samarghandi MR, Nouri J, Mesdaghinia AR, Mahvi AH, Naseri S, Vaezi F (2007) Efficiency removal of phenol, lead and cadmium by means of UV/TiO2/H2O2 processes. Int J Environ Sci Technol 4:25–19
Shokovitz ER (1976) Flocculation of dissolved organic and inorganic matter during the mixing of river water and seawater. Geochim Cosmochim Acta 40(7):831–845
Sholkovitz ER, Boyle EA, Price NB (1978) The removal of dissolved humic acids and iron during estuarine mixing. Earth Planet Sci Lett 58:423–439
Turner A, Millward GE (1994) Partitioning of trace metal in a macrotidal estuary. Implications for contaminant transport models. Estuar Coast Shelf Sci 39:45–58
Venkatramanan S, Ramkumar T, Anithamary I, Vasudevan S (2012) Heavy metal distribution in surface sediments of the Tirumalairajan river estuary and the surrounding coastal area, east coast of India. Arab J Geosci 7(1):123–130
Zhimang GU, Fang J, Deng B (2005) Preparation and evaluation of GAC-based iron-containing adsorbents for arsenic removal. Environ Sci Technol 39:3833–3843
Zhiqing LE, Jianhu Z, Jinsi C (1987) Flocculation of dissolved Fe, Al, Mn, Si, Cu, Pb and Zn during estuarine mixing. Acta Oceanol Sin 6:567–576
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karbassi, A.R., Fakhraee, M., Heidari, M. et al. Dissolved and particulate trace metal geochemistry during mixing of Karganrud River with Caspian Sea water. Arab J Geosci 8, 2143–2151 (2015). https://doi.org/10.1007/s12517-014-1267-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12517-014-1267-4