Abstract
The paper presents the uranium (U) concentration and distribution pattern in the Paleoproterozoic phosphorites of Lalitpur district of Uttar Pradesh. The study of thin sections, SEM and XRD reveal that apatite is the essential phosphate mineral while quartz and feldspars are the dominant gangue in the phosphorites of the investigated area. The collophane is observed to be mostly oolitic in form and microspherulitic in texture. The major element geochemistry indicated that the phosphorite samples are rich in P2O5, CaO, SiO2 and Fe2O3 whereas depletion of MgO, MnO, K2O and Al2O3 was observed. The CaO/P2O5 ratio ranges from 1.13 to 1.46 which is slightly lower than that of cations and anions substituted francolite (1.621) and close to that of carbonate-fluorapatite (1.318). The trace element geochemistry indicates that the phosphorites of Lalitpur have the significant range of U concentration (1.67 to 129.67 μg/g) which is more than that of Th (0.69 to 0.09 μg/g) among the analysed trace elements in the phosphorite samples of the area. The positive correlation of U with P2O5, CaO and U/P2O5 indicates a close association of U with phosphate minerals like collophane (apatite), whereas negative correlation of U with SiO2 and Fe2O3 may be due to mutual replacement. The antipathetic relationship of U with Ni may be an indication of high oxidizing conditions, whereas sympathetic relationship of U with K2O points towards higher alkaline conditions of the basin of deposition during phosphatization. The variable concentration of U and its relationship with significant major and trace elements in most of the phosphorite samples lead one to believe that the deposition of these phosphorites might have taken place in highly alkaline medium during fairly oxidizing to weakly reducing environmental conditions of geosynclinal basin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorites are widely used as a raw material for the production of phosphatic fertilizers. Major and trace element studies are important for the elucidation of general relations in the behaviour of elements in sedimentary rocks and for the practical agrochemical utilization of phosphorites. Some trace elements in phosphorites are micro-fertilizers, whereas others, for instance, uranium (U), etc., are ecologically hazardous (Grashchenko et al. 1981; Zanin and Zamirailova 2007). Phosphatic rocks distributed among Proterozoic, Mesozoic and Tertiary Formations are known to be secondary potential sources of U in the world. Early Proterozoic phosphorites of Aravalli, Bijawar and Cuddapah are reported to be rich in elements like U, V, Mo and Ni and form large tonnage of low-grade uranium deposits (Rao et al. 2004). The Proterozoic rocks of the Bijawar Group of Sonrai, bordering southern fringe of the Bundelkhand massif, constitute a metallogenic province in the southern part of Uttar Pradesh. Geological investigations by different organizations (DGM, UNDP, GSI and AMD) resulted in establishing a polymetallic (Cu–Pb–Zn–U) mineralization belt in the Bijawar Group of rocks of Sonrai Basin (Roy et al. 2004).
Generally, phosphorites which are characterized by high U concentration have attracted the attention of researchers in the second half of the twentieth century in view of the development of atomic energy industries. Phosphorites were considered as a source of radioactive materials, and the behaviour of U, therein, has been discussed by several workers (Altschuler 1980; Gulbrandsen 1966; Il’in and Volkov 1994; Sokolov 1996; Zanin et al. 2000a; Baturin and Kochenov 2001; Bejaoui et al. 2012). The high concentration of U in the apatite of sedimentary phosphorites is a common feature. Altschuler (1980) suggested the U concentration range of 30 to 260 μg/g with an average of 120 μg/g in phosphorites. U in apatite has been utilized as a dating tool (Kolodny and Kaplan 1970; Veeh et al. 1973), and because of rising fuel prices, the potential of U recovery from phosphate rocks became attractive. U in phosphorites has been found to occur in mixed oxidation states, i.e. both tetravalent and hexavalent. U is also known to be incorporated in the carbonate-fluorapatite (CFA) lattice which would consequently lead to an enrichment of U in phosphorites formed under suboxic conditions (Altschuler et al. 1958; Kolodny and Kaplan 1970; Burnett and Veeh 1977; Jarvis et al. 1994; Arning et al. 2009).
The present investigation is an attempt to study the abundance, distribution pattern and inter-relationship of U with significant major and trace elements of the Paleoproterozoic phosphorites of the Lalitpur district, Uttar Pradesh, using advance analytical instrumental techniques in order to delineate the genetic significance of U in the depositional environment of the basin.
Geological set-up
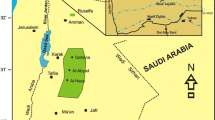
The Paleoproterozoic Bijawar Group (2–1.4 Ga) of rocks overlie the Archaean Bundelkhand Granitic Complex (3.3–2.5 Ga) and underlie the rocks of the Vindhyan Supergroup (1.4–0.5 Ga) (Jha et al. 2012). The rocks of the Bijawar–Sonrai groups were deposited in marginal basins surrounding Bundelkhand Craton (Sharma 2000; Jha et al. 2012). These basins occur in Sonrai, Hirapur and Gwalior areas, located south, south-east and north-west directions of the Bundelkhand Craton (Fig. 1), respectively. They preserve volcano-sedimentary units of Late Archaean to Early Proterozoic age (Sharma 2000; Jha et al. 2012; Khan et al. 2012a). The study area is located at the extreme south western corner of Uttar Pradesh (Fig. 1). The basin is 28 km long and 5 km wide, and shows an east–west extension. The phosphorites are found to occur at Sonrai (24° 18' 00''; 78° 46' 00''), are associated with the rocks of Jamuni and Rohini Members of the Sonrai Formation and are mainly concentrated in a stretch of 27 km with a width of 5 km between Pisnari (24° 19' 00”; 78° 44' 30'') and Berwar (24° 18' 30”; 78° 54' 00'') (Kothiyal et al. 2002; Khan et al. 2012b). In the study area, the linear Bijawar–Vindhyan sequence is unconformably resting over pre-existing Berwar Formation. The lithostratigraphic units of the Bijawar Group near Sonrai basin are better exposed and are divided into three formations: the Berwar, Sonrai and Solda Formations, as shown in Table 1 (Prakash et al. 1975; Roy et al. 2004).
Detached and lenticular phosphorite bodies having length of a few metres to 4 km and width from thin bands to about 125 m occur at four distinct stratigraphic levels in the Lalitpur district of the Bijawar Group. The base is the first horizon containing massive to brecciated phosphorites which occurs within the lower reddish shales. The second horizon occurs at the base of the brecciated quartzite member overlying the black shales and consists of brecciated phosphorites containing lensoid bodies with phosphorite cement. The phosphorite could be the basal part of the brecciated quartzite. The third horizon within the brecciated quartzite consists of massive to brecciated phosphorites. This horizon contains angular to subangular fragments of quartzite cemented by high-grade phosphorites. The fourth, the uppermost horizon, overlies the brecciated quartzite which is the most important phosphorites in the Sonrai Basin. It is massive at places but elsewhere is brecciated with angular to subangular fragments of phosphorites embedded within high-grade phosphorite matrix. This horizon is overlying by ferruginous quartzites (Pant et al. 1989; Khan et al. 2012a, b).
Methodology
Thin sections of the representative samples of different lithological units were prepared in the laboratory. The mineralogical and petrographic characters of phosphorites of the area were systematically and carefully examined under the plane polarized light and between crossed nicols. The X-ray diffractometer (XRD) and scanning electron microscope (SEM) studies were carried out at National Institute of Oceanography, Goa. The diffraction data were obtained with a RIGAKU-ULTIMA IV X-ray diffractometer using nickel filtered Cu Kα radiation. The samples were then observed under JEOL 5800LV scanning electron microscope (SEM), attached with EDS analyzer at various magnifications to see the shape, size and nature of the grains of phosphate minerals, and good images were obtained with maximum resolution at an acceleration voltage 20 kV facility.
Twenty six fresh samples of phosphorites were selected, crushed and powdered for the preparation of solution. Geochemical analyses of the samples were carried out at the laboratories of the National Geophysical Research Institute (NGRI), Hyderabad, India. Major elements were determined by X-ray fluorescence spectrometry (XRF) using a Philips MAGIX PRO Model 2440 by using pressed pellets of the samples as described by Roy et al. (2009). Trace elements including U were analysed by inductively coupled plasma mass spectrometry, Perkin Elmer SCIEX ELAN DRC II using the open acid digestion technique as described by Roy et al. (2007). National Institute of Standards and Technology (NIST) 120C, a certified reference material, was used for external calibration which was adopted for the analysis using Rh as an internal standard. Overall accuracy, by comparing the values obtained for certified reference material NIST-120C with that of certified values Govindaraju (1994), was found to be better than 2 % with a spread of ±3 in majority of the cases. The precision obtained is <2 % RSD for almost all the analyses.
Mineralogy
Megascopic study of the phosphorite samples reveals that the phosphorites are reddish brown in colour and fine to medium grained. The rock is composed essentially of innumerable angular fragments of chert and quartz embedded in a groundmass of iron oxides and secondary silica. The rock is hard, compact, bedded and ferruginous but non-laminated (Fig. 2a). Microscopic studies of these samples indicate that the collophane [3Ca3(PO4)2.Ca (CO3,F2,O)(H2O)], a cryptocrystalline mineral (Kerr, 1959), is the dominant phosphate mineral of Lalitpur phosphorite deposits. The colour of the collophane ranges from greyish yellow to greenish black. It is isotropic to weakly anisotropic between crossed nicols. The form of the collophane is oolitic which generally lacks concentric structures and called as ‘pseudo-ooliths’ or more commonly ‘pellets’ (Fig. 2b). Slansky (1986) also observed the same form in the phosphorites. The collophane groundmass appears as fibrous mass of fine apatite needles which are randomly oriented or show radiating arrangement giving the rock a ‘microspherulitic texture’ (Fig. 2c). The same has also been reported by Deer et al. (1962) and Cook (1972). Scanning electron microscopic (SEM) studies of freshly fractured phosphorites show good hexagonal apatite crystals (Fig. 2d). The SEM images of phosphorites show unaltered and pure microcrystalline apatite (Fig. 2e). X-ray diffraction study indicates that carbonate-fluorapatite (CFA) is the major apatite phase in these phosphorites (Fig. 2f), whereas quartz and feldspars are the dominant gangue. Similar findings were reported by Nagaraju et al. (2011) for the phosphatic breccia in Proterzoic metasedimetary sequence in the Betul district of Madhya Pradesh.
Photomicrographs showing a sample photographs of phosphorites. b Thin section photomicrographs showing pseudo-ooliths and pellets of collophane in plane polarized light. c Photomicrographs showing collophane groundmass of fibrous apatite needles with radiating arrangement as ‘microspherulitic texture’ between crossed nicols. d, e Scanning electron microscope (SEM) photomicrographs showing crystalline hexagonal apatite of phosphorites. f X-ray diffraction (XRD) patterns indicating carbonate-fluorapatite (CFA), quartz (Q) and feldspar (F) minerals in phosphorites of the Lalitpur district
Results
The major element geochemistry reveals that the phosphorite samples are rich in P2O5, CaO, SiO2 and Fe2O3 but depleted in MgO, MnO, K2O and Al2O3. The P2O5, SiO2, CaO, Fe2O3, MgO, Al2O3, MnO and K2O concentrations ranges from 28.78 to 31.84 wt.%, 5.20 to 21.44 wt.%, 35.80 to 44.92 wt.%, 0.55 to 2.89 wt.%, 0.27 to 0.31 wt.%, 0.12 to 3.06 wt.%, 0.01 to 0.03 wt.% and 0.01 to 1.91 wt.%, respectively (Table 2). The trace element geochemistry indicates that U has significant range of concentration (1.67 to 129.67 μg/g) which is greater than that of Th (0.69 to 0.09 μg/g) among the analysed trace elements in the phosphorite samples of the area. The other trace elements like Ni, Cu, Cr, Co, Zn, V, Sr and Pb range from 23.81 to 78.51 μg/g, 8.36 to 154.82 μg/g, 37.41to 64.57 μg/g, 7.80 to 170.61 μg/g, 16.67 to 285.36 μg/g, 4.75 to 75.18 μg/g, 4.79 to 172.98 μg/g and 20.52 to 645.74 μg/g, respectively (Table 3). The concentrations of U in phosphorites reported by many earlier workers—15–29 μg/g (Adesanwo et al. 2009), 50–80 μg/g (Rao et al. 1984), 43.6–103 μg/g (Rao et al. 2008), 6.2–139.5 μg/g (Stamatakis 2004), 2.0–194 μg/g (Rao et al. 2002), traces to 0.1 μg/g (Pitawala et al. 2003) and an average 53 μg/g (Imamoglu 2009). As reported by Baturin and Kochenov (2001), most of the phosphorites of Precambrian and Cambrian age observed to be microgranular, and granular varieties have a low concentration of U as 26 and 16 μg/g, respectively.
Discussion
The geochemical depositional environment and sources of phosphorite-hosted uranium is an essential issue. Although, there are various models of phosphate deposition, but many researchers assume that the primary formation of phosphorites and U accumulation therein occurred in a reductive medium (Altschuler et al. 1958; Baturin 1978; Burnett and Veeh 1977; Kholodov and Paul 1995; Kolodny 1981; Baturin and Kochenov 2001). Generally, the sedimentary phosphate deposits have a positive correlation between uranium (U) and phosphate concentrations (P2O5) (Lucas and Abbas 1989; Follmi 1996; Brookfield et al. 2009). In the study area, the relationship of U versus P2O5 is positive in phosphorite samples (Fig. 3a) which indicates that most of the U fraction seems to be held by the francolite phase. This is further supported by the studies of Soudry et al. (2002), Brookfield et al. (2009) and Banerjee et al. (2012). In the light of previous literature, this significant interpretation reveals that (a) the phosphorus system has a better chemical affinity with uranium in which divalent Ca+2 followed by many divalent ions with U+6 in the apatite crystal lattices; (b) high oxidizing conditions due to absence of organic matter, microorganisms and pyrite; (c) co-precipitation of phosphorus and uranium in the sedimentary environment in which the Eh and pH of the basinal waters were close to each other for their precipitation; (d) the deposition took place in the low lying and stable areas in which the influx of the clastic material was small in the shallower marine conditions; (e) the adsorption of uranium may be on the surface of apatite, ferruginous and clayey minerals by diagenesis processes; (f) the reduction of U+6 to U+4 may have taken place due to the presence of cryptocrystalline clay minerals; (g) the uranium is associated with phosphatic content and other gangue minerals by secondary enrichment processes and this may be released to minor unconformities or diastem in the area; (h) the occurrences of uraniferous phosphatic rocks formed in oxidizing conditions further indicate that the uranium might have leached, remobilized and reprecipitated by episodes of mild weathering. In addition, the sympathetic relationship of U with CaO (Fig. 3b) indicates that phosphatization may have occurred under the shallow marine conditions during which minor substitution of Ca+2 by U+4 was possible in the apatite structure. The enrichment of uranium in these phosphorites may be due to absorption and adsorption on the mineral surfaces. The mutual ionic substitution of calcium (Ca) by uranium (U) may be due to their similar/close ionic charge and ionic radii. This is in accordance with the findings of Howard and Hough (1979), Altschuler (1980) and Banerjee et al. (1982). In the phosphorite samples, the negative relationship between U and SiO2 (Fig. 3c) indicates that the SiO4 might have been replaced by PO4 during the early stages of phosphatization in which the UO4 was replaced in the apatite lattices. In the phosphorites, the SiO2 might have been weathered by groundwater and marine conditions in which the U content increased slowly. The remobilized uranium might have reprecipitated at a suitable pH and Eh of the waters. The part of the U content may be present as sorption by gangue minerals as supported by Pric and Rose (1981). The phosphorite samples show the weak positive relationship between U and K2O (Fig. 3d) indicating partial substitution of Ca+2 by K+1 in the apatite lattice and also the higher alkaline medium of the basin during phosphatization. The antipathetic relationship of U with Fe2O3 (Fig. 3e) in these phosphorites indicates the high oxidation–reduction conditions of the Bijawar basin and absence of iron sulphide minerals like pyrite, etc. The U content might have been brought by iron oxides, and the same was later on fixed on the surfaces of phosphate minerals as collophane and gangue as well. The U/P2O5 ratio (0.05 to 4.23), as given in Table 2, shows a progressive positive correlation with P2O5 content (Fig. 3f) which may be interpreted as (a) the phosphatization may have taken place during marine conditions, i.e. oxidizing state basinal waters; (b) leaching of ores by mild weathering; (c) open sedimentary environment of deposition which took place during ancient period (early Proterozoic). This is in accordance with the findings of Burnett and Gomberg (1977) and Al-Bassam et al. (1983). U/Fe2O3 shows negative relationship with Fe2O3 in the phosphorite samples (Fig. 3g) which indicates that the deposit is free from sulphide mineralisation. The phosphatization took place in the Bijawar basin after precipitation of iron bearing minerals like hematite, magnetite, limonite, etc. The goethitic and iron oxide coatings are the secondary features which took place at the time of diagenetic processes. The positive relationship between U/Fe2O3 and P2O5/Fe2O3 (Fig.3h) may be an indication of formation of phosphate and iron minerals during Proterozoic period in the highly oxidising conditions. This is also supported by the adsorption of P2O5 content by iron particularly in secondary phosphorite samples in the marine environment.
The low concentration of U (1.67 to 11.88 μg/g) in a few of the samples of the study area may be due to its removal from the crystal lattice of carbonate-apatite during catagenesis as also reported by Zanin et al. (2000) and Zanin and Zamirailova (2007). The negative correlation of U–Ni in the phosphorites (Fig. 4a) may be due to mutual ionic substitution during highly oxidizing marine conditions of the basin before the precipitation of apatite. The same findings and interpretation were given by Debrabant and Paquet (1975), Lucas et al. (1978) and Khan et al. (2012c). The meagre evidence of organic content in the phosphorites of the study area may suggest that the organisms did not play any significant role in the environment during the formation of these sediments, which is also supported by the work of Pant et al. (1989) and Khan et al. (2012a). The occurrence of large masses of clay, ferruginous and phosphatic minerals and their colloidal gels may be responsible for the adsorption of the trace elements. The weaker correlation between U and V in phosphorites (Fig. 4b) indicates that perhaps there was not a proper mutual substitution of these elements in the apatite crystal lattices during the inorganic processes of the phosphorites (Khan et al. 2012c). The antipathetic relationship of U and Cr (Fig. 4c) in the samples of phosphorites indicates the mutual substitution of these elements in the apatite crystal lattices. This is also in line of the findings of Howard and Hough (1979) and Khan et al. (2012c). U–Cu plot (Fig. 4d) in the samples of the phosphorites shows sympathetic relationship indicating the formation of these rocks during highly oxidizing to slightly reducing, shallow marine environmental conditions of the Proterozoic basin. Furthermore, it may be suggested on the basis of above findings that during the geochemical environment of the basin, there might be mutual substitution of ions vis-a-vis Ca+2 by Cu+2 and of P+5 by U+6 in the apatite crystal lattices. The relationship between U and Cu may be due to the leaching and lateritic remobilization generated by groundwater action. The weathering, remobilization and redeposition of these phosphorites may perhaps be responsible for the enrichment of secondary uranium, which is also reported by Verma (1980), Al-Bassam et al. (1983) and Khan et al. (2012c). The absence of correlation between U and Pb in the phosphate samples (Fig. 4e) supports the depositional environment accompanied by sedimentary processes during phosphatization. The absence of correlation between these elements may be due to weathering and/leaching of the ore by groundwater action. U shows positive correlation with Th (Fig. 4f) in the phosphorites of the study area, indicating close association of Th with U. According to Rao (1984), the presence of Th in phosphorites is considered to be due to the substitution of Ce in the phosphate structure.
Conclusion
On the basis of forgoing discussion, following inferences may be postulated about the genetic significance of uranium (U) content in the phosphorite deposits of the Paleoproterozoic Bijawar basin:
-
(1)
The apatite is the dominant phosphate mineral as evident from the thin section, SEM and XRD studies of these phosphorites, whereas quartz and feldspars are the dominant gangue. The pseudo-ooliths and microspherulitic texture of the phosphate minerals like collophane are prominent textural features.
-
(2)
Geochemical studies reveal that U content in most of the phosphorite samples ranges from 1.669 to 129.668 μg/g, except a few of them which have low U concentration that may be due to its removal from the crystal lattice of carbonate-apatite during catagenesis. The sympathetic relationship of U with P2O5, CaO, U/P2O5 and K2O indicates a close association of uranium with phosphate minerals like collophane (apatite), etc. and their syngenetic formation during alkaline medium, whereas antipathetic relationship of U with SiO2, Fe2O3, Ni and Cr may be due to mutual replacement during their precipitation.
-
(3)
The association, fixation, adsorption, sympathetic and antipathetic correlation of uranium (U) with significant major and trace elements lead one to believe that the precipitation and formation of these phosphorites might have taken place in fairly oxidizing to slightly reducing sedimentary environment at suitable Eh and pH in shallow marine alkaline conditions, followed by remobilization, leaching and diagenetic processes in geosynclinal basin of deposition.
References
Adesanwo OO, Adetunji MT, Dunlevey JN, Diatta S, Osiname OA, Adesanwo JK (2009) X-ray fluorescence studies of Ogun phosphate rocks. Electron J Environ Agric Food Chem 8(10):1052–1061
Al-Bassam K, Al-Dahan A, Jamil A (1983) Campanian–Maastrichtian phosphorites of Iraq. Petrology, geochemistry and genesis. Mineral Depos 18:215–233
Altschuler ZS (1980) The geochemistry of trace elements in marine phosphorites: part 1. Characteristic abundances and enrichment, marine phosphorites. SEPM Spec Publi 29:19–30
Altschuler ZS, Clarke RS, Young EY (1958) Geochemistry of uranium in apatite and phosphorite. US Geol Surv Prof Pap 314-D:45–90
Arning ET, Lückge A, Breuer C, Gussone N, Birgel D, Peckmann J (2009) Genesis of phosphorite crusts off Peru. Mar Geol 262:68–81
Banerjee DM, Khan MWY, Srivastava N, Saigal GC (1982) Precambrain phosphorites in the Bijawar rocks of Hirapur-Bassia area, Sagar district, Madhya Pradesh, India. Mineral Deposita 17:349–362
Banerjee R, Ranjan R, Roy MK, Maithani PB (2012) Geochemistry and petrogenesis of granites and associated cherty cataclasite around Parasia, district Chhindwara, Madhya Pradesh: Implications for the Uranium mineralization. Indian Mineral 46(1):41–73
Baturin GN, Dubinchuk VT (1978) On uranium forms in oceanic phosphorites. Okeanologiya 18:1036–1041
Baturin GN, Kochenov AV (2001) Uranium in phosphorites. Lithol Miner Resour 36(4):303–321
Bejaoui J, Samaali M, Baccouche S, Regugui N, Hamouda MFB, Azzouz Z, Trabelsi A, Bouhlel S, Salim B (2012) New information on radionuclides concentration in phosphorites originating from Tunisia and Algeria. Arab J Geosci. doi:10.1007/s12517-012-0536-3
Brookfield ME, Hemmings DP, Van Straaten P (2009) Paleoenvironments and origin of the sedimentary phosphorites of the Napo Formation (Late Cretaceous, Oriente Basin, Ecuador. J South Am Earth Sci 28:180–192
Burnett WC, Gomberg DN (1977) Uranium oxidation and probable subaerial weathering of phosphatised limestone from Pourtales terrace. Sedimentology 24(2):291–302
Burnett WC, Veeh HH (1977) Uranium-series disequilibrium studies in phosphorite nodules from west coast of South America. Geochim Cosmochim Acta 41:755–764
Cook PJ (1972) Petrology and geochemistry of the phosphate deposits of N.W. Queensland, Australia. Econ Geol 67(8):1193–1213
Debrabaut P, Pacquet J (1975) L’association glauconites phosphate-carbonate (Albien de La Sierra de Esfuna, Espaque mendionali). Chem Geol 15:61–75
Deer WA, Howie RA, Zussman J (1962) Rock forming minerals. Non-Silicates 5:371
Follmi KB (1996) The phosphorus cycle, phosphogenesis and marine phosphate rich deposits. Earth Sci Rev 40:55–124
Govindaraju K (1994) Compilation of working values and sample description for 383 geostandards. Geostandards Newsletter. J Geostand Geoanalysis 18:1–158
Grashchenko SM, Drechko VF, Krisyuk EM (1981) To the norms of concentrations of natural radionuclides in phosphate fertilizers. Sanit Gig 1:84–86
Gulbrandsen RA (1966) Chemical composition of phosphorites of the phosphoria formation. Geoch Cosmochim Acta 30:769–778
Howard PF, Hough HJ (1979) On the geochemistry and origin of the D Tree, Wenarch, and Sherrin Creek phosphorite deposits of the Georgine basin, Northern Australia. Econ Geol 74:260–284
Il’in AV, Volkov RI (1994) Geochemistry of uranium in Vendian–Cambrian phosphorites. Geokhimiya 7:1042–1051
Imamoglu MS, Nathan Y, Coban H, Soudry D, Glenn C (2009) Geochemical, mineralogical and isotopic signatures of the Semikan, West Kasrık Turkish phosphorites from the Derik-Mazıdagı-Mardin area, SE Anatolia. Int J Earth Sci 98:1679–1690
Jarvis I, Burnett WC, Nathan Y, Almbaydin FSM, Attia AKM, Castro LN, Flicoteaux R, Hilmy ME, Husain V, Qutawnah AA, Serjani A, Zanin YN (1994) Phosphorite geochemistry: state-of-the-art and environmental concerns. Eclogae Geol Helv 87:643–700
Jha SK, Shrivastava JP, Bhairam CL (2012) Clay mineralogical studies on Bijawars of the Sonrai basin: palaeoenvironmental implications and inferences on the uranium mineralization. J Geol Soc India 79:117–134
Kerr PF (1959) Optical Mineralogy, (3rd Edition). New York, London, Toronto: McGraw Hill., pp. xiv + 442.
Khan KF, Dar Shamim A, Khan Saif A (2012a) Geochemistry of phosphate bearing sedimentary rocks in parts of Sonrai block, Lalitpur district, Uttar Pradesh, India. Chemie der Erde 72:117–125
Khan KF, Dar Shamim A, Khan Saif A (2012b) Rare earth element (REE) geochemistry of phosphorites of the Sonrai area of Paleoproterozoic Bijawar basin, Uttar Pradesh, India. J Rare Earths 30(5):507–514
Khan KF, Khan Saif A, Dar Shamim A, Husain Z (2012c) Geochemistry of phosphorite deposits around Hirapur-Mardeora area in Chhatarpur and Sagar Districts, Madhya Pradesh, India. J Geol Min Res 4(3):51–64
Kholodov VN, Paul RK (1995) The Black Sea: a geochemical model of phosphate deposition. Litol Pol Iskop 6:563–581
Kolodny Y (1981) Carbon isotopes and depositional environment of high productivity sedimentary sequence, the case of the Mishash-Ghareb Formation, Israel. Isr J Earth Sci 29:147–156
Kolodny Y, Kaplan IR (1970) Uranium isotopes in sea floor phosphorites. Geochim Cosmochim Acta 34:3–24
Kothiyal DL, Srivastava VC, Verma RN (2002) Geology and mineral resources of the states of India. Uttar Pradesh and Uttranchal. Geol Surv of India. Misc Pub 30, Part, XIII
Lucas J, Abbas M (1989) Uranium in natural phosphorites: the Syrian example. Sci Geol Bull 42:223–236
Lucas J, Prevot J, Larnboy M (1978) Les phosphorites de la marge norde de 1' Espagne, Chimie mineralogy, Genese. Oceanol Acta 1:55–72
Nagaraju M, Shivakumar K, Verma SC (2011) A note on the uraniferous Phosphatic breccia in Proterzoic metasedimentary sequence, Betul district, Madhya Pradesh. Indian Mineral 45(1):75–82
Pant A, Khan HH, Sonakia A (1989). Phosphorite resources in the Bijawar Group of central India. Phosphate deposits of the world. In: Notholt AJG, Sheldon RP, Davidson DF (eds) Phosphate rock resources. International Geological Correlation Programme Project 156: Phosphorites. Cambridge University Press, vol 2, pp 473–477.
Pitawala A, Schidlowski M, Dahanayake K, Hofmeister W (2003) Geochemical and petrological characteristics of Eppawala phosphate deposits, Sri Lanka. Miner Deposita 38:505–515
Prakash R, Swarup P, Srivastava RN (1975) Geology and mineralization in the southern parts of Bundelkhand in Lalitpur district, Uttar Pradesh. J Geol Soc India 16:143–156
Pric S, Rose AW (1981) Uranium anomalies in palaeoaquifers near sandstone type uranium deposits in the Devonian Catskill Formation of Pennsylvania. J Geochem Explor 15(1–3):219
Rao Liaqat AK (1984) Trace-element studies of the Precambrian stromatolitic phosphorites of Udaipur, Rajasthan, India. Chem Geol 45:17–31
Rao Sesha RVS, Deshpande MSM, Shivkumar K (2004) A note on the occurrence of uraniferous phosphatic ferruginous breccias in the lower Vindhyan sediments of Son-valley, around Baskati, Sindhi district, Madhya Pradesh. J Geol Soc India 64(5):685–688
Rao VP, Michard A, Naqvi SWA, Bottcher ME, Krishnaswamy R, Thamban M, Nataraja R, Borole DV (2002) Quarternary phosphorites off the southeast coast of India. Chem Geol 182:483–502
Rao VP, Hegner E, Naqvi SWA, Kessarkar PM, Masood SA, Raju DS (2008) Miocene phosphorites from the Murray Ridge, north western Arabian Sea. Palaeogeogr Palaeoclimatol Palaeoecol 260:347–358
Roy M, Bagchi AK, Babu EVSSK, Mishra B, Krishnamurthy P (2004) Petromineragraphy and mineral chemistry of Bituminous Shale-hosted uranium mineralization at Sonrai, Lalitpur district, Uttar Pradesh. J Geol Soc India 63:291–298
Roy P, Balaram V, Kumar A (2007) New REE and trace element data on two kimberlitic reference materials by ICP-MS. Geostand Geoanalytical Res 31(3):261–273
Roy P, Balaram V, Krishna AK, Singh RS, Chavan CD, Charan SN, Murthy NN (2009) A simplified and rapid method for the determination of sulphur in kimberlites and other geological samples by wavelength-dispersive X-ray fluorescence spectrometry. At Spectrosc 30(5):178–173
Sharma KK (2000) Evolution of the Archaean–Paleoproterozoic crust of the Bundelkhand Craton, northern Indian shield. In: Verma OP, Mahadevan TM (eds), Research Highlights in Earth Sciences, DST, India Geol Cong 1:95–105
Slansky M (1986) Geology of Sedimentary phosphates. North Oxford Academic Publisher Ltd. pp. 19–35
Sokolov AS (1996) Evolution of uranium mineralization in phosphorites. Geokhimiya 11:1117–1119
Soudry D, Ehrlich S, Yoffe O, Nathan Y (2002) Uranium oxidation state and related variations in geochemistry of phosphorites from the Negev (southern Israel). Chem Geol 189:213–230
Stamatakis Michael G (2004) Phosphate deposits of Neogene age in Greece. Mineralogy, geochemistry and genetic implications. Chem der Erde 64:329–357
Veeh HH, Burnett WC, Soutar A (1973) Contemporary phosphorite on the continental margin off Peru. Science 181:844–845
Verma KK (1980) Mineral deposits related to stromatolites. G.S.I. Spec Pub 17:303–307
Zanin Yu N, Zamirailova AG (2007) Uranium in Supergene Phosphorites. Geochem Int 45:32–46
Zanin Yu N, Zamirailova AG, Gilinskaya LG (2000) Uranium of sedimentary apatite in catagenesis. Geochem Int 38:452–458
Zanin YN, Zamirailova AG, Gilinskaya LG, Fomin AN, Kireev AD (2000) Uranium in the sedimentary apatite during catagenesis. Geokhimiya 5:502–509
Acknowledgments
The authors are grateful to the Chairman, Department of Geology, Aligarh Muslim University, for providing facilities to carry out this work. Authors are thankful to the Directors, National Geophysical Research Institute, Hyderabad (NGRI) and National Institute of Oceanography, Goa, for their permission for geochemical analysis, XRD and SEM studies, respectively. We are also thankful to Dr. V.P. Rao, National Institute of Oceanography, Goa, and Dr. Masroor Alam, Department of Civil Engineering, Aligarh Muslim University, Aligarh, for their kind help in SEM, XRD and photomicrographs. First author is thankful to Dr. S. H. Jafri for his help and guidance during chemical analysis at NGRI, Hyderabad. University grants commission (UGC) is thankfully acknowledged for providing financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dar, S.A., Khan, K.F., Khan, S.A. et al. Uranium (U) concentration and its genetic significance in the phosphorites of the Paleoproterozoic Bijawar Group of the Lalitpur district, Uttar Pradesh, India. Arab J Geosci 7, 2237–2248 (2014). https://doi.org/10.1007/s12517-013-0903-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12517-013-0903-8