Abstract
One of the important indicators to show the quality of water for drinking and irrigation is nitrate values in water and soil. Nitrate enters surface water and groundwater through degradation and decomposition of human and animal wastes, industrial productions, and agricultural runoff. The present paper focuses on the concentration of nitrite (NO2 −1) and nitrate (NO3 −1) of the groundwater in Taft region, Central Iran. Sixty-one samples of the region’s aqueducts, wells, and springs were collected in September 2008 and May 2009 and analyzed by ICP-MS method. However, distribution maps of nitrate and nitrite and their frequency diagram in the pertinent samples have been generated. Then, they were compared to the US Environmental Protection Agency (EPA) and WHO international standards. The mean of nitrate content measured in the samples was 18.52 mg/l, maximum was 115 mg/l which is higher than the EPA standard (i.e., 10 mg/l), and the mean of nitrite content was about 0.06 mg/l. According to the distribution maps, concentration of these anions is high in the downstream of settlements and farmlands of Taft region. With respect to the penetration of agricultural wastes, flooding irrigation, thin layer of alluvium, sandy texture, and the amount of fertilizer consumed in the region, and also absence of any natural source for these anions and absence of the major industrial activities in the region to produce sewage, it seems that nitrate and nitrite originated from the agricultural sewage and human waste. As the content of nitrate in drinking water in the region is higher than WHO and EPA standards, so there is the risk of methemoglobinemia disease in infants. In addition, nitrate content within the stomach and lungs interacts with amine and nitrosamines are made up which are potentially the initial cause of all cancers in human.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organic and inorganic compounds used in industries, agriculture, and urban life and global distribution of some of these toxic substances have polluted water and soil. Water and soil become polluted due to several factors, including inorganic fertilizers, heavy metals, and agricultural pesticides. As far as food security aspects are concerned, the annual consumption of nitrogen and phosphorus fertilizers has increased (Gharaei et al. 2007). Although it is necessary to use chemical fertilizer and manure to increase crops, one of the important factors of nonpoint source pollution of groundwater (especially shallow groundwater) can be the extensive use of the inorganic fertilizers, particularly nitrogen fertilizers (Townsend and Young 2000).
One of the important indicators to show the quality of water for drinking and irrigation is nitrate values in water and soil (Krapac et al. 2002; Criss and Davisson 2004). Nitrate enters surface and groundwater through degradation and decomposition of human and animal wastes, industrial productions, and agricultural runoff (Di and Cameron 2002; Kraft and Stites 2003). Several researchers carried out on the last factor (Jordan and Smith 2005).
Using inorganic fertilizers, especially nitrogen-containing fertilizers, has increased dramatically in recent years, which caused the increase of groundwater pollution potential (Nishio 2004).
Among the major inorganic fertilizers used in agriculture are nitrogen containing or nitrogen (N), phosphorus, potassium (K), and sulfur fertilizers. If concentrations of PO4, SO4, K, and NO3 ions exceed the permissible amounts, they will pollute groundwater, especially shallow groundwater (Bohlke 2002). Improperly absorbed by plants and soil and rapidly washed away by water, nitrogen fertilizers cause the most pollution of groundwater (Thorbum et al. 2003).
The annual global consumption of nitrogen fertilizers is 80 million tons per year and the developing countries account for the lion’s share (Dissanayake and Chandrajith 2009). Iran’s share is about 2 million tons per year (Mahvi et al. 2005) and 80 % of the consuming fertilizer in Iran is nitrogen fertilizer (Malakooti 1999). Since nitrogen is dynamic, part of that is washed away and finally pollutes soil and groundwater (Jalali 2001).
Nitrogen in water may be in the form of nitrate, nitrite, free ammonia, and organic nitrogen salt (Wolf and Pats 2002). However, water runs through grounds with these compounds, and nitrate ions may enter into the water. Organic compounds are among the factors which cause pollution of water sources. Nitrate is the product of the final oxidation of ammonia, which, due to the bacteria’s vital activity, first changes into nitrite and then nitrate. That is why the amount of nitrate is more than the ones of ammonia and nitrite (Tchobanoglous et al. 2003).
Disposal wells in rural and densely populated places and alluvial deposits can be considered as other factors, which increase nitrate content (Chowdary et al. 2005). Nitrate concentration in surface water is mainly less than 1 mg/l, except when it is polluted by domestic and industrial sewage and decomposition of organic substances or inorganic fertilizers (Lappenbusch 1986). Protein is the major source of nitrogen in human diet. Nitrate is an unnecessary substance, which is not needed to be taken daily by drinking water (Montgomery 1985). Nitrate content in surface water is about 5 mg/l; however, in groundwater, it sometimes reaches 50 to 300 mg/l (Wolf and Pats 2002). US Environmental Protection Agency’s (EPA) offer for the initial standard of nitrate in drinking water is 10 mg in proportion to nitrogen. WHO standard also determined the maximum permissible amount of nitrate in drinking water as 50 mg/l (EPA 2011; WHO 2011). The Institute of Standards and Industrial Research of Iran, no. 1053, determined the optimal and allowed maximum of nitrate as 0 and 50 mg, respectively. During recent years, most estates and agencies supporting environment in the developed countries passed various laws and regulations to reduce pollutants, particularly nitrate, from surface water and groundwater (Talaei and Jarfi 2009).
Environmental engineers have found out that the water containing total nitrogen and ammonia is considered as newly polluted water; therefore, it has a high potential risk. The water in which nitrogen is formed as nitrate is considered as water that was polluted long time ago; therefore, it has lower risk to public health. Water containing significant amount of nitrite has unclear characteristics (Daneshvar 2009; Babaei et al. 2009).
Nitrogen in the form of nitrate with high concentrations is harmful. The relationship between nitrate concentration in drinking water and incidence of methemoglobinemia and cyanosis in children is completely known. Animals may also have different symptoms and suffer from the disease caused by high amounts of nitrate in drinking water such as methemoglobinemia, shortage of vitamin A, reproductive disorders, abortion, and reduced milk production (Fewtrell 2004; Knobeloch et al. 2000). The second aspect of concerns that relates to the increase of nitrate concentration in water is when surface water becomes rich which causes the rapid growth of aquatic plants. Its most known aspect is the harmful growth of planktons. Enrichment process should be considered as a natural activity, which is worsened by human activities, including discharge of domestic and industrial sewage, wastewater of factories, runoff, and leaching of farmlands by over-fertilization (Dorgaham et al. 2004; Lucassen et al.; 2004). Lands with high-density agriculture have the increased risk of pollution for surface water and groundwater (McIntyre et al. 2003a, b; Mcintyre and Wheater 2004).
Study area
Taft region is a plain (831 km2) enclosed by mountains with the average height of 2,373 m and 831 km2 that is 15 km southwest of Yazd, Central Iran (Fig. 1). With the average rainfall of 223.78 mm, average annual temperature of 12.96 °C, and average evaporation of 1,615 mm, this area has a dry, weak dry, and medium dry climate based on De Marten classification (Hamon Consulting Engineers 2007).
Taft region is one of the agricultural centers of Yazd province where 45.71 % of people are urbanites and 54.28 % live in rural areas. Rural employment is mainly agriculture and animal husbandry. Total area of farms and orchards in Taft region is 152.44 km2; of which 79.91 km2 is allocated for orchards and 72.53 km2 for farms. Local farmers and experts point out that they have been using nitrogen, phosphorous, and sulfate fertilizers for 35 years to increase production. Farmlands are mainly located in the northwest and south of the region.
Geological setting of the region
The study area is a part of Central Iran structural zone (Darvish Zadeh 2005). The rock units in the area consist of shale, conglomerate, sandstone, limestone, argillaceous limestone, calcareous shale and dolomite, and andesitic volcanic rocks. Intrusive rocks are composed of granodiorite–grant, which have formed Shirkooh heights and covered with Cretaceous rocks with progressive base (Fig. 2). Subvolcanic intruded in Cretaceous rocks composed of granodiorite–monzodiorite types. Dark-colored diabasic rocks, which are seen all over the area, are composed of gabbro–diorite.
Structural trend of the area has been northwest to southeast with dextral (right lateral) strike-slip faults which caused tectonization, mountain building, and disjunctive boundary of rock units. Dareh Anjir dextral (right lateral) strike-slip fault with trend from north to south, which originates diabasic dikes with gabbro–diorite composition of Deh Bala area. It seems that white color alteration of Taft’s limestone has been a result of this fault as well.
Methodology
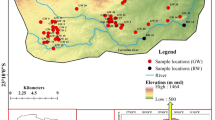
Sampling is conducted in both plain and marginal uplands within two stages (in September 2008 and September 2009). Polyethylene, 1.5 l, containers were used for sampling. Sixty-one samples were taken from wells, springs, and aqueducts throughout the area. Sampling was taken to measure anions and cations, and other chemical parameters, including pH and temperature, were measured in the site too. Coordination of the samples was specified by Carmine GPS. Tables 1 and 2 show the results obtained from the analyses.
Nitrate/nitrite distribution maps were prepared using the geographical information system (Arc GIS software) with Kriging interpolation method. As the origin of nitrate/nitrite is mostly dependent upon the human activities, thus concentration of these anions in the plain margin and uplands was compared with other area.
Discussion
Nitrate distribution map (Fig. 3) shows that sample nos. 41, 59, 105, 178, and 192 had high amounts of nitrate concentration, which are collected from northwest and center of Taft plain and south of the region. The samples were collected from wells, springs, and aqueducts of Hoseinabad Shourabeh, Chenarak, Nasrabad, Tonok, Rezaabad, and Khodaabad Paein. In addition, studying nitrite distribution map (Fig. 4) shows the concentration of this anion in the central plain and northwest of the region in the population centers and downstream of the farmlands.
Nitrogen fertilizer consumed for different products of the region is between 100 and 350 kg in a hectare. By studying the water balance in the region and with respect to flooding irrigation, thin layer of alluvium, and sandy texture, it is estimated that the amount of the penetration of agricultural wastewater was 24.8 million m3. Regression coefficients for the mountainous and lowland regions are estimated as 60 and 25 %, respectively (Hamon Consulting Engineers 2007).
Since no natural source is considered for these anions and there are no major industrial activities to produce sewage in the region, and with respect to the penetration of agricultural wastewater in the region, it seems that the source of nitrate and nitrite is caused by agricultural runoff. In addition, the sewage outlet has a considerable amount of nitrogen, which is mainly in the form of nitrate (Daneshvar 2009).
Maximum permissible amounts of nitrate and nitrite concentration for drinkable sources in EPA (2011) standard are determined as 10 and 1 mg/l, respectively. Therefore, nitrate content in most of the samples of Taft region is higher than the maximum permissible amount specified in EPA standard (Figs. 5 and 6).
The maximum permissible amounts of nitrate and nitrite concentration are WHO (2011) standard and specified as 50 and 3 mg/l, respectively. Nitrate contents in sample nos. 41, 59, 105, and 109 are higher than the ones specified in this standard. Frequency diagram of nitrite (Fig. 6) shows that nitrite concentration in all the samples does not exceed the specified standards, even though the recommended amount for this is about zero (Alizadeh 2009).
Conclusion
Based on the nitrate and nitrite distribution maps, the concentration of these anions was high in the population centers and downstream lands of northwest and center of the farmlands in Taft region. With respect to the penetration of agricultural wastewater, flooding irrigation, thin layer of alluvium, sandy texture, the amount of fertilizer consumed in the region, absence of any natural source for these anions, and absence of the major industrial activities in the region to produce sewage, it seems that nitrate and nitrite contents originated from the agricultural sewage and human waste.
Uncontrolled use of fertilizers in agricultural lands has led to the severe pollution of water and soil sources. With respect to the low absorption of anion substances by soil layers and its direct transfer to the groundwater table, the anion pollutants in the inorganic fertilizers such as nitrate are abundant in groundwater of the agricultural lands (Babaei et al. 2009).
As nitrate content in the drinkable water of the region is higher than the standard level specified by WHO and EPA, there is the risk of methemoglobinemia disease in infants. Nitrates can react with amines in the stomach or lungs to form N-nitrosamines, which have induced tumors in laboratory animals. Although the causation of human tumors is not directly linked to these compounds, exposure to them is considered as being potentially capable of initiating human cancer (Dissanayake and Chandrajith 2009).
High amounts of nitrate in animals cause abortion and reduced milk production (Malakooti et al. 2004). An increase of nitrate content causes gastritis and severe pain in monogastric animals (Dissanayake and Chandrajith 2009). In addition, uncontrolled consumption of nitrogen fertilizers causes loss of rhizobiums and nodes of plants roots (Malakooti 1999).
In order to reduce nitrite of soil and its transfer to the groundwater, following points are recommended:
-
1.
Applying management methods such as reduction of fertilizer before planting, measuring nitrate concentration near the shrub, and using fertilizer at the planting stage according to the needs of the plant
-
2.
Using organic fertilizers would reduce consumption of inorganic ones
-
3.
Allotment of nitrogen fertilizers at different stages of the plant growth
-
4.
Recommendation and consumption of fertilizer for crops is performed based on soil tests and leaf analysis
-
5.
Preventing excessive irrigation after using inorganic fertilizer
References
Alizadeh N (2009) Principles of applied hydrology. Astan Quds Razavi, Iman Reza University, Mashhad, Iran (in Persian)
Babaei A, Alavi N, Jafarzadeh H (2009) Environmental chemistry (analysis of water and sewage). Medical Science College, Jondi Shapour, Health and Marine Services, Ahvaz. Iran (in Persian)
Bohlke J (2002) Groundwater recharge and agricultural contamination. Hydrogeology J 10:153–179
Chowdary V, Rao N, Sarma PBS (2005) Decision support framework for assessment of non-point-source pollution of groundwater in large mitigation projects. J Agric water 75:194–225
Criss R, Davisson M (2004) Fertilizers, water quality and human health. Environ Heal Perspect 112(10):A536–A536
Daneshvar N (2009) Principles of water quality control. Published by Tabriz University, Tabriz, Iran (in Persian)
Darvish Zadeh A (2005) Geology of Iran. Amir Kabir, Tehran (in Persian)
Di H, Cameron K (2002) Nitrate leaching and pasture production from different nitrogen sources on a shallow stony soil under flood-irrigated dairy pasture. Aust J Soil Res 40(2):317–334
Dissanayake CB, Chandrajith R (2009) Introduction to medical geology. Springer, Berlin
Dorgham M, Abdol-Aziz E, El-Deeb Z, Okbah A (2004) Eutrophication problems in the Western Harbour of Alexandria. Egypt Oceanologia 46(1):25–44
Fewtrell L (2004) Drinking-water nitrate, methemoglobinemia, and global burden of disease. A discussion. Environ Health Perspect 112(14):1371–1374
Gharaei H, Rezaei A, Gharaei S (2007) Effects of inorganic pollution on soil and water in the lands around Doroodzan Dam, Marvdasht, 2nd National Meeting on Agriculture and Ecology.
Hamon Consulting Engineers (2007) Document of economic, social and cultural development of Taft, Government of Yazd, Yazd, Iran (in Persian)
Jalali M (2001) Water quality study of wells in Hamedan city, management and planning organization of Hamdan province, report no. 285 (in Persian)
Jordan C, Smith R (2005) Methods to predict the agricultural contribution to catchments nitrate loads: designation of nitrate vulnerable zone in Northern Ireland. J Hydro 304:316–329
Knobeloch L, Salna B, Hogan A, Postle J, Anderson H (2000) Blue babies and nitrate contaminated well water. Environ Health Perspect 108(7):675–678
Kraft G, Stites W (2003) Nitrate impacts on groundwater from irrigated-vegetable systems in a humid north-central US sand plain. Agric Ecosyst Environ 100(1):63–74
Krapac I, Dey W, Roy W, Smyth C, Storment E, Sargent S, Steele J (2002) Impacts of swine manure pits on groundwater quality. Environ Pollute 120(2):475–492
Lappenbusch W (1986) Contaminated drinking water and your health. Lappenbusch Environmental Health, Inc, Alexandria
Lucassen E, Smolders A, Van Der Salm A, Roelofs J (2004) High groundwater nitrate concentrations inhibit eutrophication of sulphate-rich freshwater wetlands. J Biogeochemistry 67(2):249–267
Mahvi A, Nori J, Babaei A, Nabizadeh R (2005) Agricultural activities impact on groundwater nitrate pollution. Int J Environ Sci Tech 2(1):41–47
Malakooti M (1999) Stable agriculture and performance improvement with optimization of fertilizer in Iran, 2nd edn. Amouzesh Keshavarzi, Iran (in Persian)
Malakooti M, Baybordi A, Tabatabaei S (2004) Optimum use of the effective quantity fertilizer to improve performance, quality improve performance, quality improvement and decrease of pollutants in vegetables and fruits and improvement of society’s health. Ministry of Jihad-e-Keshavarzi, Keshavarzi Karbord, Iran (in Persian)
McIntyre N, Wheater H (2004) A tool for risk-based management of surface water quality. J Environmental Modeling and Software 19(12):1131–1140
McIntyre N, Wagener T, Wheater H, Chapra S (2003a) Risk-based modeling of surface water quality. Environ Model Software 274(1–4):225–247
Mcintyre N, Wagener T, Whearet H, Chapra S (2003b) Risk-based modeling of surface water quality: a case study of the Charles River, Massachusetts. J Hydrol 274:225–247
Montgomery J (1985) Water treatment, principles and design. Wiley, New York
Nishio M (2004) Effect of intensive fertilizer use on groundwater quality. Institute of Agricultural and Forest Engineering, University of Tsukuba, Japan. Tennoal 1-1-1, Tsukuba 305-8572.
Talaei A, Jarfi S (2009) Study of providing drinkable water for villages of Pasargad province with the emphasis on nitrate pollution. The 12th National Meeting on Iran’s Environmental Health, Shahid Beheshti Medical Science University, Health College.
Tchobanoglous G, Burton FL, David Stensel H (2003) Waste water engineering treatment and reuse, 4th edn. McGraw Hill, New York, p 1819
Thorbum P, Biggs J, Weier K, Keating B (2003) Nitrate in groundwater of intensive agricultural areas in coastal Northeastern Australia. Agr Ecosyst Environ 94:49–58
Townsend M, Young D (2000) Factors affecting nitrate concentrations in groundwater in Stafford Country Kansas. Kansas Geology Survey.
US EPA (2011) Drinking water standards and health advisories US Environmental Protection Agency, Office of Water, 820-R-11-002, Washington, D.C.
WHO (2011) Guidelines for drinking-water quality, 4th edn. World Health Organization, Geneva
Wolf A, Pats J (2002) Reactive nitrogen and human health: acute and long term implications. Ambio 31(2):20–25
Acknowledgments
We should appreciate Mrs. Rahmani, Manager of Medical Geology Department of Geological Survey of Iran, for her sincere cooperation and reminder that the present article is derived from Taft’s Medical Geology Project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farhadinejad, T., Khakzad, A., Jafari, M. et al. The study of environmental effects of chemical fertilizers and domestic sewage on water quality of Taft region, Central Iran. Arab J Geosci 7, 221–229 (2014). https://doi.org/10.1007/s12517-012-0717-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12517-012-0717-0