Abstract
Recent technological advances have fueled the growth in hybrid radionuclide and CT imaging of the heart. Noninvasive imaging studies are reliable means to diagnose coronary artery disease (CAD), stratify risk, and guide clinical management. Myocardial perfusion scintigraphy is a robust, widely available noninvasive modality for the evaluation of ischemia from known or suspected CAD. Cardiac CT (coronary artery calcium score and coronary CT angiography) has emerged as a clinically robust noninvasive anatomic imaging test, capable of rapidly diagnosing or excluding obstructive CAD. Both anatomic and functional modalities have strengths and weaknesses, and can complement each other by offering integrated structural and physiologic information. As we discuss below, in selected patients, hybrid imaging may facilitate more accurate diagnosis, risk stratification, and management in a “one-stop shop” setting.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hybrid imaging refers to the combined imaging using CT and radionuclide imaging. This could be achieved either by co-registration and fusion of images from the two modalities performed on separate single photon emission CT (SPECT) or positron emission tomography (PET) and CT scanners or integrated imaging using a hybrid SPECT/CT or PET/CT scanner. While SPECT/PET nuclear imaging accurately reflects physiology, they lack anatomical details. On the contrary, cardiac CT provides a means to measure calcified atherosclerotic plaque burden by coronary calcium scoring (CCS), as well as coronary artery stenosis by CT coronary angiography (CTCA). Integrated PET and CCTA studies can offer the best of both modalities by providing concurrent functional and structural assessment.
PET and SPECT Myocardial Perfusion Imaging: Functional Assessment
Myocardial perfusion scintigraphy (MPS) is commonly used, clinically accounting for 90% of all noninvasive stress imaging tests in the United States [1]. Most of these studies consist of SPECT MPS, with an average sensitivity and specificity of 87% and 73%, detecting >50% coronary artery stenosis [2]. The prognostic value of SPECT MPS has been well established in a wide variety of patient cohorts [3••]. New SPECT scanners with solid-state detectors are more sensitive in detecting emission photons, and significantly reduce patient radiation doses and/or shorten imaging time. The sensitivity of this new technology is promising [4], and clinical validation in large-scale clinical trials is underway.
Presently, PET MPS is predominately performed in select academic centers. However, PET MPS is increasingly being used with the availability of generator-produced radiotracers like rubidium-82; its use will grow further with the availability of unit dose tracers such as new F-18–radiolabeled flow agents currently undergoing clinical evaluation [5]. The diagnostic accuracy of PET MPS in detecting flow-limiting coronary artery disease (CAD) is very high (sensitivity of 90%–92% and specificity of 85%–89%) [6•, 7] and similar in men and women and in obese and non-obese individuals [8]. Also, PET MPS has a superior diagnostic accuracy, improved specificity, and lower false-positive rate compared to nonattenuation-corrected SPECT MPS [9–12].
Despite their high clinical efficacy in diagnosis of CAD, risk stratification, and guiding management [13, 14], both relative SPECT [15] and PET MPS are limited in the noninvasive diagnosis of the extent of multivessel CAD and in detecting subclinical atherosclerosis [16••]. With gated rubidium-82 PET MPS, a failure to increase left ventricular ejection fraction (LVEF) with stress [17, 18•], or quantitative assessment of myocardial blood flow may help improve detection of multivessel CAD [19]. Absolute PET myocardial blood flow is well validated to detect early preclinical abnormalities in myocardial blood flow from subclinical atherosclerotic disease [20•]. However, these features provide only indirect evidence of the magnitude of ischemic burden, while coronary artery calcium score (CACS), as well as CTCA, provide a direct means to identify coronary atherosclerosis. When used in conjunction with MPS, hybrid imaging can improve diagnostic accuracy of MPS (by CT attenuation correction), and provide a comprehensive assessment of anatomic atherosclerosis (CACS and CTCA) to identify subclinical and multivessel CAD.
CT Attenuation Correction
Hybrid SPECT/CT and all PET/CT units are capable of CT attenuation correction (AC). AC has several advantages (Table 1) [21•]. It improves diagnostic accuracy of MPS, particularly specificity (Fig. 1), and is recommended by professional societies [1, 21•]. However, this technique has the potential for misregistration artifacts (erroneous perfusion defects) due to patient, cardiac, respiratory motion, or any combination of the above [22], mandating review of image registration, as part of the quality control.
For SPECT/CT MPS, AC has been demonstrated to improve specificity and normalcy rates compared to nonattenuation-corrected MPS. The multicenter study by Masood et al. [23] validated x-ray–based AC and showed that AC consistently improved the diagnostic yield of SPECT MPS for detecting significant angiographic CAD, as well as normalcy rate. SPECT MPS with CTCA has also been shown to decrease the number of equivocal studies in comparison to prone SPECT MPS without AC [24]. Furthermore, Fricke et al. [25] compared Tc-99m SPECT/CT with N-13 ammonia PET MPS and found that the concordance between the two modalities improved after AC of SPECT MPS, particularly in the inferior wall. They also found that SPECT MPS without AC tends to overestimate relative perfusion to the anterior and anterolateral walls compared to N-13 ammonia PET. Further studies with follow-up of clinical outcome, perhaps using the newer-generation hybrid scanners, would be required to validate the prognostic value of attenuation-corrected SPECT/CT MPS.
The diagnostic and prognostic values of attenuation-corrected PET/CT MPS are equivalent to those of dedicated PET MPS [3••, 6•]. Sampson et al. [16••] studied 64 intermediate-risk patients for CAD who had rubidium-82 PET/CT MPS and coronary angiography within 6 months, and demonstrated sensitivity, specificity, and normalcy rates of 93%, 83%, and 100%, respectively. The prognostic value of gated rubidium-82 PET/CT MPS was studied in 1,433 patients [26]. In this study, a normal scan was associated with an excellent outcome, whereas mild, moderate, and severely abnormal scans were associated with a progressive increase in event rates. The study also demonstrated that patients with an increase in LVEF during peak vasodilator stress had longer cardiac event-free survival compared to those with a decrease or without an increase in LVEF. Importantly, the changes in LVEF during gated PET/CT MPS have significant incremental value to clinical, stress, and perfusion data.
CT Coronary Calcium Score: Anatomic Imaging
Coronary calcium score (CCS) was initially performed using electron beam CT and more recently by multidetector CT (≥6 slice MDCT), with relatively low radiation dose (1–2 mSv) [27•]. Calcification of coronary arteries is pathognomonic for coronary atherosclerosis [28]. The amount of coronary calcium can be reliably quantified by CT, and is often expressed as Agatston score, which correlates strongly with the overall coronary plaque burden [29]. However, the magnitude of calcification does not always correlate with the severity of underlying coronary artery stenosis. The prognostic value of CCA is well studied. Absence of coronary calcification (CCS = 0) portends excellent prognosis, with 0.4% annual rate of myocardial infarction or cardiac death in men and women of diverse ethnicities [30]. On the contrary, patients with high CCS (≥400) have annual event rate of approximately 2%; approximately 20% of these patients, regardless of clinical symptoms, can have flow-limiting CAD [31, 32].
Calcium Score and MPS in the Diagnosis and Management of Patients with Suspected CAD
Several studies [31, 33–36••] have shown that CCS has incremental diagnostic value over MPS due to its ability in quantifying overall atherosclerotic burden including subclinical atherosclerotic calcifications. These studies, including predominantly asymptomatic individuals undergoing calcium score and a subsequent MPS for clinical indications, have shown that approximately 21% to 47% of the patients with normal MPS have coronary calcium score >400. Also, it has been demonstrated that patients with high CCS more frequently demonstrate ischemic MPS.
The prognostic value of CCS in patients with SPECT and PET MPS scans has been studied. Rozanski et al. [32] studied 1,153 patients (51% asymptomatic) who underwent both CCS and SPECT MPS and found that in patients with normal MPS there was no significant difference in risk-adjusted event rates in patients with CCS >400 or <400 (mean follow-up of 32 ± 16 months with cardiac death or myocardial infarction in 11 patients). In another study of CCS and SPECT MPS [33] in 1,175 patients (83% asymptomatic), over a median follow-up of 6.9 years, there were 145 cardiac events (cardiac death, myocardial infarction, or coronary revascularization) and 109 total events (death or myocardial infarction). Risk-adjusted survival was significantly better in patients with normal MPS and calcium score (CCS = 0–10) compared to patients with normal MPS and high calcium score (CCS >400) at 3 and 5 years after the SPECT MPS [33]. Schenker et al. [34] evaluated 636 patients who underwent rubidium-82 PET/CT MPS and concomitant CCS for clinical indications. The frequency of ischemic MPS was 16% in those with CCS of 0, and 46% in patients with CCS >400. The annualized event rate in patients with normal PET MPS and negative calcium score was substantially lower than in those with normal MPS and CCS ≥1,000. Lastly, in a recent study of 1,031 patients from the emergency room referred for SPECT MPS, the median CCS was 0 and patients with a calcium score of 0 had a low event rate (0.3% of myocardial infarction or acute coronary syndromes) over a mean follow-up of 7.4 months [37].
The findings of the above studies are complementary and suggest the following. Firstly, in patients with normal SPECT MPS and a high calcium score (Fig. 2), short-term risk is very low [32, 35]; however, these individuals may carry an intermediate risk in longer term as opposed to the individuals who have normal MPS and no/little coronary calcifications. Next, in symptomatic patients with normal clinically indicated PET MPS, those with calcified atherosclerotic plaques have a worse overall prognosis. Patients in the emergency room with atypical chest pain and a calcium score of 0 appear to have a low event rate.
Static images of SPECT myocardial perfusion scintigraphy demonstrating normal myocardial perfusion indicate very low risk for cardiac events. Coronary calcium study revealed extensive calcified plaques in the left main (LM) and anterior descending (LAD) coronary arteries (coronary calcium score = 735). The patient has higher risk for cardiac events in the intermediate to long term than those without calcified plaques
Clinical Applications of MPS and CACS
Based on the evidence from several studies, American Society of Nuclear Cardiology/American College of Cardiology/American Heart Association appropriate-use criteria [3••] recommend that a SPECT MPS study may be considered appropriate in asymptomatic patients with CCS >400 or in patients with high clinical risk and a CAC of >100 AU. The results from prior studies of the CCS screening population suggest that extensive coronary calcification may influence the physicians in prescribing more aggressive medical treatment [38–40]. We routinely perform and report CCS in patients undergoing MPS when the patient has no known CAD, myocardial infarction, or prior revascularization.
There are, however, several caveats to the use of CCS with MPS. Calcium score provides no information about stenosis severity. Low CCS may be less reliable in lowering individual risk for patients with acute symptoms, especially in young patients and women. This is probably because CCS misses noncalcified plaque, which is often the culprit lesion in acute coronary syndromes. Rosen et al. [41] demonstrated that among 175 patients who had CCS and CT angiography for chest pain, up to 4% had significant coronary obstruction despite having a zero CAC score at baseline. Calcium score is generally not performed when CTCA is going to be part of the assessment or if the patient has established CAD (ie, prior coronary artery bypass graft [CABG] or stenting). Also, CCS acquired during high resting heart rates can be degraded by motion and should be avoided. Unlike with CTCA, intravenous β-blockers are not routinely used for CCS.
CTCA: Anatomic Imaging
Multidetector CT (≥16-detector row) technology has enabled reliable visualization coronary artery stenosis and atherosclerotic plaque. Recently, three large clinical trials of 64-multidetector row cardiac CT angiography (ACCURACY [42], CORE64 [43], and European Trial [44]) demonstrated good diagnostic accuracy and excellent negative predictive values. These studies showed 75% to 88% sensitivity and 90% to 93% specificity for detection of >50% stenosis on a per-vessel basis. More importantly, the excellent negative predictive values (89%–99%) of CTCA enable rapid exclusion of clinically significant CAD in low-to-intermediate risk patients. Several studies have shown that patients with normal or minimally abnormal CTCA have extremely low risk for cardiac events [45, 46].
However, the stenosis severity cannot be accurately assessed by current multidetector CT technology [47, 48]. CTCA has a tendency to overestimate the degree of stenosis [49]. This is most marked in vessels with densely calcified plaques or coronary stents <3 mm due to blooming, beam-hardening, and metallic artifacts. In fact, according to the guidelines of the Society of Cardiovascular Computed Tomography, proceeding with CTCA in the presence of extensive calcification is “controversial.” Many centers do not proceed with CTCA in the presence of a calcium score >600. The spatial resolution, contrast-to-noise ratio, and temporal resolution of current multidetector CT technology remain limiting factors in accurate plaque quantification.
Similarly, imaging coronary artery stents with CTCA remains challenging, and its use should be carefully considered. A published study based on 64-slice multidetector CT has reported 91% sensitivity, 93% specificity, 77% positive predictive value, and 98% negative predictive value in diagnosing >50% in-stent stenosis [50]. This indicates that a negative result can exclude significant in-stent stenosis, while there is a moderate false-positive rate. The main determinants of stent visualization include the diameter, design, and location of the stent. For example, 64-slice multidetector CT has been shown to diagnose left main coronary artery in-stent stenosis with a high accuracy of 98% [51].
Also, the degree of coronary artery stenosis on CTCA correlates poorly with the presence of myocardial ischemia on nuclear MPS. For greater than 70% stenosis on CTCA, only 53% [52] to 66% [53] have inducible ischemia in the same vessel territories. For nonobstructive stenoses on CTCA (<50%–60%), 5% [53] to 14% [52] could still show inducible ischemia in the vessel territories. Therefore, CTCA is an excellent tool to exclude CAD or nonobstructive plaques. However, it cannot be used alone to guide revascularization decisions.
Integrated MPS/CTCA
The integration of CTCA with MPS offers several potential advantages. CTCA provides anatomical information that enables the detection of multivessel CAD, obstructive disease in left main and proximal LAD, as well as subclinical atherosclerosis. All of these important factors in risk stratification and therapeutic planning cannot be determined on MPS alone.
On the other hand, MPS provides functional information about ischemic burden [2] and flow reserve [54, 55], which are critical for determining benefits from revascularization. MPS can also be very helpful in evaluation of ischemia in the distal coronary segments or coronary segments obscured by multidetector CT artifacts. In addition, gated MPS provides physiologic information about LVEF [17, 18•, 26, 56], which is a powerful predictor of outcomes; indeed, for the same degree of anatomic stenosis, therapeutic benefit of revascularization is higher in patients with depressed LVEF compared to patients with preserved LVEF [57]. Therefore, it makes intuitive sense that combined MPS and CTCA provides significantly better characterization of underlying CAD than either alone.
Diagnostic Value of Integrated MPS and CTCA
The hybrid approach to the diagnosis of CAD has been studied for both PET and SPECT MPS. Rispler et al. [58] reported a significant improvement in specificity (63% to 95%) and positive predictive value (31% to 77%) for the detection of obstructive CAD in patients with known or suspected CAD undergoing hybrid SPECT/CTCA. Namdar et al. [59] detailed their experience with integrated PET and CTCA in 25 patients with known CAD and recurrent symptoms. Using PET MPS and invasive coronary angiography as gold standards, integrated PET MPS and CTCA hybrid imaging correctly predicts the revascularization decisions with 90% sensitivity, 98% specificity, 82% positive predictive value, and 99% negative predictive value. Di Carli et al. [52] also demonstrated that PET MPS and concomitant CTCA have complementary roles in diagnosing CAD. Only 47% of significant angiographic stenoses are associated with coronary flow limitation, whereas about half of patients with the normal MPS have evidence of non-flow-limiting CAD. In summary, when several studies evaluating MPS and CTCA are considered, the negative predictive value of CTCA appears to be robust, whereas the positive predictive value of CTCA in predicting ischemia is only modest (45%–67%; Fig. 3) [60]. Of note, these study populations are relatively small, and there are currently no patient outcome data on the utility of hybrid MPS and CT imaging.
Results of five representative studies, demonstrating the modest positive predictive values (PPVs) and consistently high negative predictive values (NPVs) of 64-multidetector CT coronary CT angiography in detecting ischemia on myocardial perfusion scintigraphy [45, 53, 72–74]. (Adapted from Min et al. [61]; with permission)
Prognostic Value of Hybrid MPS and CTCA
Evidence evaluating the combined prognostic value of CTCA and stress MPS assessment is emerging. Van Werkhoven et al. [36••] studied 541 patients followed for a median of 672 days. In this cohort, about 25% of patients with normal MPS had obstructive CAD and 5% of these had high-risk CAD on CTCA. The study outcomes suggest that patients with normal MPS and no or mild CAD (<50% stenosis on CTCA) have best outcomes, while those with significant CAD (≥50% stenosis on CTCA) and abnormal MPS have the worst outcomes. This study, though limited in power for adequate multivariable analysis (only 23 events, including eight all-cause mortality, eight nonfatal myocardial infarction, and seven unstable angina), was the first attempt to evaluate outcomes based on the results of both MPS and CTCA.
Min et al. [61] used decision analysis and Markov models to study cost effectiveness of diagnostic approaches using CTCA, MPS, and invasive coronary angiography, or combinations of the above. This very intriguing analysis demonstrated that CTCA alone may be a cost effective strategy in patients with an intermediate pretest likelihood of CAD. However, notably, this is decision analysis modeling of outcomes, but not real patient data. For instance, pathways included binary options such as MPS SPECT followed by invasive coronary angiography for positive or equivocal findings for CAD at MPS. Also, they did not include LVEF in the models. In clinical practice, decisions for invasive angiography are based on degree of ischemic burden and LVEF [60] rather than angiography for all positive or equivocal MPS. Nonetheless, this is the first reporting attempting to understand cost effectiveness of these approaches. We await the results of the ongoing SPARC trial [62] that will provide more information on the clinical efficacy and overall cost effectiveness of PET, SPECT, CTCA, and hybrid imaging in an intermediate-risk cohort.
Potential Clinical Applications of Combined MPS and CTCA
There are several scenarios wherein CTCA and MPS could theoretically add value to the other. For example, MPS following a CTCA could improve the identification of patients who would benefit from revascularization to reduce unnecessary procedures caused by “oculostenotic reflex” based on anatomic imaging by CTCA alone. Evidence of ischemia determined by exercise stress testing, myocardial perfusion imaging, or fractional flow reserve on coronary angiography remains superior to anatomic information of coronary stenosis for identifying patients who are most likely to benefit from revascularization [13, 54, 63, 64••].
Likewise, CTCA can potentially add prognostic value to the MPS [36••]. For instance, identification of coronary plaques with positive remodeling, spotty or no calcification could prompt escalated medical therapy and risk factor control since the morphologies are features of vulnerable plaques [65]. Similarly, identification of left main and multivessel disease has important clinical and therapeutic implications [66, 67]. Vivid CT images may also motivate lifestyle modifications and improve adherence to medical therapy in patients with subclinical atherosclerosis.
In selected patients with equivocal or inconclusive MPS, CTCA can clarify diagnosis [3••]. In particular, normal or near normal CTCA results can effectively exclude significant CAD in epicardial vessels. In patients with a mildly abnormal MPS and a high clinical suspicion of significant multivessel CAD, CTCA may clarify the diagnosis noninvasively.
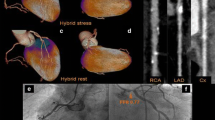
Integrated PET/CTCA can also be advantageous in the evaluation of patients with coronary artery anomalies or other structural abnormalities such as coronary artery fistulas, or myocardial muscle bridges. In patients with coronary artery anomalies (Fig. 4), the nature of the anomaly as well as ischemia is important in determining the need for intervention. CTCA can provide the overview of the coronary anatomy as well as the fine details that may be important to surgical planning, such as ostial take-off angles and epicardial versus transmural courses. Exercise MPS could provide information about hemodynamic significance of the anomaly.
a–c, A 55-year-old woman with prior aortic valve replacement for bicuspid aortic valve presented with typical anginal symptoms 6 months after surgery. The stress myocardial perfusion scintigraphy (MPS) images (a) demonstrated a reversible perfusion defect in the inferior wall. Retrospective review of the preoperative invasive angiography (c) revealed faint contrast filling the right coronary artery (red arrows) during the left coronary injection, suggesting anomalous origin of the right coronary artery. CT angiography acquired following MPS confirmed an anomalous origin of the right coronary artery from the left coronary cusp with possible compression of the proximal segment of the right coronary artery by the prosthetic valve (b; yellow arrows)
For patients with prior coronary bypass surgery scheduled for repeat left thoracotomy, combined structural and functional evaluations could be valuable since the coronary artery anatomy has been altered. Integrated imaging with MPS to facilitate estimation of ischemia and viability and CTCA to localize the native vessels and bypass grafts can help prevent damage to these structures during repeat CABG surgery.
Currently there is no in vivo imaging method capable of directly visualizing the coronary microcirculation in humans. With the ability to quantify myocardial blood flow with PET MPS and the use of CTCA to exclude epicardial CAD, hybrid PET/CTCA is an excellent tool for diagnosing microvascular dysfunction from structural (remodeling process) or functional (endothelial dysfunction or vasoconstriction) processes.
Lastly, hybrid imaging using radionuclide techniques and CT imaging can provide advantages for molecular cardiology research applications. The hybrid techniques capitalize on the exquisite sensitivity of radionuclide techniques (to quantify minute physiological processes using specific radiolabeled ligands) and the high spatial resolution of CT, to precisely localize regional small areas of radiotracer uptake (Fig. 5) [68].
a–c, Positron emission tomography (PET)–CT imaging of morphology and biology. Representative short-axis tomographic images are shown. Study animal after regional injection of adenovirus carrying HSV1-sr39tk reporter gene together with VEGF121 gene (AdTk-VEGF). Images: Panel a shows contrast-enhanced multislice CT depicting location of titanium clip markings (yellow arrows), along with circumferential wall thickness; Panel c shows the PET image of the reporter probe [18F]fluoro-hydroxymethylbutyl-guanine (FHBG) with significant accumulation of FHBG (unclear location of the radiotracer uptake). In the middle (Panel b) is the PET-CT fusion of morphologic CT with PET image of the reporter probe FHBG, showing significant accumulation of FHBG, co-localizing with clip markings in areas expressing the HSV1-sr39tk reporter gene (CT image providing the road map for the PET image). (Adapted from Wagner et al. [75]; with permission)
Although there are several proposed advantages of hybrid imaging, the clinical utility of this approach requires further validation by large-scale clinical trials and an understanding of how to utilize these results in clinical practice. Presently, CTCA is considered appropriate in patients with equivocal MPS or stress testing results, and MPS is considered appropriate in patients with equivocal CTCA results [3••, 69•]. Hybrid imaging should be performed in a sequential manner based on individual patients to maximize the diagnostic advantages of each modality. For instance, in low-risk patients, CTCA can be performed first to exclude significant CAD, and MPS can be reserved for lesions with indeterminate functional significance. For intermediate-risk to high-risk patients, MPS may be performed first and CTCA can be performed for equivocal cases or for further risk stratification. On the other hand, in patients with high-risk MPS and known coronary stents/calcified vessels, CTCA would lead to unnecessary contrast and radiation exposure without providing incremental diagnostic or prognostic information. In all cases, effective communication with the patient and the referring physician is critical to assure that the appropriate test is performed.
Challenges with Hybrid Imaging
The appropriate use of cardiac imaging has recently received a great deal of attention due to concerns about the costs and radiation exposure. Conventional spiral CTCA protocols with retrospective gating were associated with a wide range of radiation exposures, ranging from 9 to 21 mSv [68]. In combination with rest-stress Tc-99m sestamibi SPECT MPS, radiation dose is estimated to be as high as 41.5 mSv, which heightened concern about excessive radiation dosage with this approach [58]. Rapid advances in scanner technology have addressed some of these concerns. With newer low-dose CTCA acquisition protocols using prospective ECG gating, the radiation dose can be reduced up to 70% with no reduction in diagnostic accuracy [70, 71]. A combined SPECT/CT or PET/CT protocol, including rest and stress perfusion and prospective ECG-gated CTCA, could result in an estimated mean effective dose of approximately 9 to 15 mSv, respectively (Table 2). With new SPECT scanners using semiconductor detectors, lower-dose imaging is also feasible.
Conclusions
MPS and CACS are established modalities with extensive evidence to support their clinical utility in the diagnosis, risk stratification, and management of patients with known or suspected CAD. CTCA is a clinically robust noninvasive test capable of detecting early calcified and noncalcified plaque burden and significant multivessel obstructive CAD. Data on the prognostic value of CTCA are now emerging. Hybrid MPS and CT imaging offers several attractive clinical and research applications. Attenuation correction with CT transmission imaging improves the specificity and diagnostic accuracy of MPS. In conjunction with MPS, CCS can refine intermediate to long-term risk stratification for patients with normal MPS. The importance of hybrid radionuclide and CT imaging in molecular cardiology applications is indisputable. However, the evidence base for the clinical role of hybrid CTCA and MPS imaging in the overall diagnostic paradigm of CAD remains to be established.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance•• Of major importance
Heller GV, Links J, Bateman TM, et al.: American Society of Nuclear Cardiology and Society of Nuclear Medicine joint position statement: attenuation correction of myocardial perfusion SPECT scintigraphy. J Nucl Cardiol 2004, 11:229–230.
Klocke FJ, Baird MG, Lorell BH, et al.: ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). J Am Coll Cardiol 2003, 42:1318–1333.
•• Hendel RC, Berman DS, Di Carli MF, et al.: ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 Appropriate Use Criteria for Cardiac Radionuclide Imaging. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. Circulation 2009, 1–114. The article outlines the most up-to-date multosociety recommendations for the appropriate use criteria of cardiac radionuclide imaging.
Maddahi J, Mendez R, Mahmarian JJ, et al.: Prospective multicenter evaluation of rapid, gated SPECT myocardial perfusion upright imaging. J Nucl Cardiol 2009, 16:351–357.
Nekolla SG, Martinez-Moeller A, Saraste A: PET and MRI in cardiac imaging: from validation studies to integrated applications. Eur J Nucl Med Mol Imaging 2009, 36(Suppl 1):S121–S130.
• Di Carli MF, Hachamovitch R: New technology for noninvasive evaluation of coronary artery disease. Circulation 2007, 115:1464–1480.
Nandalur KR, Dwamena BA, Choudhri AF, et al.: Diagnostic performance of positron emission tomography in the detection of coronary artery disease: a meta-analysis. Acad Radiol 2008, 15:444–451.
Bateman TM, Heller GV, McGhie AI, et al.: Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: comparison with ECG-gated Tc-99m sestamibi SPECT. J Nucl Cardiol 2006, 13:24–33.
Berman DS, Hachamovitch R, Shaw LJ, et al.: Roles of nuclear cardiology, cardiac computed tomography, and cardiac magnetic resonance: Noninvasive risk stratification and a conceptual framework for the selection of noninvasive imaging tests in patients with known or suspected coronary artery disease. J Nucl Med 2006, 47:1107–1118.
Go R, Marwick T, MacIntyre W, et al.: A prospective comparison of rubidium-82 PET and thallium-201 SPECT myocardial perfusion imaging utilizing a single dipyridamole stress in the diagnosis of coronary artery disease. J Nucl Med 1990, 31:1899–1905.
Stewart RE, Schwaiger M, Molina E, et al.: Comparison of rubidium-82 positron emission tomography and thallium-201 SPECT imaging for detection of coronary artery disease. Am J Cardiol 1991, 67:1303–1310.
Bateman TM, Berman DS, Matloff JM: Radionuclide ventriculography for assessing cardiac shock early after open-heart surgery. Acute Care 1985, 11:48–52.
Boden WE, O’Rourke RA, Teo KK, et al.: Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007, 356:1503–1516.
Hachamovitch R, Berman DS, Kiat H, et al.: Value of stress myocardial perfusion single photon emission computed tomography in patients with normal resting electrocardiograms: an evaluation of incremental prognostic value and cost-effectiveness. Circulation 2002, 105:823–829.
Lima RSL, Watson DD, Goode AR, et al.: Incremental value of combined perfusion and function over perfusion alone by gated SPECT myocardial perfusion imaging for detection of severe three-vessel coronary artery disease. J Am Coll Cardiol 2003, 42:64–70.
•• Sampson UK, Dorbala S, Limaye A, et al.: Diagnostic accuracy of rubidium-82 myocardial perfusion imaging with hybrid positron emission tomography/computed tomography in the detection of coronary artery disease. J Am Coll Cardiol 2007, 49:1052–1058. The article is currently the largest clinical study that has validated the diagnostic efficacy of rubidium PET imaging.
Brown TLY, Merrill J, Volokh L, et al.: Determinants of the response of left ventricular ejection fraction to vasodilator stress in electrocardiographically gated (82)rubidium myocardial perfusion PET. Eur J Nucl Med Mol Imaging 2008, 35:336–342.
• Dorbala S, Vangala D, Sampson U, et al.: Value of vasodilator left ventricular ejection fraction reserve in evaluating the magnitude of myocardium at risk and the extent of angiographic coronary artery disease: a 82Rb PET/CT study. J Nucl Med 2007, 48:349–358.
Parkash R, deKemp RA, Ruddy TD, et al.: Potential utility of rubidium 82 PET quantification in patients with 3-vessel coronary artery disease. J Nucl Cardiol 2004, 11:440–449.
• Camici PG, Crea F: Coronary microvascular dysfunction. N Engl J Med 2007, 356:830–840.
• Dilsizian V, Bacharach SL, Beanlands RS, et al.: PET myocardial perfusion and metabolism clinical imaging. J Nucl Cardiol 2009, 16:651.
Goetze S, Brown TL, Lavely WC, et al.: Attenuation correction in myocardial perfusion SPECT/CT: effects of misregistration and value of reregistration. J Nucl Med 2007, 48:1090–1095.
Masood Y, Liu TH, Depuey G, et al.: Clinical validation of SPECT attenuation correction using x-ray computed tomography-derived attenuation maps: multicenter clinical trial with angiographic correlation. J Nucl Cardiol 2005, 12:676–686.
Malkerneker D, Brenner R, Martin WH, et al.: CT-based attenuation correction versus prone imaging to decrease equivocal interpretations of rest/stress Tc-99m tetrofosmin SPECT MPI. J Nucl Cardiol 2007, 14:314–323.
Fricke E, Fricke H, Weise R, et al.: Attenuation correction of myocardial SPECT perfusion images with low-dose CT: evaluation of the method by comparison with perfusion PET. J Nucl Med 2005, 46:736–744.
Dorbala S, Hachamovitch R, Curillova Z, et al.: Incremental prognostic value of gated Rb-82 positron emission tomography myocardial perfusion imaging over clinical variables and rest LVEF. JACC Cardiovasc Imaging 2009, 2:846–854.
• Einstein AJ, Moser KW, Thompson RC, et al.: Radiation dose to patients from cardiac diagnostic imaging. Circulation 2007, 116:1290–1305.
Blankenhorn DH, Stern D: Calcification of the coronary arteries. Am J Roentgenol Radium Ther Nucl Med 1959, 81:772–777.
Kaufmann RB, Peyser PA, Sheedy PF, et al.: Quantification of coronary artery calcium by electron beam computed tomography for determination of severity of angiographic coronary artery disease in younger patients. J Am Coll Cardiol 1995, 25:626–632.
Bellasi A, Lacey C, Taylor AJ, et al.: Comparison of prognostic usefulness of coronary artery calcium in men versus women (results from a meta- and pooled analysis estimating all-cause mortality and coronary heart disease death or myocardial infarction). Am J Cardiol 2007, 100:409–414.
Berman DS, Wong ND, Gransar H, et al.: Relationship between stress-induced myocardial ischemia and atherosclerosis measured by coronary calcium tomography. J Am Coll Cardiol 2004, 44:923–930.
Rozanski A, Gransar H, Wong ND, et al.: Clinical outcomes after both coronary calcium scanning and exercise myocardial perfusion scintigraphy. J Am Coll Cardiol 2007, 49:1352–1361.
Chang SM, Nabi F, Xu J, et al.: The coronary artery calcium score and stress myocardial perfusion imaging provide independent and complementary prediction of cardiac risk. J Am Coll Cardiol 2009, 54:1872–1882.
Schenker MP, Dorbala S, Hong ECT, et al.: Interrelation of coronary calcification, myocardial ischemia, and outcomes in patients with intermediate likelihood of coronary artery disease: a combined positron emission tomography/computed tomography study. Circulation 2008, 117:1693–1700.
Uebleis C, Becker A, Griesshammer I, et al.: Stable coronary artery disease: prognostic value of myocardial perfusion SPECT in relation to coronary calcium scoring—long-term follow-up. Radiology 2009, 252:682–690.
•• van Werkhoven JM, Schuijf JD, Gaemperli O, et al.: Prognostic value of multislice computed tomography and gated single-photon emission computed tomography in patients with suspected coronary artery disease. J Am Coll Cardiol 2009, 53:623–632. This important article first validated the incremental prognostic values of calcium score and CTCA in addition to SPECT MPI.
Nabi F, Chang SM, Pratt CM, et al.: Coronary artery calcium scoring in the emergency department: identifying which patients with chest pain can be safely discharged home. Ann Emerg Med 2010. doi:10.1016/j.annemergmed.2010.01.017
Taylor AJ, Bindeman J, Feuerstein I, et al.: Community-based provision of statin and aspirin after the detection of coronary artery calcium within a community-based screening cohort. J Am Coll Cardiol 2008, 51:1337–1341.
Thompson RC, McGhie AI, Moser KW, et al.: Clinical utility of coronary calcium scoring after nonischemic myocardial perfusion imaging. J Nucl Cardiol 2005, 12:392–400.
Blankstein R, Dorbala S: Adding calcium scoring to myocardial perfusion imaging: Does it alter physicians’ therapeutic decision making? J Nucl Cardiol 2010, 17:168–171.
Rosen BD, Fernandes V, McClelland RL, et al.: Relationship between baseline coronary calcium score and demonstration of coronary artery stenoses during follow-up MESA (Multi-Ethnic Study of Atherosclerosis). JACC Cardiovasc Imaging 2009, 2:1175–1183.
Budoff M, Dowe D, Jollis J, et al.: Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol 2008, 52:1724–1732.
Miller JM, Rochitte CE, Dewey M, et al.: Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 2008, 359:2324–2336.
Meijboom WB, Meijs MFL, Schuijf JD, et al.: Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 2008, 52:2135–2144.
Gaemperli O, Valenta I, Schepis T, et al.: Coronary 64-slice CT angiography predicts outcome in patients with known or suspected coronary artery disease. Eur Radiol 2008, 18:1162–1173.
Gilard M, Le Gal G, Cornily JC, et al.: Midterm prognosis of patients with suspected coronary artery disease and normal multislice computed tomographic findings: a prospective management outcome study. Arch Intern Med 2007, 167:1686–1689.
Leber AW, Knez A, von Ziegler F, et al.: Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol 2005, 46:147–154.
Raff GL, Gallagher MJ, O’Neill WW, et al.: Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol 2005, 46:552–557.
Husmann L, Gaemperli O, Schepis T, et al.: Accuracy of quantitative coronary angiography with computed tomography and its dependency on plaque composition: plaque composition and accuracy of cardiac CT. Int J Cardiovasc Imaging 2008, 24:895–904.
Ehara M, Kawai M, Surmely JF, et al.: Diagnostic accuracy of coronary in-stent restenosis using 64-slice computed tomography: comparison with invasive coronary angiography. J Am Coll Cardiol 2007, 49:951–959.
van Mieghem CAG, Cademartiri F, Mollet NR, et al.: Multislice spiral computed tomography for the evaluation of stent patency after left main coronary artery stenting: a comparison with conventional coronary angiography and intravascular ultrasound. Circulation 2006, 114:645–653.
Di Carli MF, Dorbala S, Curillova Z, et al.: Relationship between CT coronary angiography and stress perfusion imaging in patients with suspected ischemic heart disease assessed by integrated PET-CT imaging. J Nucl Cardiol 2007, 14:799–809.
Sato A, Hiroe M, Tamura M, et al.: Quantitative measures of coronary stenosis severity by 64-slice CT angiography and relation to physiologic significance of perfusion in nonobese patients: comparison with stress myocardial perfusion imaging. J Nucl Med 2008, 49:564–572.
Hachamovitch R, Hayes SW, Friedman JD, et al.: Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003, 107:2900–2907.
Tonino PAL, De Bruyne B, Pijls NHJ, et al.: Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009, 360:213–224.
Lertsburapa K, Ahlberg AW, Bateman TM, et al.: Independent and incremental prognostic value of left ventricular ejection fraction determined by stress gated rubidium 82 PET imaging in patients with known or suspected coronary artery disease. J Nucl Cardiol 2008, 15:745–753.
Eagle KA, Guyton RA, Davidoff R, et al.: ACC/AHA Guidelines for Coronary Artery Bypass Graft Surgery: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1991 Guidelines for Coronary Artery Bypass Graft Surgery). American College of Cardiology/American Heart Association. J Am Coll Cardiol 1999, 34:1262–1347.
Rispler S, Keidar Z, Ghersin E, et al.: Integrated single-photon emission computed tomography and computed tomography coronary angiography for the assessment of hemodynamically significant coronary artery lesions. J Am Coll Cardiol 2007, 49:1059–1067.
Namdar M, Hany TF, Koepfli P, et al.: Integrated PET/CT for the assessment of coronary artery disease: a feasibility study. J Nucl Med 2005, 46:930–935.
Sharir T, Germano G, Kang X, et al.: Prediction of myocardial infarction versus cardiac death by gated myocardial perfusion SPECT: risk stratification by the amount of stress-induced ischemia and the poststress ejection fraction. J Nucl Med 2001, 42:831–837.
Min JK, Gilmore A, Budoff MJ, et al.: Cost-effectiveness of coronary CT angiography versus myocardial perfusion SPECT for evaluation of patients with chest pain and no known coronary artery disease. Radiology 2010, 254:801–808.
Hachamovitch R, Johnson J, Hlatky M, et al.: The study of myocardial perfusion and coronary anatomy imaging roles in CAD (SPARC): design, rationale, and baseline patient characteristics of a prospective, multicenter observational registry comparing PET, SPECT, and CTA for resource utilization and clinical outcomes. J Nucl Cardiol 2009, 16:935–948.
Gaemperli O, Husmann L, Schepis T, et al.: Coronary CT angiography and myocardial perfusion imaging to detect flow-limiting stenoses: a potential gatekeeper for coronary revascularization? Eur Heart J 2009, 30:2921–2929.
•• Shaw LJ, Berman DS, Maron DJ, et al.: Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008, 117:1283–1291. The nuclear substudy of the COURAGE trial has shown the clinical benefit of revascularization over medical therapy alone is only significant in chronic stable angina patients with moderate to severe ischemia.
Motoyama S, Kondo T, Sarai M, et al.: Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol 2007, 50:319–326.
Min JK, Shaw LJ, Devereux RB, et al.: Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol 2007, 50:1161–1170.
Myers WO, Blackstone EH, Davis K, et al.: CASS Registry long term surgical survival. Coronary Artery Surgery Study. J Am Coll Cardiol 1999, 33:488–498.
Hausleiter J, Meyer T, Hadamitzky M, et al.: Radiation dose estimates from cardiac multislice computed tomography in daily practice: impact of different scanning protocols on effective dose estimates. Circulation 2006, 113:1305–1310.
• Hendel RC, Patel MR, Kramer CM, et al.: ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol 2006, 48:1475–1497.
Hirai N, Horiguchi J, Fujioka C, et al.: Prospective versus retrospective ECG-gated 64-detector coronary CT angiography: assessment of image quality, stenosis, and radiation dose. Radiology 2008, 248:424–430.
Husmann L, Valenta I, Gaemperli O, et al.: Feasibility of low-dose coronary CT angiography: first experience with prospective ECG-gating. Eur Heart J 2008, 29:191–197.
Hacker M, Jakobs T, Hack N, et al.: Sixty-four slice spiral CT angiography does not predict the functional relevance of coronary artery stenoses in patients with stable angina. Eur J Nucl Med Mol Imaging 2007, 34:4–10.
Haramati LB, Levsky JM, Jain VR, et al.: CT angiography for evaluation of coronary artery disease in inner-city outpatients: an initial prospective comparison with stress myocardial perfusion imaging. Int J Cardiovasc Imaging 2009, 25:303–313.
Schuijf JD, Wijns W, Jukema JW, et al.: Relationship between noninvasive coronary angiography with multi-slice computed tomography and myocardial perfusion imaging. J Am Coll Cardiol 2006, 48:2508–2514.
Wagner B, Anton M, Nekolla SG, et al.: Noninvasive characterization of myocardial molecular interventions by integrated positron emission tomography and computed tomography. J Am Coll Cardiol 2006, 48:2107–2115.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hsiao, E.M., Ali, B. & Dorbala, S. Clinical Role of Hybrid Imaging. Curr Cardiovasc Imaging Rep 3, 324–335 (2010). https://doi.org/10.1007/s12410-010-9033-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12410-010-9033-9