Abstract
This is a conducted study of the physicochemical and the bacteriological characteristics of groundwater from 24 wells in the six departments of Algeria: Mostaganem, Mecheria, Naama, Tiaret, Bechar, and Adrar. The six departments and surrounding areas depend heavily on water from wells as a resource for drinking water, as well as domestic, industrial, and agricultural uses. Understanding the groundwater chemistry provides insight into the interactions of water with the environment and contributes to better resource management. In total, 24 water samples from wells have been analyzed for major physical–chemical elements and metals. The results show that the water have pH values ranging between 7.1 and 8.2, salinity between 213 and 1,273 mg/L, and nitrate concentrations between 30 and 48.7 mg/L. The high concentration of nitrates is explained by the utilization of chemical fertilizers in agriculture. Almost all the samples are of type Na–SO4 and Na–HCO3, and the trace metal concentrations are within the admissible standard ranges. The bacteriological analysis of 24 samples analyzed showed that nine samples (nos. 3, 5, 8, 12, 14, 16, 17, 22, and 23) are contaminated. We conclude that the groundwater samples may safely be used for drinking, domestic, agricultural, and industrial purposes with the exception of the following samples: nos. 3, 5, 8, 12, 14, 16, 17, 22, and 23.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The deterioration of the natural environment, in particular the aquiferous medium became gradually a world concern. In Algeria, the principal source of satisfaction in the demand for water is the subsoil water because of its easy exploitation. The demographic growth and the modernization of agriculture involve a major problem of deterioration of the quality of this underground source, already in limited quantity. Currently, the rivers of studied area permanently receive the urban rejections and industrialists charged with chemical and organic elements, often toxic. These polluting substances can affect in a dangerous way the public health, if it continues to be poured in nature without preliminary treatment. The mechanism of pollution of underground waters is an evolutionary process in space and time, not easily controllable. The geographical information systems make it possible to carry out a space analysis with an aim of having synthesis charts and of clearly seeing the deterioration of the quality of subsoil waters in the urban grouping of some localities in Western and Southern Algeria (Mostaganem, Mecheria, Naama, Tiaret, Bechar, and Adrar). The chemical composition of groundwater is impacted by several sources, such as atmospheric input, interaction of water with rocks, and human activities (Bahri and Saibi 2010).
The present study deals with the characterization, the classification, and the bacteriological evaluation of 24 groundwater samples collected during 2009 in the six departments in Algeria. We discussed also in the paper the possibility of utilization of these groundwaters in drinking, agriculture, and industry. Measurements are made on the concentrations of inorganic nonmetals (HCO3, Cl, SO4, PO4, NO3, and pH value), metals (Ca, Cr, Cu, Co, Fe, Na, K, Mg, Mn, Pb, Ni, Sr, and Zn), and the physical parameters of electrical conductivity (EC), total hardness (TH), and dissolved solids (DS).
Materials and methods
Sampling sites and sample collecting
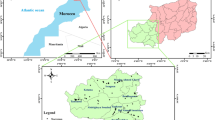
The water samples were collected in September, October, and November of 2009 from the locations presented in Fig. 1 (Table 1). The samples were kept in polyethylene bottles that had been pre-cleaned to remove traces of previous samples, to leach any contaminations from vessel walls and, for trace analysis, to help prevent adsorption of species onto the bottle walls. The 1 L bottles were soaked in 10% nitric acid and washed many times in deionized water and finally watched with a proportion from the sampling water. All the samples were tightly sealed and transported immediately to the laboratory for analysis. The samples designated for heavy metal analyses were filtrated by using 0.45 μm filter membranes. The filtered samples acidified to pH 2 in order to keep the metals in solution. All the samples were stored in the dark at 4°C.
a Geographical location of the sampled waters in the different departments of Algeria. b Location of the samples in the Algerian geological map. Mostaganem, Tiaret, Naama, and Mechria are located in the Alpine Algeria domain in the north. Bechar and Adrar are located in the Saharan Platform in the south of the country (Askri et al. 1995)
Measurements and analysis
All chemicals used were purchased from Sigma, Aldrich, and E. Merck (A.R., 99.9%). The various parameters were determined using standard procedures (AFNOR 1986, 1999). Measurements were taken in situ of pH (pH meter, WTW, Multi Line, Type F/SET-3); conductivity (conductivity meter HI 8033, HANNA Instruments), CO 2−3 , and HCO −3 (titrimetrically) were measured in situ as soon as the samples were collected. In addition, concentrations of Cl− (Mohr’s method), SO 2−4 (nephelometric method), PO 3−4 (colorimetric method), and NO −3 (salicylate method) were analyzed after filtration of the water samples by following the procedures recommended by AFNOR (1986, 1999). Ca2+, Mg2+, and the TH were determined by complexometric titration. DS were determined by evaporating progressively 1 L of filtered water sample at 100°C. The residue was dried at 180°C for 2 h (AFNOR 1986, 1999). K, Fe, Cu, Cd, Co, Cr, V, Mn, Na, Sr, Ni, Pb, and Zn were determined with atomic emission spectroscopy (ICP-AE.S; Varian; Liberty Serie II, Observation aviale). The precision of the measurements was checked taking three replicates from the sample and including a blank in each batch.
Statistical analysis
The statistical analysis of the physicochemical parameters was performed using the Matlab program data, version 6.5, Tool Books.
The bacteriological quality of water
The water while playing an important role in the life, health, access to hygiene, and comfort, is another side of many a vector-borne diseases such as brucellosis, tuberculosis, typhoid fever, cholera, and diarrhea, to name only a few diseases that kill thousands of people each year worldwide (Ouahdi 1995). The physicochemical analysis alone cannot inquire about the status of water quality. Similar samples were subjected to bacteriological analysis at the Laboratory of Microbiology, Department of Biology (University of Mostaganem). Germs sought in this analysis are: total coliforms, fecal coliforms, fecal streptococci, sulfite-reducing Clostridium, Salmonella, and Vibrio cholerae.
Results and discussions
Characterization of the ground water samples
The average values for the various parameters are summarized in Table 2. The accuracy of analyses was obtained from ion-balance error [15] according to the following equation:
where: rc = cation sum as meq/L, ra = anion sum as meq/L, and e = error percent. Applying this equation on the present analyses indicates that the e values (ranged from 1.25 to 4.97) are less than the permissible limit (5%).
The examined ground water samples were characterized by slight to moderate alkalinity (pH values ranged from 7.1 to 8.2) and moderate salinity (DS = 213–508 mg/L) except sample (3) with DS = 635 mg/L, sample (5) with 642 mg/L, sample (6) with 1023 mg/L, sample (10) with 1,082 mg/L, sample (16) with 1,063 mg/L, sample (18) with 1,273 mg/L, sample (21) with 1,152 mg/L, and sample (23) with 1,085 mg/L.
The relatively high salinity may be due to the lithological composition of these localities and to the leaching and dissolution of soil salts and chemical fertilizers by irrigation waters. At the basin center of the studied area, gypseous conglomerates constitute the base of sandy and gray marls sedimentation.
The utilization of nitrogen, phosphorous, and potash (potassium) fertilizers (local products of Algeria) are heavily used by farmers in large quantities (Bahri and Saibi 2010). Especially, the water for irrigation in some agricultural areas comes from the rivers that are affected by the waters coming from the sanitation network (Bahri and Saibi 2010).
The EC ranged from 314 to 1,658 μS/cm; these values are related to total DS. The primary anions in the groundwater samples are, in order, HCO −3 > Cl− > SO 2−4 > NO −3 , and the primary cations are Ca > Na > Mg > K. These are the rankings in most localities due to the lithological composition of this region (Polvêche 1960).
High concentrations of nitrate (30–48.7 mg/L) in different localities (sample nos. 3, 6, 18, and 23) result from the presence of cultivation zones surrounding these samples, where ammonium nitrate is used as a chemical fertilizer. The increases in other anions and cations occur due to the concentration of these ions by recycling the groundwater as irrigation water. This continuous cycle of groundwater is a result of the leaching and washing processes of the soil.
The phosphate concentrations in most of the ground water samples (0.02–0.1 mg/L) were relatively low, which may be due to the lithologic composition, but relatively high concentrations were measured at some of the cultivation regions (0.2–0.38 mg/L); these high values were due to the effect of the phosphate fertilizers.
With respect to trace metals, their concentrations (Co, 0.002–0.007 mg/L; Cr, 0.004–0.007 mg/L; Cu, 0.009–0.02 mg/L; Fe, 0.06–0.27 mg/L; Pb, 0.007–0.031 mg/L; Mn, 0.005–0.108 mg/L; Ni, 0.005–0.024 mg/L; V, 0.001–0.004; Zn, 0.25–1.45 mg/L; and F−, 0–0.20 mg/L) are within the permissible limit (AFNOR 1986). Relatively low values of trace elements in these samples indicate that the samples of groundwater under study are far from pollution sources, so these values represent ordinary or background conditions (for the lithologic composition of these regions).
Classification of the groundwater samples
Groundwater samples were classified according to total DS (AFNOR 1999). Most samples (19) are freshwater samples, but the water from the five samples (nos. 10, 12, 14, 16, and 18) is brackish (Table 3). With respect to water hardness (Handler 1968), the water samples were classified as moderately hard water (predominant class, 17 samples), very hard water (sample nos. 4, 10, 12, 14, 16, and 18), and soft water (sample no. 24).
Based on Cl−, SO 2−4 , and HCO −3 + CO −3 concentrations (Journal de la République Algérienne Démocratique et Populaire (JORADP) 2000), the samples were classified as normal chloride (<7 meq/L), normal sulfate (<2.5 meq/L), and normal carbonate and bicarbonate (3–7.86 meq/L) water types (Table 3). According to base exchange (Matthes 1982), the predominant water type of sample nos. 4, 5, 7, 8, 10, 14, 16, 17, 18, 20, 22, and 23 is Na–SO4, while the Na–HCO3 water type is predominant in other samples (nos. 1, 2, 3, 6, 9, 11, 12, 13, 15, 19, 21, and 24). The classification of water samples into two types (Na–SO4 and Na–HCO3) is due to the geological composition of these regions. The presence of a calcareous sandstone crust on the surface could explain the presence of bicarbonate in the sampling waters in the studied area. The presence of sulfate is explained by the dissolution of anhydrite in the circulating waters.
The values of hydrochemical parameters rK/rCl, rNa/rCl, rMg/rCl, rCa/rCl, and rSO4/rCl of the groundwater samples under study (24 samples; r = meq/L for these parameters in the sample) are greater than the standard values of sea water (Polvêche 1960). This is an indication of the meteoric genesis (Table 3) of groundwater in these localities. According to the classification of the meteoric genesis of groundwater into two types, the sample nos. 1, 4, 5, 7, 8, 12, 14, 16, 17, 18, 20, 21, 22, and 23 are characterized by deep meteoric water percolation, and the sample nos. 2, 3, 6, 9, 10, 11, 13, 15, 19, and 24 are characterized by surface and shallow meteoric water percolation types.
Evaluation of the groundwater samples
Suitability of the groundwater for drinking and domestic purposes
According to the World Health Organization (WHO; 2004) standards, results of the sample analyses indicate that the samples are suitable for drinking and domestic uses.
Suitability of the groundwater for drinking and domestic purposes
The sodium adsorption ratio (SAR) is used as an index referring to the suitability of water for irrigation. If we have high amounts of sodium in the soils, this could impact plant growth by (1) specific toxicity to sodium-sensitive plants, (2) nutrient deficiencies or imbalances, (3) high pH, and (4) spread of soil particles that causes poor physical condition of soil. A SAR plot was added, which presents the salinity hazard vs the sodium hazard. It is represented as follows:
The ionic symbols indicate concentrations of the ions in the irrigation water in milliequivalents per liter (Ciaccio 1971). The calculation of the SAR for a given water source provides a useful index of the sodium hazard to soils and crops. Applying this index on the sample yields excellent results (SAR values ranged from 1.0 to 3), such that the sampled waters can be recommended for irrigation purposes on any soil type (Table 3).
Suitability of the groundwater for industrial purposes
The saturation index is an important factor introduced to test water quality (Rhoades and Bernstein 1971), indicating the extent to which waters flowing through pipes will precipitate or dissolve calcium carbonate. This index is defined as the actual pH of water (pHw) minus the calculated pH at which the water would be in equilibrium with calcium carbonate (pHc) as follows:
pHc is calculated by the following equation:
From the negative logarithm of the second dissociation constant of H2CO3 (pK2), the solubility product constant of calcium carbonate (pKs), the molar concentration of CO 2−3 plus HCO −3 (pAlk). If the saturation index is positive, CaCO3 will precipitate, and if it is negative, CaCO3 will dissolve. Applying this index to the sample data (Table 3) showed that sample nos. 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 14, 16, 17, 18, 19, 20, 21, 22, 23, and 24 exhibit a tendency for CaCO3 precipitation (positive values), while the other samples, nos. 11, 13, and 15, exhibit a tendency for the dissolution of CaCO3 (negative values).
The statistical analysis of the physicochemical parameters
A statistical analysis of the groundwater samples (Table 4) exhibits excellent positive correlation values (r > 0.9) between EC, DS, and SO 24 and also, high positive correlation (r = 0.84) between EC and Na. This is due to the conductivity being dependent on total DS, the main constituents of which are Na and SO 2−4 in our samples.
Also excellent correlation value (r = 0.95) between Na and SO 2−4 , indicate that the main water type in samples is Na–SO 2−4 . High positive correlations (r ranged from 0.95 to 0.79) are observed between Na and SO 2−4 , Na and NO −3 , K and SO 2−4 , and Na and Cl−; this indicates that these soluble salts are predominant in the most samples. Also, high positive correlations (r ranged from 0.99 to 0.55) are observed between Ca and TH, Mg and TH, and Ca and Mg; this is due to the presence of Ca and Mg as dolomite (CaMg CO3) in sandstone, and in addition, the Ca and Mg are main causes for hardness (TH). The positive and significant correlation coefficient values between Ca and PO 3−4 (r = 0.69) may be due to the presence of chlorapatite mineral [Ca5 (PO4)3 Cl] in the rocks, which is surrounding these wells (Fig. 2). Cl− shows positive correlation with K (r = 0.79); this may be due to the presence of these metals as chloride in some samples. High positive correlation coefficients (r = 0.90–0.76) are observed between electric conductivity and SO 2−4 , Na, K, and NO −3 and indicate the high mobility of these ions. The data show positive correlation coefficient between the chemical in the well waters (Zn and K, Zn and Na, Mg and Mn, and Ca and Fe); this indicates that these metals may exist as soluble nitrates, sulfates, chlorides, and bicarbonates or humates (Rashed et al. 1995).
Bacteriological quality
The bacteriological analysis of the 24 samples showed that five samples (nos. 1, 8, 12, 13, and 18) are contaminated. Table 5 below includes all the germs encountered.
The results show that contamination of waters studied coliforms differs from one region to another (Table 5). The sample nos. 3, 5, 8, 12, 14, 16, 17, 22, and 23 are more contaminated than the other samples with, respectively, 42, 22, 24, 34, 54, 15, 42, 42, and 54 CT/100 ml. As for fecal coliforms, no germ was detected in the sample nos. 1, 4, 6, 7, 5, 10, 13, 15, 19, 20, 21, and 24. The highest contamination with these germs has been observed (12 to 19 CF/100 ml) in the following samples: nos. 3, 5, 8, 14, 17, 22, and 19. For fecal streptococci, low contamination was revealed in sample nos. 3, 5, 8, 12, 14, 16, 17, 22, and 23. No withdrawals were positive for the spores of sulfite-reducing Clostridium, nor for the genus Salmonella, or V. cholerae for the species in all samples studied.
According to Champiat and Larpent (1988), the fecal contamination is due to infiltration of fecal organic matter. Indeed, the composition of the bacterial flora depends on the richness of nutrients from human waste and animals. The number of the total coliforms and the fecal coliforms in the waters analyzed sometimes exceeding standards recommended by WHO (2004) and the Algerian standards (PNAE-DD 2002; 10 CT in 100 ml of the sample and 0 CF in 100 ml of the sample) shows the pollution of sample nos. 3, 5, 8, 12, 14, 16, 17, 22, and 23. These results confirm the impact of discharges of domestic sewage and industrial.
Conclusions
The results obtained from the 26 analyzed samples show that the groundwaters in some localities in Western and Southern Algeria area exhibit slight to moderate alkalinity with medium salinity, due to the characteristics of the superficial aquifer lithology. The samples of groundwater in some localities in Western and Southern Algeria area are far from the obvious pollution sources. The samples were classified as moderately hard water, normal chloride (<6 meq/L), normal sulfate (<2 meq/L), and normal carbonate and bicarbonate (3.36–7.13 meq/L) water types. The predominant water types (according to base exchange) of samples are Na–SO4, and Na–HCO3. Values of the hydrochemical parameters indicate the meteoric genesis of these samples (surface, shallow, and deep meteoric water percolation). The groundwaters in some localities in Western and Southern Algeria area can be concluded to be suitable for drinking and domestic, agricultural, and industrial uses. Statistical analysis confirms the results of the chemical analyses and exhibits good and interesting correlations that lead to the interpretation these results.
The presence of indicator organisms of fecal contamination and the presence of other bacteria responsible for infections transmitted by water is undoubtedly a threat to the inhabitants of sample nos. 1, 8, 12, 13, and 18. A monitoring network must be taken to avoid any health risk.
References
AFNOR (1986) Eaux, méthodes d’essais, recueil de normes Française. (in French language)
AFNOR (1999) La qualité de l’eau. Recueil Environnement. AFNOR Edition, volume 3. (in French language)
Askri H, Belmecheri A, Benrabah B, Boudjema A, Boumendjel K, Daoudi M, Drid M, Ghalem T, Docca AM, Ghandriche H, Ghomari A, Guellati N, Khennous M, Lounici R, Naili H, Takherist D, Terkmani M (1995) Geology of Algeria. In: Delsol D (ed) Well Evaluation Conference Algeria. Schlumberger, Houston, TX, pp 1–93
Bahri F, Saibi H (2010) Characterisation, classification, and evaluation of some groundwater samples in the Mostaganem area of northwestern Algeria. AJG 3(1):79–89
Champiat D, Larpent JP (1988) Biologie des eaux; méthodes et techniques. Masson Edition, Paris, pp 374. (in French language)
Ciaccio LL (1971) The water and water pollution handbook, 1st edn. Marcel Dekker, New York, p 149
Journal de la République Algérienne Démocratique et Populaire (JORADP) (2000) Les eaux minérales naturelles. N°51 du 20 Août 2000. (in French language)
Handler J (1968) Le livre de la santé « Médecines parallèles, thermalisme, le médecin et son malade», Monte-Carlo: Rencontre Edition, André Sauret. 19, 113. (in French language)
Matthes G (1982) The properties of groundwater. Wiley, New York, p 397
Ouahdi A (1995) Les maladies à transmission hydrique—santé plus Alger N°45. (in French language)
PNAE-DD (2002) Plan d’Actions pour l’Environnement et le Développement Durable (PNAE-DD). Ministère de l’Aménagement du territoire et de l’Environnement, pp 148. (in French language)
Polvêche J (1960) Contribution à l’étude géologique de l’Ouarsenis Oranais Tome I et II. Bulletin N°24. Publications du service de la carte géologique de l’Algérie. (in French language)
Rashed MN, Awadallah RM, Soltan ME, Hassan SM (1995) Interrelationship between major, minor and trace elements in Kalabsha wells and High Dam Lake waters. J Environ Sci Health, A: Environ Sci Eng 30(10):2205–2219
Rhoades JD, Bernstein L (1971) Chemical, physical and biological characteristics of irrigation and soil waters. In: Ciaccio LL, Richards LA (eds) Water and water pollution handbook, 1. Marcel and Dekker Incorporation, N.Y, pp 141–215
World Health Organization (WHO) (2004) Guidelines for drinking-water quality. Vol. 1. Recommendation, 3rd edn. World Health Organization, Geneva
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bahri, F., Saibi, H. Characterization, classification, bacteriological, and evaluation of groundwater from 24 wells in six departments of Algeria. Arab J Geosci 5, 1449–1458 (2012). https://doi.org/10.1007/s12517-011-0329-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12517-011-0329-0