Abstract
Myocardial perfusion imaging is important for the management of patients with suspected or known coronary artery disease. Nuclear cardiology is the most widely used noninvasive approach for the assessment of myocardial perfusion. The available single-photon emission CT (SPECT) flow agents are characterized by a rapid myocardial extraction and by a cardiac uptake proportional to blood flow. In addition, different positron emission tomography (PET) tracers may be used for the absolute quantitative measurement of myocardial blood flow and coronary flow reserve. However, the available SPECT and PET tracers for myocardial perfusion imaging have some limitations that must be considered to maximize their clinical applications and there is still a well-recognized need for the development of new perfusion tracers with more ideal properties. This review illustrates the current status and the future perspectives of blood flow tracers for SPECT and PET myocardial perfusion imaging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In current clinical practice the assessment of myocardial perfusion with radiotracers is an integral component of the evaluation of patients with suspected or known coronary artery disease (CAD) and it has been successfully performed for over 30 years. More than 10 million studies per year are executed worldwide with a rich literature confirming both high diagnostic and prognostic values of this approach. Its strengths include standardized protocols, ease of use, and well-established guidelines [1••] and, over these years, considerable technological advancement has been made to improve image quality and optimize acquisition protocols. However, the assessment of myocardial perfusion is closely related to development of more suitable radiopharmaceuticals. In particular, a myocardial perfusion imaging agent should ideally exhibit a high first-pass extraction linearly correlated with increasing flow in the hyperemic range, and must be labeled with a radioisotope having optimal half-life, suitable energy, and favorable dosimetry. Moreover, the clearance rates of the compound from the myocardium after initial uptake should be slow enough in comparison to background to allow acquisition of high-quality images. Finally, the technical feasibility of performing an absolute quantitative measurement of myocardial blood flow would be highly desirable.
First historical attempts to perform myocardial perfusion imaging with potassium-43 and rubidium-81 in the early 1970s, despite a wide number of technical limitations, provided a conceptual framework for future developments in this field [2, 3]. The introduction of thallium-201 (201Tl) in the mid-1970s represented a milestone in the development and widespread clinical use of myocardial perfusion imaging [4]. Indeed, this radiotracer had a great impact on diagnostic evaluation, risk stratification, and therapeutic decision-making in patients with CAD over the next two decades. However, 201Tl has important limitations, like a relatively low energy of emitted photons, a physical half-life of 73 h, and an unfavorable biodistribution in testes and kidneys. These restrictions affect the amount of administrable dose to approximately 150 MBq, limiting image quality, in association with a relatively high-absorbed dose and thereby stimulating the research for better myocardial perfusion imaging agents.
When technetium-99 m (99mTc)–labeled compounds, such as methoxyisobutyl isonitrile (sestamibi) and 1,2-bis[bis(2-ethoxyethyl)phosphino] ethane (tetrofosmin), were approved for clinical use in the early 1990s, there was a rapid adoption of these radiopharmaceuticals [5]. Despite a lower first-pass extraction in the myocardium and higher uptake in bowel and liver in comparison to 201Tl, these tracers have relatively higher-energy photons, a shorter physical half-life (6 h), and can be used in much higher doses limiting attenuation artifacts with superior single-photon emission CT (SPECT) image quality and providing a lower radiation burden than that achieved through 201Tl imaging. Another major advantage of 99mTc-labeled agents over 201Tl is that, in addition to perfusion data, post-stress assessment of left ventricular function (motion, thickening, and ejection fraction) can be accurately determined with ECG-triggered acquisition (gated-SPECT) using one injection and one imaging sequence. By providing this additional information gated-SPECT helps to significantly increase both specificity and prognostic value of myocardial scintigraphy [6•]. However, despite the success of these single-photon tracers, there is still the room for further improvement in myocardial perfusion imaging. In particular the presence of diffuse disease in all three main coronary arteries may decrease the sensitivity of conventional SPECT images for each individual vessel, and “balanced ischemia” may mask or minimize the presence of disease [7]. In this context, myocardial perfusion positron emission tomography (PET) offers many theoretical advantages over traditional single-photon techniques. These advantages include higher spatial and contrast resolution, resulting in higher image quality and improved diagnostic accuracy. Moreover, the possibility to quantify myocardial perfusion in absolute terms (ie, milliliters per gram per minute), that allows the noninvasive evaluation of coronary microcirculation and the identification of three-vessel disease, represents the major goal of PET. Despite the fact that clinical value of cardiac PET imaging was demonstrated more than 30 years ago [8], its use has been minimal because of several reasons. The main ones are represented by the need for an on-site cyclotron, high costs of PET scanners, lack of reimbursement, and limited availability of user-friendly software for cardiac image processing and display [9]. Nowadays, however, the increasing availability of PET facilities in a large number of nuclear medicine centers worldwide is rapidly changing many of these issues.
Current Status of SPECT Perfusion Tracers
The principal characteristics of the currently used SPECT perfusion tracers are illustrated in Table 1. Despite its several virtues and a rich history as a myocardial perfusion agent, 201Tl has suffered in recent decades the increasing popularity of 99mTc-labeled agents. In fact, due to its favorable chemical and physical characteristics, 99mTc can be incorporated into a wide range of organic as well as inorganic molecules, and therefore, it became the radioisotope of choice for the development of the last generation of myocardial perfusion SPECT agents. In the attempt to obtain the ideal myocardial perfusion tracer a wide number of 99mTc compounds have been synthesized and experimentally tested in animals and men during the last 30 years. Based on their structural characteristics, these 99mTc-labeled myocardial perfusion imaging agents were divided into two main categories: lipophilic cationic agents, consisting of 1) isonitriles (sestamibi), 2) diphosphines (tetrofosmin), and 3) Q complexes; and lipophilic neutral agents, consisting of 1) teboroxime and 2) N-NOEt.
Although showing a high myocardial extraction and favorable biodistribution in animals, neutral compounds were affected by relatively rapid myocardial clearance and prominent sustained liver uptake. This unfavorable combination turns into a “challenge” the identification of best timing for acquisition and, de facto, precludes a routinely clinical use of these tracers. Q complexes even compare favorably with exercise-redistribution 201Tl showing good preliminary biodistribution data in man, as for the 99mTc-N-NOEt. However, these complexes have not been submitted for FDA approval. Because of good myocardial image quality due to high contrast in uptake between left ventricle and lungs, sestamibi and tetrofosmin are the only compounds based on 99mTc chemistry that achieved the clinical routine. Therefore, nowadays the spectrum of single-photon-emitting radiopharmaceuticals approved for clinical use as myocardial perfusion tracers is limited to 201Tl-chloride, 99mTc-sestamibi, and 99mTc-tetrofosmin. Several studies have attempted to highlight the possible superiority of a tracer on the other, analyzing and comparing their diagnostic and prognostic potential in various clinical settings and different patient subgroups. 201Tl was used as a reference for evaluating clinical performance of 99mTc-based radiopharmaceuticals. As previously reported, there are technical differences between these tracers mainly related to the uptake modalities of the compounds [10] and to the imaging characteristics. These aspects, in particular if a pharmacologic stress is performed, may affect in different ways the biodistribution of the tracers accounting for the differences reported in literature in radiopharmaceuticals behavior, mainly in studies with limited groups of selected patients and specific clinical settings [11–13]. However, in data emerging from large prospective controlled trials performed over the past 20 years in order to assess the diagnostic accuracy of myocardial perfusion scintigraphy for the detection of CAD, a good correlation and a broadly comparable clinical behavior of the three tracers has been shown. In particular, in the largest study of 2560 patients randomized to each of the three compounds [14], overall sensitivity in the subset of patients undergoing angiography was 91% and specificity 87%, with no significant difference between the three tracers. However, according to the authors’ conclusions, in obese patients and in women with large breasts the use of 99mTc agents should be preferred to reduce the impact of soft tissue attenuation artifacts. Moreover, the use of radiopharmaceutical-specific normal data files is recommended for quantitative analysis of SPECT images [15].
Current Status of PET Perfusion Tracers
PET has many advantages over SPECT, including higher spatial resolution and the ability to provide absolute quantitative measurements of physiologic parameters, such as regional myocardial blood flow and coronary flow reserve (Fig. 1). The most common tracers currently used for the assessment of myocardial perfusion with PET are nitrogen-13 (13N) ammonia, rubidium-82 (82Rb), and oxygen-15 (15O) water [16]. The principal characteristics of these tracers are illustrated in Table 1. When injected, 13N-ammonia is extracted by myocardial tissue with a very high extraction fraction where it is converted to 13N-glutamine [17]. The clearance half time of 13N-ammonia activity from the myocardium is slow enough that one can wait until blood pool activity is significantly lower than myocardial activity. 13N-ammonia myocardial extraction is nonlinear and inversely related to blood flow. 13N-ammonia provides excellent-quality images of the myocardium, because of the high single-pass extraction (approximately 70%–80% at physiologic flow rates), the relatively prolonged retention of tracer by the heart (biological half-life of 80–400 min) after intravenous administration, and the rapid blood-pool clearance (Fig. 2). Imaging with 13N-ammonia requires either an on-site cyclotron or close proximity to a regional positron radiopharmaceutical source centre. 82Rb is a cation and its uptake depends on myocardial perfusion [18]. An advantage of 82Rb over 13N-ammonia is the production by a generator without the need for a costly cyclotron. The single-pass extraction of 82Rb by the myocardium is inversely and nonlinearly related to coronary blood flow. The quality of images obtained after intravenous administration of 82Rb depends on the tracer infusion duration and imaging protocol. Although disappearance of tracer from arterial blood is rapid, infusion system with prolonged administration time results in high myocardial blood-pool activity. 15O-water is a freely diffusible tracer with a short physical half-life (2.1 min) requiring an on-site cyclotron. To effectively obtain myocardial images, 15O-water (because of its short half-life) requires administration with rapid data acquisition. Because water is distributed in both the vascular space and myocardium, visualization of myocardial activity with this tracer requires correction for activity in the vascular compartments, which makes the images difficult to interpret visually. The correction can be performed based on the early 15O-water kinetic information or is accomplished by acquiring a separate scan that identifies either the intravascular compartment [19].
Example of myocardial blood flow quantification with stress (dipyridamole) and rest 13N-ammonia PET imaging in a patient with nonischemic dilated cardiomyopathy: 17-segment model polar maps of rest blood flow (lower left; color scale: 0–3.0 mL/min/g), stress blood flow (upper left; scale: 0–3.0 mL/min/g), flow reserve (upper right; scale: 0–3.0), and flow difference (lower right; scale: 0–1.5), which demonstrate a global impairment (absolute and percentage values are displayed in the tables below)
Transaxial, short-axis, and long-axis images of PET myocardial perfusion obtained at stress (dipyridamole) and at rest with 13N-ammonia in a patient with ischemic dilated cardiomyopathy and atypical chest pain. There is a moderately large flow deficit in the lateral and anterolateral walls (arrows) with stress a that is normal at rest b
PET has a high sensitivity and specificity for the detection of myocardial ischemia. Studies that compared perfusion imaging with PET and SPECT in the assessment of CAD found PET to be superior to SPECT in terms of sensitivity, specificity, and predictive accuracy [20, 21]. Myocardial perfusion PET is particularly useful in reducing the number of false-positive SPECT studies due to attenuation artifacts. The higher sensitivity of PET can also be useful in the evaluation of quantitative myocardial blood flow. Gated-PET may provide additional information regarding peak stress ventricular function, which is an advantage to gated-SPECT. Patients in whom stress imaging with exercise is neither required nor feasible, and patients with a high likelihood of false-positive or false-negative studies by SPECT, are likely to benefit from PET imaging. This includes obese individuals and women with large breasts, where SPECT imaging is less effective due to attenuation artifacts. Many patients with end-stage renal and liver disease have edema, ascites, and high-elevated diaphragms, sometimes with pericardial effusions, which may lead to non-uniform attenuation abnormalities [22]. Furthermore, patients with equivocal results with other noninvasive tests or conflicting results can benefit from PET imaging.
Future Perspectives of SPECT Perfusion Tracers
An ideal myocardial perfusion single-photon imaging agent has been sought in nuclear cardiology. The commercial agents 99mTc-sestamibi and 99mTc-tetrofosmin, despite the excellent and comparable clinical results achieved [14], do not have all the traits of an ideal tracer. In particular, a high first-pass extraction and linear relationship between flow and tracer uptake are key indicators of ideal behavior. As previously reported [23], sestamibi and tetrofosmin do not exhibit optimal values for these parameters. The major drawback in image quality for 99mTc compounds is the early hepatic uptake that may interfere with assessment of the inferior left ventricular wall, mostly in studies at rest or after dipyridamole or adenosine administration. Various strategies, such as delayed image acquisition or ingestion of a fatty meal, milk, chilled or carbonated water, have been proposed to deal with this limitation, with often contradictory results [24].
Thus, there is a clinical need for a radiotracer with high heart uptake and a substantially better heart-to-liver ratio than that of sestamibi and tetrofosmin. In fact, as underlined in an excellent editorial by Zaret [25], these radiopharmaceuticals “…stand in the middle, rather than at the end, of a journey in the pursuit of the ideal!” Despite this need, there has not been a great impetus in the development of new perfusion agents in the 10 years after FDA tetrofosmin approval in 1996. However, in the last few years with the goal of speeding up hepatic clearance while maintaining high myocardial image quality, a number of experimental studies addressed the issue of developing new radiopharmaceuticals toward the improvement of myocardial and liver kinetics in comparison with those of clinically available 99mTc compounds. In particular, attention is now focused on two distinct classes of radiopharmaceuticals: the first including an electrically neutral lipophilic compound labeled with 125I, rotenone, and the second, grouping a class of cationic perfusion imaging agents, all labeled with 99mTc.
7’-Z-iodorotenone (ZIROT) is an inhibitor of complex I of the mitochondrial transport electron chain that has been labeled with 125I. Z-iodorotenone is a neutral and lipophilic compound and has been shown to have high first-pass extraction (~ 84%) and better retention in comparison to sestamibi (~ 48% first-pass extraction) in isolated perfused rabbit heart studies [26]. While the absolute heart uptake of iodorotenone was similar to that of sestamibi and tetrofosmin, ZIROT demonstrated significantly superior (P < 0.001) heart-to-lung ratios in rodents at 1 h post-injection [27]. However, biodistribution experiments in adult male Sprague–Dawley rats indicated that Z-iodorotenone heart-to-liver ratio was similar to that of sestamibi but significantly lower than that of tetrofosmin [26]. Despite these preliminary experimental results suggesting favorable distribution characteristics of ZIROT in comparison to the currently used 99mTc tracers, no data are yet available in human models.
As mentioned above, 99mTc-N-NOEt represents the first reported example of a heart imaging agent characterized by the presence of a terminal Tc'N multiple bond. It has a square-pyramidal geometry in which two identical bidentate dithiocarbamate ligands are symmetrically bound to a Tc'N group in the basal plane of the square-pyramid [28]. Thereafter, a new class of asymmetric, cationic perfusion imaging agents has been synthesized in order to optimize the balance between myocardial extraction and liver uptake [29]. The molecular structure of this class of compounds has a common chemical feature that lies in a Tc ≡ N core bound to two different bidentate ligands, thereby giving rise to asymmetrical complexes carrying a single positive charge [30]. The first in order of time was the monocationic nitrido 99mTc complex DBODC whose biodistribution behavior was extensively studied in rats [29, 31] and dogs [32]. These preclinical results in animals showed that heart uptake of 99mTc-N-DBODC and washout from blood and lungs, as well as kinetic features, as determined in dogs, are similar to those of the commercial radiopharmaceuticals sestamibi and tetrofosmin. However, a prominent difference was observed in liver washout, which was remarkably rapid and quantitative, leading to a heart-to-liver ratio at 60 min after injection in rats approximately 10 times higher than that of sestamibi and tetrofosmin [30, 31, 33]. Based on these promising results a phase 1 study has been carried out [34••] showing that this tracer has the desirable characteristics of the highest heart uptake observed for a monocationic myocardial perfusion tracer in humans (Fig. 3) and a fast and quantitative washout from non-target organs surrounding the heart region (Table 2). Under these conditions, the heart region is quickly visualized at 5 min after injection, and liver activity appears to be significantly reduced also 30 min after tracer administration both in rest and in stress images, making 99mTc-N-DBODC a promising myocardial perfusion agent with improved imaging properties. Representative planar whole-body images of the biodistribution of 99mTc-N-DBODC in healthy volunteers are shown in Fig. 4.
Another monocationic nitrido-compound under evaluation is 99mTc-N-MPO [35]. In rats it accumulates in the mitochondrial fraction to the same extent as sestamibi as demonstrated by a myocardial subcellular distribution study. Moreover, its biodistribution data in Sprague–Dawley rats showed a heart-to-liver ratio significantly higher than that of sestamibi at all time points, and also slightly higher than that of 99mTc-N-DBODC. It has been postulated that multidrug resistance transport function of hepatocytes and renal cells may be responsible for the fast clearance of 99mTc-N-MPO from liver and kidneys, respectively. However, additional comparison studies in guinea pigs [36] and dogs [37•] showed results below expectations: myocardial and liver uptake values were similar for the two tracers, resulting in only a mild to moderate advantage in heart-to-liver ratio of 99mTc-N-MPO over sestamibi. Unfortunately, no clinical data are yet available regarding the myocardial and liver kinetics of 99mTc-N-MPO in patients.
The last promising compound belonging to the core Tc ≡ N class is 99mTc-15 C5-PNP, a tricarbonyl complex with high solution stability and rapid clearance from blood and non-target organs in rodents [38]. This agent was recently evaluated in comparison with 99mTc-sestamibi in a male Sprague–Dawley rat heart model and despite heart-to-liver ratio values lower than that observed in a previous study [39], in vivo micro-SPECT acquisitions revealed cardiac perfusion images of similar quality with both tracers. In particular, a good visualization of the normal left ventricular wall and perfusion defects could be achieved 15 to 20 min after intravenous administration of 99mTc-15 C5-PNP. Moreover, the hepatic washout of this compound was significantly faster and greater than that of sestamibi leading to images with a significantly lower liver activity. If these encouraging preliminary data will be reproduced in humans, 99mTc-15 C5-PNP will be a welcome addition to currently approved perfusion agents.
It should be noted, however, that scientific research directed toward identifying new myocardial perfusion tracers never stops. An Italian group recently published the synthesis and biological evaluation of a new series of bidentate ligands aimed to increase the first-pass extraction of Tc ≡ N monocationic compounds, trying to keep unaltered their favorable imaging properties [40•]. The study shows that the incorporation of alicyclic dithiocarbamate in the [99mTc(N)(PNP)]+ building block yields to a significant increase of the heart uptake at early injection point, suggesting that the first-pass extraction fraction of these novel complexes may be increased with respect to the other cationic 99mTc agents keeping almost unaltered the favorable target/non-target ratios.
Future Perspectives of PET Perfusion Tracers
82Rb has the advantage of not requiring an on-site cyclotron and is currently the most widely used tracer for assessment of myocardial perfusion with PET. The kinetic properties of this radionuclide are similar to those of 201Tl and it is appropriate for myocardial perfusion imaging using qualitative or semiquantitative evaluation. On the other hand, its potential for absolute quantification of blood flow is still under investigation [41••]. 13N-ammonia and 15O-water are the most often used and validated PET tracers for quantification of myocardial perfusion [16]. However, they have short half-lives (10 and 2 min, respectively) and require on-site cyclotron, which limits their clinical use. Therefore, tracer availability still remains a major determinant for cardiac studies with PET.
Recently, many myocardial perfusion tracers labeled with fluorine-18 (18F) have been developed: fluorodihydrorotenone, fluorobenzyl-triphenylphosphonium, fluorophenyl-triphenylphosphonium, fluoroethylrhodamine B, and BMS747158-02 [42, 43]. Due to half-life of 110 min, their availability is not limited to laboratories with on-site cyclotron, facilitating larger clinical use of PET cardiac imaging.
The mechanisms of retention of 18F-fluorodihydrorotenone [44] and 18F-fluorobenzyl triphenyl phosphonium [45] in myocytes have not yet been completely clarified and may partly represent nonspecific binding to mitochondrial membranes. Recently, Shoup et al. [42] reported an excellent heart-to-blood ratio of 18F-fluorophenyl-triphenylphosphonium and a good correlation with 13N-ammonia distribution in normal and LAD-occluded rabbits, suggesting that this radiopharmaceutical may have potential as a PET agent for characterizing mitochondrial damage and/or myocardial blood flow. 18F-fluoroethylrhodamine B is another compound proposed as blood flow tracer [43]. However, with both these tracers no human studies have been reported.
A more promising myocardial perfusion tracer is 18F-BMS747158-02, a pyridaben analogue and inhibitor of mitochondrial complex I (MTC I) of the electron transport chain in the inner mitochondrial membrane [46–48]. Because of its lipophilicity and high binding affinity to MCT I this tracer shows effective and rapid uptake in cardiomyocytes followed by very slow washout. In vivo PET studies with 18F-BMS747158-02 have demonstrated excellent quality of myocardial images in different animal models. 18F-BMS747158-02 first-pass myocardial extraction fraction in isolated rat and rabbit hearts is more than 90% and persistent even at very high flow rates. High, essentially flow independent extraction fraction of 18F-BMS747158-02 implies an almost linear relationship between uptake and myocardial blood flow, which is an important tracer feature for assessment of flow reserve in stress testing. Experiences on the use of 18F-BMS747158-02 in diseased myocardium are still limited. When injected during transient coronary artery ligation, the resulting perfusion defects were clearly delineated by 18F-BMS747158-02 PET in rat and pig models [49•, 50••]. Because 18F-BMS747158-02 binds to a mitochondrial enzyme, it is important to clarify whether alterations in the metabolic state of the myocardium, such as those related to work load, substrate supply, and ischemia, affect its regional retention. The effects of various chronic cardiac disease conditions, such as heart failure, on the relationship between tracer uptake and blood flow still remain to be studied. Although detailed pharmacokinetics of the tracer need to be determined in human studies, the results of the experimental studies demonstrating good image quality, stable kinetics, and high extraction over wide flow range indicate suitability of 18F-BMS747158-02 for clinical protocols similar to those used with SPECT tracers. In particular, the tracer could be injected during physical exercise on a treadmill remote from the PET scanner followed by post-injection imaging similar to the SPECT protocols. Therefore, the important information on exercise-provoked symptoms as well as prognostic information related to exercise capacity can be explored. Provided that myocardial blood flow assessment can be improved, the need for doing rest and stress protocols would become less important as the absolute blood flow together with functional evaluation in gated images would provide diagnosis of inducible ischemia independently of coronary flow reserve. Finally, the use of 18F-BMS747158-02 for assessment of myocardial infarction and viability in combination with FDG could also be an option in PET centers, which have FDG available.
Conclusions
Despite all of the successes achieved with the current generation of SPECT and PET myocardial perfusion tracers, there is still a recognized need for the development of new perfusion tracers with improved properties. All the available single-photon tracers for myocardial perfusion imaging have suboptimal extraction and biodistribution characteristics that must be carefully considered to maximize their clinical applications. In the last few years, the design of new 99mTc-labeled tracers has been guided by observation of the relationships between basic biological and chemical properties and corresponding myocardial images. Although 82Rb has played an important role in expanding PET myocardial perfusion imaging to more clinical centers, this tracer also has some suboptimal characteristics, including the roll-off in tracer extraction at high flow rates, which is comparable to the other potassium analog, 201Tl. In addition, the very short half-life of 82Rb requires the use of pharmacologic stress. Thus, the quest for the ideal myocardial blood flow tracer is still in progress.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Hendel RC, Berman DS, Di Carli MF, et al.: ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 Appropriate Use Criteria for Cardiac Radionuclide Imaging. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. Circulation 2009, 1–114. This article outlines the most recent recommendations for the appropriate use criteria of cardiac radionuclide imaging.
Zaret BL, Strauss HW, Martin ND, et al. Noninvasive evaluation of regional myocardial perfusion with radioactive potassium: study of patients at rest, exercise, and during anginal pectoris. N Engl J Med. 1973;288:809–12.
Berman DS, Salel AF, DeNardo GL, Mason DT. Noninvasive detection of regional myocardial ischemia using rubidium-81 and the scintillation camera: comparison with stress electrocardiography in patients with arteriographically documented coronary stenosis. Circulation. 1975;52:619–26.
Kaul S, Boucher CA, Newell JB, et al. Determination of the quantitative thallium imaging variables that optimize detection of coronary artery disease. J Am Coll Cardiol. 1986;7:527–37.
Maddahi J, Kiat H, Berman DS. Myocardial perfusion imaging with technetium-99 m-labeled agents. Am J Cardiol. 1991;67:27D–34D.
• Cuocolo A, Petretta M, Acampa W, De Falco T: Gated SPECT myocardial perfusion imaging: the further improvements of an excellent tool. Q J Nucl Med Mol Imaging 2010, 54:129–144. This article outlined the recent methodological improvements of gated SPECT imaging and provided a description of the clinical role of this technique.
Aarnoudse WH, Botman KJ, Pijls NH. False-negative myocardial scintigraphy in balanced three-vessel disease, revealed by coronary pressure measurement. Int J Cardiovasc Intervent. 2003;5:67–71.
Gould KL, Schelbert H, Phelps M, et al. Noninvasive assessment of coronary stenoses with myocardial perfusion imaging during pharmacologic coronary vasodilatation. V. Detection of 47 percent diameter coronary stenosis with intravenous nitrogen-13 ammonia and emission-computed tomography in intact dogs. Am J Cardiol. 1979;43:200–8.
Cuocolo A, Breatnach E. Multimodality imaging in Europe: a survey by the European Association of Nuclear Medicine (EANM) and the European Society of Radiology (ESR). Eur J Nucl Med Mol Imaging. 2010;37:163–7.
Arbab AS, Koizumi K, Toyama K, Arai T, Araki T. Technetium-99 m-tetrofosmin, technetium-99 m-MIBI and thallium-201 uptake in rat myocardial cells. J Nucl Med. 1998;39:266–71.
Wackers FJTh, Berman DS, Maddahi J, et al. Technetium-99 m Hexakis 2-Methoxyisobutyl Isonitrile: human biodistribution, dosimetry, safety, and preliminary comparison to thallium-201 for mocardial perfusion imaging. J Nucl Med. 1989;30:301–11.
Zaret BL, Rigo P, Wackers FJ, et al. Myocardial perfusion imaging with 99mTc tetrofosmin. Comparison to 201Tl imaging and coronary angiography in a phase III multicenter trial. Tetrofosmin International Trial Study Group. Circulation. 1995;91:313–9.
Matsunari I, Fujino S, Taki J, et al. Comparison of defect size between thallium-201 and technetium-99 m tetrofosmin myocardial single-photon emission computed tomography in patients with single-vassel coronary artery disease. Am J Cardiol. 1996;77:350–4.
Kapur A, Latus KA, Davies G, et al. A comparison of three radionuclide myocardial perfusion tracers in clinical practice: the ROBUST study. Eur J Nucl Med Mol Imaging. 2002;29:1608–16.
Naruse H, Daher E, Sinusas A, et al. Quantitative comparison of planar and SPECT normal data files of thallium-201, technetium-99 m-sestamibi, technetium-99 m-tetrofosmin and technetium-99 m-furifosmin. J Nucl Med. 1996;37:1783–8.
Cuocolo A, Acampa W, Imbriaco M, et al. The many ways to myocardial perfusion imaging. Q J Nucl Med Mol Imaging. 2005;49:4–18.
Tamaki N, Senda M, Yonekura Y, et al. Dynamic positron computed tomography of the heart with a high sensitivity positron camera and nitrogen-13 ammonia. J Nucl Med. 1985;26:567–75.
Selwyn AP, Allan RM, L'Abbate A, et al. Relation between regional myocardial uptake of rubidium-82 and perfusion: absolute reduction of cation uptake in ischemia. Am J Cardiol. 1982;50:112–21.
Bergmann SR, Fox KA, Rand AL, et al. Quantification of regional myocardial blood flow in vivo with 0–15 water. Circulation. 1984;70:724–33.
Stewart RE, Schwaiger M, Molina E, et al. Comparison of Rb-82 PET and Tl-201 SPECT imaging for detection of CAD. Am J Cardiol. 1991;67:1303–10.
Tamaki N, Yonekura Y, Senda M, et al. Value and limitation of stress thallium-201 single-photon emission computed tomography: comparison with nitrogen-13 ammonia positron tomography. J Nucl Med. 1988;29:1181–8.
Tallaj JA, Kovar D, Iskandrian AE. The use of technetium-99 m sestamibi in a patient with liver cirrhosis. J Nucl Cardiol. 2000;6:722–3.
Beller GA, Bergmann SR. Myocardial perfusion imaging agents: SPECT and PET. J Nucl Cardiol. 2004;11:71–86.
Dahlberg ST. Assessment of myocardial perfusion with Tc-99 m: image is everything. J Nucl Cardiol. 2009;16:493–6.
Zaret L. Pursuit of the ideal perfusion agent. J Nucl Cardiol. 2002;9:149–50.
Marshall RC, Powers-Risius P, Reutter BW, et al. Kinetic analysis of 125I-iodorotenone as a deposited myocardial flow tracer: comparison with 99mTc-sestamibi. J Nucl Med. 2001;42:272–81.
VanBrocklin HF, Hanrahan SM, Enas JD, et al. Mitochondrial avid radioprobes. Preparation and evaluation of 70(Z)-[125I]iodorotenone and 70(Z)-[125I]iodorotenol. Nucl Med Biol. 2007;34:109–16.
Pasqualini R, Duatti A, Bellande E, et al. Bis(dithiocarbamato) nitrido technetium-99 m radiopharmaceuticals: a class of neutral myocardial imaging agents. J Nucl Med. 1994;35:334–41.
Boschi A, Bolzati C, Uccelli L, et al. A class of asymmetrical nitrido 99mTc heterocomplexes as heart imaging agents with improved biological properties. Nucl Med Commun. 2002;23:689–93.
Kim YS, He Z, Hsieh WY, et al. Impact of bidentate chelators on lipophilicity, stability, and biodistribution characteristics of cationic 99mTc-nitrido complexes. Bioconjug Chem. 2007;18:929–36.
Hatada K, Riou LM, Ruiz M, et al. 99mTc-N-DBODC5, a new myocardial perfusion imaging agent with rapid liver clearance: comparison with 99mTcsestamibi and 99mTc-tetrofosmin in rats. J Nucl Med. 2004;45:2095–101.
Hatada K, Ruiz M, Riou LM, et al. Biodistribution and myocardial uptake, washout, and redistribution kinetics of Tc-99 m N-DBODC5 when injected during vasodilator stress in canine models of coronary stenoses. J Nucl Cardiol. 2006;13:779–90.
Boschi A, Uccelli L, Bolzati C, et al. Synthesis and biological evaluation of monocationic asymmetric 99mTc-nitride heterocomplexes showing high heart uptake and improved imaging properties. J Nucl Med. 2003;44:806–14.
•• Cittanti C, Uccelli L, Pasquali M, et al.: Whole-body biodistribution and radiation dosimetry of the new cardiac tracer 99mTc-N-DBODC. J Nucl Med 2008, 49:1299–1304. This is the first study evaluating the safety profile and biodistribution behavior in human volunteers of the new myocardial perfusion tracer 99m Tc-N-DBODC.
Kim YS, Shi J, Zhai S, Hou G, Liu S. Mechanism for myocardial localization and rapid liver clearance of Tc-99 m-N-MPO: A new perfusion radiotracer for heart imaging. J Nucl Cardiol. 2009;16:571–9.
Kim YS, Wang J, Broisat A, Glover DK, Liu S. Tc-99 m-N-MPO: Novel cationic Tc-99 m radiotracer for myocardial perfusion imaging. J Nucl Cardiol. 2008;15:535–46.
• Bu L, Li R, Jin Z, et al.: Evaluation of 99mTcN-MPO as a new myocardial perfusion imaging agent in normal dogs and in an acute myocardial infarction canine model: comparison with 99mTc-sestamibi. Mol Imaging Biol. 2011;13:121–7. This study compared the biodistribution and pharmacokinetics of (99) (m)TcN-MPO and (99) (m)Tc-Sestamibi in normal dogs, and evaluated the potential of (99) (m)TcN-MPO as a myocardial perfusion agent in canines with acute myocardial infarction.
He Z, Hsieh WY, Kim YS, Liu S. Evaluation of novel cationic 99mTc(I)-tricarbonyl complexes as potential radiotracers for myocardial perfusion imaging. Nucl Med Biol. 2006;33:1045–53.
• Liu Z, Chen L, Liu S, et al.: Kinetic characterization of a novel cationic 99mTc(I)-tricarbonyl complex, 99mTc-15 C5-PNP, for myocardial perfusion imaging. J Nucl Cardiol 2010, 17:858–867. This study demonstrated that the cationic (99m)Tc(I)-tricarbonyl complex, (99m)Tc-15C5-PNP, has potential for rapid myocardial perfusion imaging with low liver uptake.
• Bolzati C, Cavazza-Ceccato M, Agostini S, et al.: Biological in vitro and in vivo studies of a series of new asymmetrical cationic [99mTc(N)(DTC-Ln)(PNP)]+ complex (DTC-Ln = Alicyclic Dithiocarbamate and PNP = Diphosphinoamine). Bioconjug Chem 2010, 21:928–939. This study showed that the incorporation of alicyclic dithiocarbamate in the [(99m)Tc(N)(PNP)](+) building block yields to a significant increase of the heart uptake at early injection point suggesting that the first-pass extraction fraction of these novel complexes may be increased with respect to the other cationic (99m)Tc-agents keeping almost unaltered the favorable target/nontarget ratios.
•• El Fakhri G, Kardan A, Sitek A, et al.: Reproducibility and accuracy of quantitative myocardial blood flow assessment with (82)Rb PET: comparison with (13)N-ammonia PET. J Nucl Med 2009, 50:1062–1071. The study demonstrated that MBF quantitation by 82 Rb PET has excellent reproducibility and interobserver reliability, providing comparable results with that achieved with 13 N-ammonia.
Shoup TM, Elmaleh DR, Brownell AL, et al.: Evaluation of (4-[(18)F]Fluorophenyl)triphenylphosphonium ion. A potential myocardial blood flow agent for PET. Mol Imaging Biol 2010, Epub ahead of print.
Gottumukkala V, Heinrich TK, Baker A, et al. Biodistribution and stability studies of [18 F]fluoroethylrhodamine B, a potential PET myocardial perfusion agent. Nucl Med Biol. 2010;37:365–70.
Marshall RC, Powers-Risius P, Reutter BW, et al. Kinetic analysis of 18 F-fluorodihydrorotenone as a deposited myocardial flow tracer: Comparison to 201Tl. J Nucl Med. 2004;45:1950–9.
Madar I, Ravert H, Dipaula A, et al. Assessment of severity of coronary artery stenosis in a canine model using the PET agent 18 F-fluorobenzyl triphenyl phosphonium: Comparison with 99mTc-tetrofosmin. J Nucl Med. 2007;48:1021–30.
Yalamanchili P, Wexler E, Hayes M, et al. Mechanism of uptake and retention of F-18 BMS-747158-02 in cardiomyocytes: A novel PET myocardial imaging agent. J Nucl Cardiol. 2007;14:782–8.
Yu M, Guaraldi MT, Mistry M, et al. BMS-747158-02: A novel PET myocardial perfusion imaging agent. J Nucl Cardiol. 2007;14:789–98.
Huisman MC, Higuchi T, Reder S, et al. Initial characterization of an 18 F-labeled myocardial perfusion tracer. J Nucl Med. 2008;49:630–6.
• Higuchi T, Nekolla SG, Huisman MM, et al.: A new 18 F-labeled myocardial PET tracer: Myocardial uptake after permanent and transient coronary occlusion in rats. J Nucl Med 2008, 49:1715–1722. This study investigated the potential of a new (18)F-labeled pyridazinone analog ((18)F-BMS-747158-02) and showed that this tracer may allow for assessment of flow using exercise-rest protocols similar to those used in combination with exercise and rest perfusion SPECT.
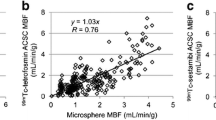
•• Nekolla SG, Reder S, Saraste A, et al.: Evaluation of the novel myocardial perfusion positron-emission tomography tracer 18 F-BMS-747158-02: comparison to 13 N-ammonia and validation with microspheres in a pig model. Circulation 2009, 119:2333–2342. This study demonstrated that 18 F-BMS-747158-02 allows quantitative assessment of regional myocardial perfusion over a wide flow range, suggesting that this tracer might be useful for clinical PET applications in patients with suspected or proven CAD.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cuocolo, A., Cittanti, C., Acampa, W. et al. Current and Future Status of Blood Flow Tracers. Curr Cardiovasc Imaging Rep 4, 227–236 (2011). https://doi.org/10.1007/s12410-011-9081-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12410-011-9081-9