Abstract
Potentially toxic elements (PTEs) are known to threat human health due to exposure to contaminated groundwater. Some of these PTEs can lead to long-term carcinogenic and non-carcinogenic health risks. The Estarreja Chemical Complex (ECC), NW Portugal, has had an intense industrial activity since the early 1950s, which lead to high levels of soil and groundwater contamination. Local populations traditionally rely on groundwater for human and agricultural uses. Although rehabilitation measures have been implemented for the last 20 years, groundwater contamination levels remain high for some PTEs, whose concentrations may be several orders of magnitude higher than human consumption. Two groundwater-sampling campaigns were conducted showing the temporal evolution of groundwater quality and allowing for the calculation of non-cancer and cancer risks due to exposure to PTEs by the ECC-surrounding population, considering groundwater ingestion and dermal contact as exposure pathways. Hair and urine PTE contents were collected during of the second sampling groundwater campaign and were used as biomonitoring to validate the exposure of local population to PTEs. The results show that As is the contaminant with highest non-cancer and cancer health risks for the exposed population, presenting high values particularly in Veiros, Beduído and Pardilhó localities. The most groundwater-contaminated areas coincided with the localities in which inhabitants exhibit higher hair and urinary PTE concentrations. Hair samples show high levels of As, Hg and Ni, while urine samples show high levels for Al, As, Cd, Hg, Pb, Ni and Zn are elevated in localities close to the ECC. Urine and hair proved to be suitable to evaluate short- and long-term exposure to PTEs, and are strongly correlated groundwater PTEs concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater, which is an important source of drinking and irrigation water supply, is closely linked to the spread of chemical elements that have the potential to influence and impact human health. However, groundwater is still a neglected component of the hydrological cycle in what regards to water investigations and management (Re 2015). Moreover, groundwater has longer residence times than most of the other hydrological-cycle components and the impacts of aquifer degradation can remain unnoticed for decades, which can lead to an overlook of the long-term impacts of groundwater quality on human health. There are indeed many sources of potentially toxic elements (PTEs) to groundwater bodies, in turn threatening human health if this groundwater is ingested (e.g. through showering, cooking) or gets in contact with skin (e.g. through showering, irrigation or recreational activities) (Sun et al. 2010; Xie et al. 2011). Long-term shower exposure to PTE-contaminated water may pose a significant risk for the development of diseases, e.g. central nervous system neurotoxicity (Elsner and Spangler 2005). Dietary intake may be an important pathway of exposure to PTE (Ericson et al. 2008; Eggers et al. 2018), and groundwater PTE content has indirect implications on the dietary intake because PTE content of cultivated agricultural products may depends on the quality of the irrigation water (Inácio et al. 2014). Some of these PTEs can lead to long-term carcinogenic and non-carcinogenic health risks (Tartaglione et al. 2016; Yan et al. 2016; WHO 2017; Cabral Pinto et al. 2017a, b, 2018).
In many situations, single-discipline research approaches are not adequate to solve many of the current and pressing health issues that society faces (Hammond and Dubé 2012), and complex problems are not typically circumspect to disciplinary boundaries (Bellotti 2017). The effect of groundwater contamination on human health is arguably a critical and complex problem involving different disciplines such as geochemistry, hydrogeology and health sciences. Tackling such a complex problem requires an inter-disciplinary approach, which aims to integrate data and methods from different disciplines that can combine to solve a complex problem (Putzrath 2000; U.S. Environmental Protection Agency 2003; Komatina 2004; Callahan and Sexton 2007; Ryker and Small 2008). Groundwater chemical composition may cause metabolic changes, which may favour the occurrence of endemic diseases in humans. Consequently, the investigation of groundwater geochemistry is needed to understand the causes of such diseases (Panaullah et al. 2009; Yao et al. 2011).
Exposure to PTEs has been related to oncological disease and mortality (Hopenhayn-Rich et al. 1998; Villanueva et al. 2006; Karagas et al. 1998; Alavanja et al. 2004; Kavcar et al. 2009; Phan et al. 2010; Wongsasuluk et al. 2014; Rapant et al. 2017; Cabral Pinto and Ferreira da Silva 2019), breathing difficulties (Bouchard et al. 1992; Frampton 2001; Ize-Iyamu and Bernard 2007; Rahman et al. 2009; Golash and Gogate 2012; Schneider et al. 2013), cognitive problems among children (Needleman et al. 1990; Bouchard et al. 2011) and brain development damages (Liu et al. 2005). Early exposure to PTEs can interfere with developmental programming and induce subclinical alterations that may result in pathophysiology at a later life stage (Gorell et al. 1999; Centeno et al. 2002; Tchounwo et al. 2003; Zatta et al. 2003; Komatina 2004; Gupta et al. 2005; Charlet and Polya 2006; Dissanayake and Chandrajith 2009; Johnson and Atchison 2009; Exley 2012; Chin-Chan et al. 2015; Ahlskog 2016; Wu et al. 2016; Cabral Pinto et al. 2014, 2017a, 2018). Environmental and occupational exposure to Al, Cu, Fe, Hg, Mn, Pb and Zn appears to be a risk factor for Parkinson’s and Alzheimer’s diseases (Zatta et al. 2003; Elsner and Spangler 2005; Erikson et al. 2008; Schneider et al. 2006; Santamaria et al. 2007; Flynn and Susi 2009).
Biomonitoring is largely used to control the health risk of people exposed to contaminants (Alimonty and Mattei 2008). It evaluates the human exposure by comparison with appropriate reference values and goes by the knowledge of the relationship between environmental exposure and deriving degree of adverse health effects. Epidemiological studies often use hair, nails and urine as biological material of exposure because they are less invasive and the samples are easy to obtain in large populations. Although urine generally reflects recent exposures the chemicals (days/weeks), this is not straightforward for all elements. Metal biological half-life characterizes its elimination period from the body (Nriagu 2007). PTE of this study have the following half-lives: 10 to 12 years for Cd and Pb (inter-individual variability of about 30%); 9 months for Zn; 60 days for Hg, 30 days for Al, and 4 days for As and Ni (Ljunggren et al. 1991; Sällsten et al. 1994; Petersen et al. 2000). Hence, concentrations of different PTEs in urine may provide valuable information on exposure within different time spans, thus helping to better assess the health effects. Most PTEs are excreted via the kidney in the urine, and to a much lesser extent by the gastrointestinal tract (Dorne et al. 2011).
The use of hair for biomonitoring purposes offers the possibility to integrate a relatively long-term exposure in a single specimen analysis (WHO 2015). Studies on industrial workers and populations exposed to high levels of environmental contaminants have shown that there is a relationship between human hair contents of some metals and estimates of their exposure via ingestion, inhalation or dermal contact (Bencko 1995; Wright et al. 2006; Amaral et al. 2008; Eastman et al. 2013). Rodushkin and Axelsson (2000) suggested that human hair in trace elements analysis may provide suitable data that point towards diseases such as diabetes mellitus, coronary artery disease, and other cardiovascular diseases (Coelho et al. 2014) and many epidemiological studies use human hair (Rodushkin and Axelsson 2000, 2003; Brima et al. 2006; Gault et al. 2008; Ayodele and Bayero 2009; Sthiannopkao et al. 2010; Wu and Chen 2010; Coelho et al. 2012; Mohmand et al. 2015) as biological markers of long-exposure periods (Slotnick and Nriagu 2006).
The present study attempts to understand the relationships between environmental geochemistry and public health in the contaminated area surrounding the ECC, by analysing the evolution of groundwater quality data, calculating health risks and evaluating the metal body burden by determining hair and urine PTE content. The concentrations of PTE in the groundwater were used to evaluate the non-cancer and cancer risks for the residents that have lived long-term in the region of Estarreja. The methodology and guidelines used are those described by the USEPA (2011) for risk assessment of contaminated sites. This inter-disciplinary study was delineated with the main objectives of (i) evaluating groundwater quality in the ECC surroundings from 2006 to 2013; (ii) investigating the relationships between exposure to PTE groundwater contamination and inhabitants’ cancer and non-cancer risks, including their temporal evolution; and, (iii) evaluating the effectiveness of PTE levels in hair and urine to detect relationships between environmental exposures and cancer and non-cancer risk in humans due to contaminated groundwater. To the best of our knowledge, this is the first time an inter-disciplinary approach is adopted aiming at integrating groundwater hydrochemistry and biomonitoring data to assess cancer and non-cancer risks in a contaminated aquifer. Pham et al. (2017) and Park et al. (2016) studied As concentration in urine as indication of exposure to groundwater contamination, although not in the context of cancer and non-cancer risks. Other studies correlate PTEs hair and/or urine concentration with health risks (including cancer), although not evaluating the environmental exposure to the contaminants (e.g. Kim et al. 2004; Iarmarcovai et al. 2005; McElroy et al. 2006).
Study Area
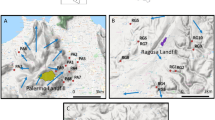
The study area is located in the surroundings of the ECC (Fig. 1a and b), which is a chemical–industrial complex located about 3 km to the north of the city of Estarreja. Surrounding populations historically rely on groundwater as a source of domestic water supply (preparing food, bathing and personal hygiene needs, washing clothes and dishes) and agricultural uses (despite the fact that the principal source of potable water supply in the region is currently surface water from the Vouga river). The ECC has been in operation since the 1930s, although its development was mainly triggered after the World War II, in the early 1950s. Nowadays the main companies within the ECC produce the following contaminants: polyvinyl chloride (CIRES); nitrobenzene, aniline, cyclohexylamine, cyclohexanol, sulphuric acid, nitric acid and sulphanilic acid (CUF), polymeric methylene diphenyl diisocyanate (DOW CHEM); pure and mixed gases for industrial and medical uses (Air Liquide); and, aluminium sulphates and polyaluminum chlorides in liquid form (AQP). The production of these chemicals within the ECC requires the use of a long list of potentially hazardous substances, which have left their environmental legacy in the surrounding environment. In the past, liquid effluents containing aniline, benzene compounds, NO3, Cl, Al, As, Cd, Cr, Hg, Ni, Zn and Pb were discharged without any previous treatment into manmade permeable water channels, contaminating agricultural fields, surface water and groundwater (Inácio et al. 1998; Pereira et al. 1998; Castro et al. 1997; Leitão 1996; Borrego 1993; Barradas et al. 1992; Aristides Hall et al. 1987). Solid wastes comprised sludge containing pyrite, calcium hydroxide, Hg and As were deposited in permeable areas with no pre-treatment (Costa and Jesus-Rydin 2001). Despite the rehabilitation actions taken during the last 20 years, including the ERASE project (ERASE 2000), the footprint of the ECC industrial activity may still be identified in impacted air, soils, sediments, surface and groundwater quality around this Complex (Leitão 1996; Pereira et al. 2009; Van der Weijden and Pacheco 2006; Ordens 2007; Cachada et al. 2012; Inácio et al. 2014; Reis et al. 2015; Neves 2015; Patinha et al. 2015; Cabral Pinto et al. 2017a, b, 2018).
The Estarreja municipality has about 26,997 inhabitants (INE 2015) and is in the northern inland margin of the Aveiro lagoon (locally known as “Ria de Aveiro”), a shallow coastal lagoon of the western Portuguese coast and a protected ecosystem. The Aveiro Lagoon has unique environmental, cultural and socio-economic features facing increasing pressures and changes that put its ecological balance and heritage at risk (Lillebø et al. 2015). It is an area of large wetlands and lagoons with a low altitude and a flat topography. It has high environmental and economic importance, and intense touristic, agricultural, fishing and industrial activities. It provides wintering areas for aquatic birds and has been classified as Special Protection Area under the Council Directive 2009/147/EC on the conservation of wild birds. Recently, Brower et al. (2018) have conducted a groundwater protection evaluation study in the Aveiro Lagoon area, which highlights the economic importance of the region’s groundwater resources according to its quality.
Hydrogeological Characterization
The study area is part of the Aveiro Quaternary aquifer (AQA) (INAG 2011), and the shallow multilayer aquifer system has been described in detail by Ordens (2007) and Neves (2015). It is over 650 km2 and is an integral part of the Lower Vouga sedimentary basin. The AQA consists of unconsolidated deposits of sand and gravel, with interlayered mud and fine silt lenses, deposited by fluvial and eolian processes. It is composed of two aquifer layers separated by a low permeability aquitard, which may locally confine or semi-confine the lower part of the aquifer system.
The AQA’s natural baseline composition (Condesso de Melo and Marques da Silva 2008) is determined by the infiltration of rainwater of Atlantic origin, the reaction with aquifer matrix minerals and active redox processes. Human-related contamination (mainly nitrates, heavy metals and organic compounds) can be detected all over the extent of the shallow unconfined aquifer, where aerobic conditions prevail. The deeper semi-confined aquifer layer presents reducing conditions, which attenuate the high nitrate concentrations but can increase As, Fe and Mn concentrations. The study area is highly vulnerable to groundwater contamination due to the high permeability of the sediments, the reduced thickness of the unsaturated zone, the flat topography and high rates of groundwater recharge (> 300–400 mm/year). The groundwater flow is in general from east to west, crossing the ECC-related contamination sources before reaching the agricultural fields and streams/trenches to the west of the ECC (Fig. 1).
Methodologies
Groundwater Sampling
The two groundwater-sampling campaigns included in this study to characterize the temporal and spatial variability of the groundwater quality are part of large research projects aiming at characterizing the hydrogeochemistry of the study area, and consequently inferring aquifer contamination and attenuation processes in the surroundings of ECC. The campaigns were carried out in May 2006 and May 2013, and a total of 29 and 22 groundwater samples (Fig. 1b), respectively, were collected. The groundwater samples have been collected along the day at different times and the equipment does the automatic compensation for the different temperatures measured. The sampling procedure included measurements of physico-chemical field parameters (temperature, pH, electrical conductivity (EC), redox potential (Eh) and dissolved oxygen (DO) concentration) in a flow cell using HANNA instruments. Stabilization of physico-chemical parameters means that these parameters do not change over time. In practical terms, it means that all the water contained in the borehole has been flushed out, and water from the aquifer is flowing and the groundwater sample is representative of the aquifer formation. After the stabilization of the field parameters, a 100-mL volume water sample was titrated for on-field alkalinity analysis with a HACH alkalinity kit and two other water samples were collected and filtered through a 0.45 µm membrane for major, minor and trace elements by ICP-MS and ion chromatography at the Activation Laboratories (Ontario, Canada) which are ISO 17025 accredited and certified to ISO 9001: 2008. Analytical blanks and potential instrumental drifts were carefully monitored and instrument standardization and reproducibility were performed with certified standard reference materials from the National Institute of Standards and Technology (NIST 1643 and SLRS-5 for ICP). QA/QC results within an error of less than 2% indicated that the analytical instruments were operating within pre-defined specifications. The sampling and analyses methodologies were identical for both campaigns, and the same Laboratory was used.
The sampling design was based on a geophysical campaign conducted to map the spatial distribution of the groundwater contamination plume (Ordens et al. 2007). Local residents/farmers and ERASE administrators were engaged for a preliminary assessment of types and levels of contamination throughout the area, and for identifying sampling points. This engagement process was critical for local knowledge-gaining on the hydrogeology and contamination history, and securing access to sampling wells, which is a process strongly advocated under the concept of socio-hydrogeology (Re 2015; Re et al. 2017). The groundwater-sampling points included ERASE monitoring wells, irrigation wells and domestic-use wells.
Hair and Urine Sampling and Analysis
Human biomonitoring, defined as “the method for assessing human exposure to chemicals or their effects by measuring these chemicals, their metabolites or reaction products in human specimens”, involves the measurement of biomarkers in different body fluids (e.g. blood, urine, breast milk) or tissues (e.g. hair, nails) (WHO 2015). The biomonitoring programme was conducted from January to September 2013 in 75 (hair) and in 103 (urine) permanent residents (> 55 years old) living in or around the city of Estarreja. Participants were recruited to participate through Private Institutions of Social Solidarity. This included collecting samples in different localities of the Estarreja municipality: Avanca, Beduído, Canelas, Estarreja, Pardilhó, Veiros, and Salreu. The intention was to sample potentially exposed population groups with different degrees of vulnerability to contaminants. The participants were clearly informed about the aims of the study and those who have agreed to participate gave their written consent. Ethical approval for this study was obtained from the National Committee for Data Protection (No. 11726/2017).
Hair
Hair samples (ranging from 100 to 300 mg) were collected near the scalp, according to the international recommendations (Mayo Clinic Laboratories 2018). Only hair samples with their natural colour and from individuals residing permanently in the study area were considered for analysis. Following a procedure reported elsewhere (Bass et al. 2001), hair were duly washed to completely remove exogenous contamination without significantly altering endogenous trace elements content in the sample. After washing, samples were dried in a laboratory drying oven (Raypa, Barcelona, Spain) at 95 °C for 40 h (the time required to achieve a constant weight). Dried samples (~ 0.1 mg) were mineralized through a microwave-assisted acid digestion procedure with 1 ml of concentrated HNO3 (> 65% m/m; TraceSELECT®, Honeywell, Fluka, Seelze, Germany) and 0.5 ml of H2O2 (≥ 30% v/v; TraceSELECT®, Fluka, Buchs, Switzerland) in a Milestone, mls 120 mega, Sorisole, Italy, high-performance microwave digestion unit equipped with an HPR 1000/10 rotor. The following microwave oven programme (W/min) was used: 250/1, 0/2, 250/5, 400/5 and 600/5. After cooling, sample solutions were made up to 8.5 mL with ultrapure water and stored in closed propylene tubes at 4 °C until analysis. Ultrapure water (at 25 °C the resistivity value is 18.2 MΩ cm) produced in aarium® pro (Sartorius, Gottingen, Germany) water purification system was used throughout the work. All the lab ware was duly decontaminated by 24 h immersion in a 10% HNO3 bath and thoroughly rinsing with ultrapure water. A sample blank was prepared in each digestion run (10 samples). Average blank levels were subtracted from the samples values. For analytical quality control, the certified reference material (CRM) ERM-DB001 (Joint Research Centre, Institute for Reference Materials and Measurements (IRMM), Geel, Belgium)—human hair was used, acid digested using the same procedure.
Trace elements’ concentrations were determined through Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) using a Thermo Fisher Scientific (Waltham, MA) iCAP™ Q instrument, equipped with standard components and accessories: a MicroMist™ nebulizer (Glass Expansion, Port Melbourne, Australia), a Peltier-cooled baffled cyclonic spray chamber, a standard quartz torch and a two-cone interface design (nickel sample and skimmer cones). High-purity argon (99.9997%; Gasin, Leça da Palmeira, Portugal) was used as the nebulizer and plasma gas. Before each analytical series, the ICP-MS instrument was tuned for maximum sensitivity and signal stability while keeping the formation of oxides and double-charged ions to a minimum. Commercially available multi-element standard solutions (PlasmaCAL, SCP Science, Baie D’Urfé, Canada) were used to prepare calibration standards. Internal standard solution was prepared from Isostandards Material (Madrid, Spain).
The limits of detection were calculated as the concentration corresponding to three times the standard deviation of 10 replicate measurements of the blank solution (2% v/v HNO3).
Urine
Urine samples were collected in polyethylene containers and stored at − 20 °C until analysis. All reagents used were of trace analysis grade or equivalent. All aqueous solutions were prepared using ultrapure water. Urine samples were defrosted 24 h before the analysis and 10-fold diluted with 1% v/v HNO3 for elemental analysis of 7 PTEs (Al, As, Cd, Ni, Hg, Pb, and Zn) using a Thermo (Waltham, MA) X-series inductively coupled plasma-mass spectrometry (ICP-MS) instrument. Samples with concentrations above 200 µg L−1 were reanalysed after further 10x dilution. Samples with extremely high concentrations were also analysed by inductively coupled plasma-mass spectrometry-optical emission spectrometry (ICP-OES) using a Horiba JobinYvon Activa M instrument although the results were not significantly different. Freeze-dried human urine Seronorm™ Trace Elements were used in experimental studies on the validation of the analytical procedure used for PTE quantification in urine samples. This material was also analysed during each analytical run as a quality control (QC) sample. Results were well within the acceptable range for all metals. Urinary data are usually adjusted to a constant creatinine concentration to correct for factors unrelated to exposure, particularly the variable dilutions among spot samples (Barr et al. 2004). Hence, the results of urine samples (µg L−1) were adjusted to creatinine (g L−1) and reported as µg g−1. The creatinine was analysed in AvLab commercial laboratory, in the city of Aveiro, Portugal and the analysis of metal(loid)s were performed in Central Laboratory of Analysis of University of Aveiro, Portugal.
Risk Assessment Model
The non-carcinogenic and carcinogenic risks for residents of the ECC surroundings were estimated according the United States Environmental Protection Agency methodology (USEPA 2001, 2011). The equations used to determine the exposure to toxic elements via ingestion and dermal contact are as follows:
where CDI denotes the chronic daily intake (mg.kg−1 day−1); C denotes the concentration of chemical in groundwater (mg L−1); IR denotes the ingestion rate of groundwater (L.day−1)—in the studied region, most households have public water supply by ADRA (2019), and the water quality is according to the Portuguese legislation (Portuguese Decree-Law 152/2017), as such groundwater is not usually used for drinking as the population is aware of potential risks due to contamination, but it is still used in many households for cooking, and for this reason, it was considered a lower daily ingestion rate of 0.15 L day−1; EF: Exposure Frequency (250 days year−1); ED: exposure duration, and it was assumed that a resident will spend half of his/her life exposed, so it was assumed 35 years; BW: body weight (70 kg); AT: averaging time (35 years × 250 days); SA: skin surface area available for contact (19652 cm2); EV: the exposure time (0.2 h/day), AF: skin adherence factor (0.07 mg.cm−2); ABS: dermal absorption factor (0.03 for As and 0.001 for other metals); CF; conversion factor (10−6 kg mg−1).
The human health risk caused by exposure to PTEs is expressed as hazard quotient (HQ) = ADD/RfD. The ADD is the average daily dose to which a person is exposed. RfD is the reference dose which is the daily dosage that enables the exposed individual to sustain this level (USDE 2013). The HI is the chronic hazard index that is the sum of HQs for multiple exposure pathways. When HI values > 1, there is a chance that non-carcinogenic risk may occur; otherwise, HI < I the opposite applies. The carcinogenic risks were calculated for As exposure of ECC surrounding population, according to the Exposure Factors Handbook (USEPA 2011) and using the oral slope factor of 1.5. For carcinogens in drinking water, the USEPA considers risk levels of up to 10−6 to be protective limit of human health (USEPA 2010). The USEPA accepts cancer risk policies from states in the range of 10−6 to 10−4 (USEPA 1992). USEPA has not derived a reference dose for Al; therefore, the human health risk assessment was not performed for this metal.
Statistical Techniques
The Kruskal–Wallis test was used to compare the values of Al, As, Cd, Hg, Ni, Pb and Zn between the different sampling campaigns [2006, 2010 (Neves 2015) and 2013] in which measurements were made due to the large deviations to normality observed in the distribution of the measurements at each of the moments of analysis, as evidenced by the test of Shapiro–Wilk. Whenever statistically significant differences were found between the three moments, post hoc tests were applied to identify pairs that differed from each other. These comparisons were made using the Mann–Whitney test and subsequent application of the Bonferroni correction and are presented in the Supplementary file (Table S1).
The Kruskal–Wallis test was also used to compare the values of PTEs in hair and in urine between the residents of the different localities in which measurements were made due to the large deviations to normality observed in the distribution of the measurements at each of the moments of analysis, as evidenced by the test of Shapiro–Wilk. Whenever statistically significant differences were found between the seven localities, post hoc tests were applied to identify pairs that differed from each other. These comparisons were made using the Mann–Whitney test and subsequent application of the Bonferroni correction and are presented in the Supplementary file (Tables S2 and S3). The software used was SPSS, version 25.
Results and Discussion
Groundwater PTE Concentrations
Table 1 (i) summarizes groundwater quality results for the samples collected in 2006 and 2013, and for the samples collected by Neves (2015) in 2010; (ii) shows the AQA’s natural baseline APA (2012); and, (iii) shows drinking water values form WHO guidelines (2011), USEPA (2009) and Portuguese legislation values (PV) (Portuguese Decree-Law 152/2017), which are only available for selected elements. WHO (2011) and EPA (2009) do not provide guideline values for Al and Zn. Nonetheless, the Portuguese legislation presents 200 μg L−1 for Al for reference.
PTE concentrations are well above the standards for human consumption provided by WHO (2011), USEPA (2009) and PV (2017), except for Pb (Table 1), for all sampling campaigns. These concentrations are well above natural background values for the aquifer (Table 1, NB), and have been shown to be associated with contaminant activities from ECC (Ordens 2007). Means and maximum values were often one–two orders of magnitude higher than the guidelines values, namely for Al, As, Cd, Hg and Zn. However, median values are significantly lower than mean levels, in most cases falling below human consumption standards. It is worth noting that the large difference between mean and median values found for some PTEs is due to a significant number of samples collected in low- or non-contaminated areas, which skews the median for lower values. According to Table 1, the percentage of samples above WHO (2011) guideline values is high: Al (48), As (35), Cd (21), Ni (31), Pb (10) and Zn (52) in 2006; Al (66), As (24), Cd (15), Ni (18) and Zn (36) in 2010; and, Al (26), As (37) and Zn (16) in 2013. Figure 2 shows the samples with PTE concentrations above WHO (2011) guideline values. All percentages are below 50%, with the exception of Zn and Al in 2006 and 2010, respectively. Highly variable contamination levels are due to complex hydrogeochemical processes occurring in the area, location/type of contaminant discharge to the environment, and the temporal evolution of contaminant activities (and attenuation processes).
Sample locations with PTE concentrations above WHO (2011) guideline values
Results show a decrease in contamination from 2006 to 2013, for some elements of up to one order of magnitude. This is particularly evident looking at the percentage of samples above WHO (2011) guideline values (Table 1), which, however, remain high for Al, As and Zn in 2013. Only the chemical elements Cd, Pb and Zn do not show significant differences (Kruskal–Wallis p ≥ 0.05) between the sampling campaigns (supplementary Table S1); all the other studied PTEs show significant differences (Kruskal–Wallis p < 0.05) between campaigns, particularly between the first and the last one (Table S1). This seems to indicate that groundwater contamination in the study area is decreasing, which is most probably related to minimization measures that occurred in the mid-2000s (ERASE 2000). A seven-year interval gives an indication of the changing contamination conditions in the local aquifer. Further studies and new sampling campaigns in the area are urgently needed to assess whether the contamination levels are decreasing to more acceptable levels, and what are the current risks for humans and for the environment in the ECC surroundings. These studies will also assist in identifying possible PTE sources, transport processes controlling the contamination spatial–temporal evolution in the aquifer and fates of elements of concern, as all these factors impact human exposure risk. Nonetheless, this is important to assess contamination conditions that have a potential impact for human health.
Hair PTE Concentrations
The use of hair for biomonitoring purposes offers the possibility to integrate a relatively long-term exposure in a single specimen analysis, reflecting exposures occurring in the last months (WHO 2015). Hair PTE concentrations (µg g−1) in Estarreja inhabitants according to their residence are shown in Table 2. Reference concentrations (median and minimum and maximum) available from the study of Rodushkin and Axelsson (2000) for non-exposed people are also shown in Table 2. Figure 3 shows the ratio of PTE hair content in subjects to reference values, per locality and for median values.
Ratio of hair potentially toxic elements (PTE) concentrations of Estarreja inhabitants (according to their residence) and hair PTE reference concentrations (median) for non-exposed people from Rodushkin and Axelsson (2000)
In general, hair trace elements’ median contents in this study with an environmentally exposed population fall within the range of values reported for non-exposed people, except for Hg (Table 2). Pardilhó, Beduído and Veiros’ inhabitants show higher levels of Hg in hair (3–6 times higher than that of non-exposed people) than inhabitants from Canelas and Salreu (p < 0.05, Table S2). These three localities are closer to ECC and more exposed to contaminated groundwater than Canelas and Salreu. Maximum values of As from inhabitants of Beduído and Veiros were above the maximum values found for non-exposed populations (Table 2). When observing median values, As hair contents are around 10 times higher than that for non-exposed people across the board (Fig. 3). Maximum Zn levels were also above the maximum contents found for non-exposed populations across the board (Table 2). The PTE body burden of the study group suggests a potential environmental exposure related to the ECC (Table 2 and Fig. 3).
Urine PTE Concentrations
Concentrations of different PTE in urine may provide valuable information on exposure within different time spans, thus helping to better assess potential health effects of contamination (Cabral Pinto et al. 2017a). Most PTE are excreted via the kidney in the urine, and to a much lesser extent by the gastrointestinal tract (Dorne et al. 2011). Summary statistics (median (50th percentile) and the 5th to 95th percentiles (P5–P95) values for PTE content in urine samples of residents in the different localities within Estarreja region are shown in Table 3. Table 3 also presents the same statistical values for healthy people available from a comprehensive study of Goullé et al. (2005). Wide ranges in urine element concentrations can be found in the literature (e.g. Goullé et al. 2005; Kazi et al. 2008; Kuiper et al. 2014), and as such, we found appropriate to compare the range and not only median values. Participants’ PTE urinary levels largely exceed those reported in the literature for healthy people for all the studied elements, with Al, Cd and Zn being the elements that show urinary median levels out of P5–P95 reference values from the study of Goullé et al. (2005). Remarkably, P95 PTE concentrations are around ten times higher than median concentrations, except for Al that is hundred times higher. This is an indication that there is a large range of PTE urinary concentrations in people exposed to contamination, and the sampling campaign covered exposed and non-exposed subjects (and locations). Interestingly, P5 values for the studied population are generally similar or slightly above those of healthy people from Goullé et al. (2005), while P95 are 2–53 times above for most elements and 3300 for Al, which is also an indication of the wide variety of PTE urinary concentrations in the sampled subjects.
Figure 4 shows the ratio of PTE urinary content in subjects to reference value per locality, using medians. When analysing PTE urinary concentrations per locality within the Estarreja region (Table 3 and Fig. 4), a large discrepancy can be observed, in which medians and P5–P95 PTE urinary concentrations from the study subjects and the values of Goullé et al. (2005) for healthy people are shown. Beduído and Veiros inhabitants have very high urinary contents for all PTEs. This is not surprising, as reported before, they are the ones closer to ECC and more exposed to contaminated groundwater due to the groundwater flow direction (Ordens 2007). Pardilhó, Estarreja and Avanca subjects also show high levels of contamination. Canelas inhabitants show much lower levels of PTEs, particularly As and Hg (p < 0.05), in urine than other subjects for most of chemical elements, and are more similar to those reported by Goullé et al. (2005) for healthy people, which indicate that those inhabitants are exposed to much lower contamination levels. In fact, these localities are not directly affected by the contamination from ECC. The exceptions are Cd in Canelas, and Al in Salreu and Canelas inhabitants, suggesting that the PTEs’ enrichment in the urine could not be only affected by groundwater, but could also result from other sources (e.g. air, soils, road dust and diet). Interestingly, these concentrations are very high across almost all localities. Canelas and Salreu inhabitants show As levels below reference values, which is a PTE of major concern in the area and with special high concentrations in Beduído inhabitants.
Ratio of urinary potentially toxic elements (PTE) concentrations of Estarreja inhabitants (according to their residence) to urinary PTE reference concentrations (median) for healthy people from Goullét et al. (2005)
Risk Assessment—Cancer and Non-cancer Risks
The results of the non-carcinogenic and carcinogenic health risks due to PTE exposure in groundwater collected in domestic-use wells in the Estarreja area are provided in Figs. 5 and 6.
Non-cancer Risk
Non-cancer risk is associated with long-term health problems (e.g. neurological or cardiovascular diseases). HI values are always lower than 1 for Ni and Pb, implying non-cancer low risk. Figure 5 shows the spatial and temporal evolutions of HI for Al, As, Cd and Hg. In 2006, these four PTEs are higher than 1 in some collected points, implying non-cancer high risk. In 2010 and 2013, Al and Cd concentrations do not imply risk. It can be noted that the non-cancer risk values for all PTEs decreased temporally and spatially, decreasing as the distance of the ECC increases. However, As and Hg maintained a high (> 1) risk in 2013. Therefore, the non-carcinogenic health risks via dermal and oral routes of groundwater from domestic-use wells were not within USEPA’s acceptable risks.
Cancer Risk
Figure 6 shows the spatial and temporal evolutions of cancer risk for As. It can be noted that the cancer risk values decreased temporally and spatially, tending to decrease as the distance of the chemical complex increases. However, the risk associated with exposure to groundwater falls out of the threshold values above which environmental and regulatory agencies consider the risk unacceptable, in all sampling campaigns.
Integrative Discussion of PTE Content in Groundwater, Hair, Urine and Cancer and Non-cancer Risks
PTE contents in hair and urine show a clear enrichment in As, Hg and Zn, particularly in the localities where the groundwater quality was directly affected by the ECC (Tables 1, 2, and 3). Considering that As, Hg, and Zn contents in hair and urine have been suggested as being effective biomarkers of environmental exposure (e.g. Bencko 1995; Mortada et al. 2002; Morton et al. 2004; Amaral et al. 2008; Gault et al. 2008; Cabral Pinto et al. 2017a), these high PTE contents observed in Veiros, Beduído, Avanca, Estarreja and Pardilhó residents’ hair indicate environmental exposure. Arsenic was the element showing higher non-cancer risk and high cancer risk in the region in all sampling campaigns (Figs. 5 and 6), which is compatible with high groundwater contamination levels, and hair and urine concentrations (above non-exposed and healthy populations) (Tables 1, 2, and 3). Mercury also shows high non-cancer risk in all sampling campaigns, which is in agreement with high groundwater contamination levels, and hair and urine high contents (Tables 1, 2, and 3). WHO (2011) and EPA (2009) and PV (2017) do not provide Zn limits for drinking water and suggest that Zn has no negative health impact in humans. Zinc shows very high concentration in groundwater, particularly in the two first campaigns, and in hair and urine samples (Tables 1, 2 and 3), and results in non-cancer risk in a sample collected from a well in 2006 (Fig. 5).
In contrast to hair, urinary concentrations of Al and Ni, elements with short half-lives, were elevated relatively to typical ranges for healthy people (Goullé et al. 2005), and groundwater concentrations for these elements are above WHO (2011), EPA (2009) and Portuguese DV (2017) in all sampling campaigns (Tables 1, 2, and 3). However, the health risk assessment was not calculated for Al because USEPA has not derived a reference dose for this element. Nickel shows no non-cancer risk, and their concentrations in groundwater and urine, although high, are not as elevated as other PTEs.
Urinary concentrations of Cd, and to a lesser extent Pb, were elevated relatively to typical ranges reported for healthy people by Goullé et al. (2005), even though recent groundwater concentrations were not above the guidelines for these elements. This could suggest that the enrichment of Cd and Pb in the urine could not be only influenced by groundwater, but could also result from other sources, such air, soils, cigarette fumes, exhaust from vehicles and diet. However, long-term exposure to contaminated groundwater could still be reflected in urine samples because Cd and Pb have long biological half-lives (Nriagu 2007; Dorne et al. 2011). Interestingly, of these elements, only Cd shows non-cancer risk in a sample from a well collected in 2006. For example, although Cd concentrations in groundwater samples were relatively low (below the permissible values), especially in the more recent sampling campaigns, a long-term exposure to Cd-containing water could still be reflected in urine samples because Cd has a long biological half-life and accumulates over time in the human body. Therefore, urine is likely to be a suitable specimen to evaluate short- and long-term PTE exposures, both for elements with low- and high-excretion rates.
Conclusions
The inter-disciplinary approach applied in this study comprising evaluation of groundwater contamination, calculation of cancer and non-cancer risks, and determination of contaminants concentration in inhabitants’ hair and urine was successful in identifying trends and links between the different datasets, and consequently in investigating the potential effects of groundwater contamination on human health in ECC’s surroundings. The local population strongly depends on agriculture and groundwater for their livelihood. The analysis of groundwater, hair and urinary PTE concentrations, and the calculation of cancer and non-cancer risks in the region of Estarreja show high health risks for the local population, mainly due to exposure to As. The most groundwater-contaminated areas generally coincided with the localities (Veiros, Beduido, and Pardilhó) in which inhabitants exhibit higher hair and urinary As, Hg and Zn contents, and localities not affected by ECC-related groundwater contamination are the ones with lower hair and urinary PTE concentrations (Canelas, Salreu). It is likely that PTE-elevated hair and urinary concentrations are related to the ingestion and dermal contact with contaminated groundwater, although this assumption requires further investigation, including that on other potential sources of contaminants for the local population, such as dietary intake of foodstuffs exposed to contaminated groundwater. This correlates well with cancer and non-cancer risks for As in groundwater in ECC surroundings, showing that the health risk to the local population is high, particularly due to exposure to As.
Despite contamination remediation measures from the early 2000s, ECC surroundings remain a highly contaminated area, especially in As, and a serious concern for the environment and for human health. There is potential scope for further research in the study area in aspects that there were beyond the scope of this manuscript. This can include evaluation of the risks associated with dietary intake of products (e.g. vegetables) exposed to contaminated groundwater; evaluation of groundwater contamination and attenuation processes, including the current state of aquifer contamination; and the incidence of cancer and non-cancer diseases in the region (and how they may relate to groundwater contamination). The results of this study can be used by environmental and health authorities to better manage the complex situation created by groundwater contamination in Estarreja, which would require a joint effort and not isolated measures.
References
ADRA (2019) Plano de Segurança de Abastecimento de Água. https://adra.pt/template-simples/4573/plano-de-seguran%C3%A7a-da-%C3%A1gua. Accessed 11 Jan 2019
Ahlskog JE (2016) New and appropriate goals for Parkinson disease physical therapy. JAMA Neurol 73(3):1–2
Alavanja MC, Hoppin JA, Kamel F (2004) Health effects of chronic pesticide exposure: cancer and neurotoxicity. Annu Rev Public Health 25:155–197
Alimonty A, Mattei D (2008) Biomarkers for human biomonitoring. In: Conti ME (ed) Biological monitoring: theory and applications: bioindicators and biomarkers for environmental quality and human exposure assessment. WIT Press, Boston
Amaral AF, Arruda M, Cabral S, Rodrigues AS (2008) Essential and non-essential trace metals in scalp hair of men chronically exposed to volcanogenic metals in the Azores, Portugal. Environ Int 34(8):1104–1108
APA (2012) Planos de Gestão das Bacias Hidrográficas dos Rios Vouga, Mondego e Lis integradas na Região Hidrográfica 4. Parte 2—Caracterização Geral e Diagnóstico. 1.4.2. Caracterização das Massas de Água Subterrânea. Report. Ex-ARH Centro. https://www.apambiente.pt/?ref=16&subref=7&sub2ref=9&sub3ref=834. Accessed 10 Sept 2018
Ayodele JT, Bayero AS (2009) Lead and zinc concentrations in hair and nail of some Kano inhabitants. Afr J Environ Sci Technol 3(6):164–170
Barradas JM, Cardoso Fonseca E, Ferreira da Silva EA, Garcia Pereira H (1992) Identification and mapping of pollution indices using a multivariate statistical methodology, Estarreja, central Portugal. Appl Geochem 7(6):563–572
Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL (2004) Urinary creatinine concentrations in the US population: implications for urinary biologic monitoring measurements. Environ Health Perspect 113(2):192–200
Bass DA, Hickok D, Quig D, Urek K (2001) Trace element analysis in hair: factors determining accuracy, precision, and reliability. Altern Med Rev 6(5):472–481
Bellotti BA (2017) Research agenda for food systems. The Global Change Institute, University of Queensland Avaiable at: https://gci.uq.edu.au/filething/get/14491/Discussion-Paper-food-systems-No1-V7-4OCT2017-FINAL-LR.pdf. Accessed 3 May 2018
Bencko V (1995) Use of human hair as a biomarker in the assessment of exposure to pollutants in occupational and environmental settings. Toxicology 101(1–2):29–39
Borrego C (1993) Water, air and soil pollution problems in Portugal. Sci Total Environ 129(1):55–70
Bouchard DC, Williams MK, Surampalli RY (1992) Nitrate contamination of groundwater: sources and potential health effects. J Am Water Works Assoc 84(9):85–90
Bouchard MF, Sauvé S, Barbeau B, Legrand M, Brodeur MÈ, Bouffard T, Mergler D (2011) Intellectual impairment in school-age children exposed to manganese from drinking water. Environ Health Perspect 119(1):138
Brima EI, Haris PI, Jenkins RO, Polya DA, Gault AG, Harrington CF (2006) Understanding arsenic metabolism through a comparative study of arsenic levels in the urine, hair and fingernails of healthy volunteers from three unexposed ethnic groups in the United Kingdom. Toxicol Appl Pharmacol 216(1):122–130
Brower R, Ordens CM, Pinto R, Condesso de Melo MT (2018) Economic valuation of groundwater protection using a groundwater quality ladder based on chemical threshold levels. Ecol Ind 88:292–304
Cabral Pinto MMS, Ferreira da Silva E (2019) Heavy Metals of Santiago Island (Cape Verde) alluvial deposits: baseline value maps and human health risk assessment. Int J Environ Res Health. https://doi.org/10.3390/ijerph16010002
Cabral Pinto MMS, Ferreira da Silva E, Silva MMVG, Melo-Gonçalves P, Candeias C (2014) Environmental risk assessment based on high-resolution spatial maps of potential toxic elements sampled on stream sediments of Santiago, Cape Verde. Geosciences (Switzerland), vol 4, pp 297–315, MDPI. https://doi.org/10.3390/geosciences4040297
Cabral Pinto MMS, Marinho-Reis AP, Almeida A, Ordens CM., Silva MM, Freitas S, Ferreira da Silva EA (2017a) Human predisposition to cognitive impairment and its relation with environmental exposure to potentially toxic elements. Environ Geochem Health 1–18. https://doi.org/10.1007/s10653-017-9928-3
Cabral Pinto MMS, Silva MMVG, Ferreira da Silva EA, Marinho-Reis P (2017b) The Cancer and Non-Cancer Risk of Santiago Island (Cape Verde) Population due to Potential Toxic Elements Exposure from Soils. Urban Environmental and Medical Geochemistry, Geosciences (Switzerland). https://doi.org/10.3390/geosciences7030078
Cabral Pinto MMS, Marinho-Reis AP, Almeida A, Freitas S, Simões MR, Diniz ML, Moreira PI (2018) Fingernail trace element content in environmentally exposed individuals and its influence on their cognitive status in ageing. Exposure Health 1–14
Cachada A, Pereira ME, Ferreira da Silva E, Duarte AC (2012) Sources of potentially toxic elements and organic pollutants in an urban area subjected to an industrial impact. Environ Monit Assess 184:15–32
Callahan MA, Sexton K (2007) If cumulative risk assessment is the answer, what is the question? Environ Health Perspect 115(5):799
Castro IV, Ferreira EM, McGrath SP (1997) Effectiveness and genetic diversity of Rhizobium leguminosarum bv. trifolii isolates in Portuguese soils polluted by industrial effluents. Soil Biol Biochem 29(8):1209–1213
Centeno JA, Mullick FG, Martinez L, Page NP, Gibb H, Longfellow D, Ladich ER (2002) Pathology related to chronic arsenic exposure. Environ Health Perspect 110(Suppl 5):883
Charlet L, Polya DA (2006) Arsenic in shallow, reducing groundwaters in southern Asia: an environmental health disaster. Elements 2(2):91–96
Chin-Chan M, Navarro-Yepes J, Quintanilla-Vega B (2015) Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front Cell Neurosci 9:124
Coelho P, Costa S, Costa C, Silva S, Walter A, Ranville J et al (2012) Metal(loid)s levels in biological matrices from human populations exposed to mining contamination. J Toxicol Environ Health A 75(13–15):893–908. https://doi.org/10.1080/15287394.2012.690705
Coelho P, Costa S, Costa C, Silva S, Walter A, Ranville J, Zoffoli R (2014) Biomonitoring of several toxic metal (loid) s in different biological matrices from environmentally and occupationally exposed populations from Panasqueira mine area, Portugal. Environ Geochem Health 36(2):255–269
Condesso de Melo MT, Marques da Silva MA (2008) The Aveiro Quaternary and Cretaceous aquifers. In: Edmunds WM, Shand P (eds) The natural baseline quality of groundwater. Blackwell Publishers, Oxford
Costa C, Jesus-Rydin C (2001) Site investigation on heavy metals contaminated ground in Estarreja—Portugal. Eng Geol 60(1–4):39–47
Dissanayake CB, Chandrajith R (2009) Phosphate mineral fertilizers, trace metals and human health. J Natl Sci Found Sri Lanka 37(3):153–165
Dorne JL, Kass GE, Bordajandi LR, Amzal B, Bertelsen U, Castoldi AF et al (2011) Human risk assessment of heavy metals: principles and applications. Metal Ions Life Sci 8:27–60
Eastman RR, Jursa TP, Benedetti C, Lucchini RG, Smith DR (2013) Hair as a biomarker of environmental manganese exposure. Environ Sci Technol 47(3):1629–1637
Eggers MJ, Doyle JT, Lefthand MJ, Young SL, Moore-Nall AL, Kindness L, Ford TE, Dietrich E, Parker AE, Hoover JH, Camper AK (2018) Community engaged cumulative risk assessment of exposure to in Putzrath well water contaminants, Crow Reservation, Montana. Int J Environ Res Public Health 15(1):76. https://doi.org/10.3390/ijerph15010076
Elsner RJ, Spangler JG (2005) Neurotoxicity of inhaled manganese: public health danger in the shower? Med Hypotheses 65(3):607–616
EPA (2009) http://esdat.net/Environmental%20Standards/US/Federal/US%20Federal%20MLCs.pdf. Accessed 10 May 2017
ERASE (2000) Estratégia de redução dos impactes ambientais associados aos resíduos industriais depositados no Complexo Químico de Estarreja. Estudo de impacte ambiental, memória geral. Aveiro, Portugal
Ericson I, Martı-Cid R, Nadal M, Van Bavel B, Lindström G, Domingo JL (2008) Human exposure to perfluorinated chemicals through the diet: Intake of perfluorinated compounds in foods from the Catalan (Spain) market. J Agric Food Chem 56(5):1787–1794
Exley C (2012) The coordination chemistry of aluminium in neurodegenerative disease. Coord Chem Rev 256(19):2142–2146
Flynn MR, Susi P (2009) Neurological risks associated with manganese exposure from welding operations–a literature review. Int J Hyg Environ Health 212(5):459–469
Frampton MW (2001) Systemic and cardiovascular effects of airway injury and inflammation: ultrafine particle exposure in humans. Environ Health Perspect 109(Suppl 4):529
Gault AG, Rowland HA, Charnock JM, Wogelius RA, Gomez-Morilla I, Vong S, Polya DA (2008) Arsenic in hair and nails of individuals exposed to arsenic-rich groundwaters in Kandal province, Cambodia. Sci Total Environ 393(1):168–176
Golash N, Gogate PR (2012) Degradation of dichlorvos containing wastewaters using sonochemical reactors. Ultrason Sonochem 19(5):1051–1060
Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Kortsha GG et al (1999) Occupational exposure to manganese, copper, lead, iron, mercury and zinc and the risk of Parkinson’s disease. Neurotoxicology 20:239–248
Goullé JP, Mahieu L, Castermant J, Neveu N, Bonneau L, Lainé G, Lacroix C (2005) Metal and metalloid multi-elementary ICP-MS validation in whole blood, plasma, urine and hair: reference values. Forensic Sci Int 153(1):39–44
Gupta VB, Anitha S, Hegde ML, Zecca L, Garruto RM, Ravid R et al (2005) Aluminium in Alzheimer’s disease: are we still at a crossroad? Cell Mol Life Sci 62(2):143–158
Hall A, Costa Duarte A, Caldeira MTM, Lucas MF (1987) Sources and sinks of mercury in the coastal lagoon of Aveiro, Portugal. Sci Total Environ 64:75–87
Hammond RA, Dubé L (2012) A systems science perspective and transdisciplinary models for food and nutrition security. Proc Natl Acad Sci 109(31):12356–12363
Hopenhayn-Rich C, Biggs ML, Smith AH (1998) Lung and kidney cancer mortality associated with arsenic in drinking water in Cordoba, Argentina. Int J Epidemiol 27(4):561–569
Iarmarcovai G, Sari-Minodier I, Chaspoul F, Botta C, De Meo M, Orsiere T, Botta A (2005) Risk assessment of welders using analysis of eight metals by ICP-MS in blood and urine and DNA damage evaluation by the comet and micronucleus assays; influence of XRCC1 and XRCC3 polymorphisms. Mutagenesis 20(6):425–432
Inácio MM, Pereira V, Pinto MS (1998) Mercury contamination in sandy soils surrounding an industrial emission source (Estarreja, Portugal). Geoderma 85(4):325–339
Inácio M, Neves O, Pereira V, Silva EF (2014) Levels of selected potential harmful elements (PHEs) in soils and vegetables used in diet of the population living in the surroundings of the Estarreja Chemical Complex (Portugal). Appl Geochem 44:38–44
INAG (2011) Sistema Nacional de Informação de Recursos Hídricos – Instituto da Agua. http://snirh.inag.pt/. Accessed 10 May 2015
INE (2015) Statistics Portugal. XV Ressenciamento Geral da População, CENSUS 2001. http://censos.ine.pt/xportal/xmain?xpid=CENSO Sandx pgid=censos_quadros_populacao
Ize-Iyamu OK, Bernard AE (2007) The effects of petroleum exploration and production operations on the heavy metals contents of soil and groundwater in the Niger Delta. Int J Phys Sci 2(10):271–275
Johnson FO, Atchison WD (2009) The role of environmental mercury, lead and pesticide exposure in development of amyotrophic lateral sclerosis. Neurotoxicology 30(5):761–765
Karagas MR, Tosteson TD, Blum J, Morris JS, Baron JA, Klaue B (1998) Design of an epidemiologic study of drinking water arsenic exposure and skin and bladder cancer risk in a US population. Environ Health Perspect 106(Suppl 4):1047
Kavcar P, Sofuoglu A, Sofuoglu SC (2009) A health risk assessment for exposure to trace metals via drinking water ingestion pathway. Int J Hyg Environ Health 212(2):216–227
Kazi TG, Afridi HI, Kazi N, Jamali MK, ArainMB Jalbani N et al (2008) Copper, chromium, manganese, iron, nickel, and zinc levels in biological samples of diabetes mellitus patients. Biol Trace Elem Res 122(1):1–18
Kim JY, Mukherjee S, Ngo LC, Christiani DC (2004) Urinary 8-hydroxy-2′-deoxyguanosine as a biomarker of oxidative DNA damage in workers exposed to fine particulates. Environ Health Perspect 112(6):666
Komatina MM (2004) Medical geology—effects of geological environments on human health. Developments in Earth and Environmental Sciences, vol. 2. Elsevier, Amsterdam
Kuiper N, Rowell C, Nriagu J, Shomar B (2014) What do the trace metal contents of urine and toenail samples from Qatar’s farm workers bioindicate? Environ Res 131:86–94
Leitão TBE (1996) Metodologia para a reabilitação de aquíferos poluídos. Ph.D. thesis. Faculdade de Ciências da Universidade de Lisboa
Lillebø AI, Ameixa OMCC, Sousa LP, Sousa AI, Soares JA, Dolbeth M, Alves FL (2015) The physio-geographical background and ecology of Ria de Aveiro. Coastal lagoons in Europe, 21
Liu QR, Walther D, Drgon T, Polesskaya O, Lesnick TG, Strain KJ, Uhl GR (2005) Human brain derived neurotrophic factor (BDNF) genes, splicing patterns, and assessments of associations with substance abuse and Parkinson’s Disease. Am J Med Genet B 134(1):93–103
Ljunggren KG, Lidums V, Sjögren B (1991) Blood and urine concentrations of aluminium among workers exposed to aluminium flake powders. Occup Environ Med 48(2):106–109
Mayo Clinic Laboratories. https://www.mayocliniclabs.com/ Accessed 28 Sept 2018
McElroy JA, Shafer MM, Trentham-Dietz A, Hampton JM, Newcomb PA (2006) Cadmium exposure and breast cancer risk. J Natl Cancer Inst 98(12):869–873
Mohmand J, Eqani SA, Fasola M, Alamdar A, Mustafa I, Ali N, Shen H (2015) Human exposure to toxic metals via contaminated dust: bio-accumulation trends and their potential risk estimation. Chemosphere 132:142–151
Mortada WI, Sobh MA, El-Defrawy MM, Farahat SE (2002) Reference intervals of cadmium, lead, and mercury in blood, urine, hair, and nails among residents in Mansoura city, Nile delta, Egypt. Environ Res 90(2):104–110
Morton J, Mason HJ, Ritchie KA, White M (2004) Comparison of hair, nails and urine for biological monitoring of low level inorganic mercury exposure in dental workers. Biomarkers 9(1):47–55
Needleman HL, Schell A, Bellinger D, Leviton A, Allred EN (1990) The long-term effects of exposure to low doses of lead in childhood: an 11-year follow-up report. N Engl J Med 322(2):83–88
Neves C (2015) Estudo da contaminação das águas subterrâneas e respectivos processos de atenuação natural na zona industrial de Estarreja. PhD thesis, University of Lisbon
Nriagu G (2007) Zinc toxicity in humans, pp 1–7. Elsevier, New Yrok. https://pdfs.semanticscholar.org/a9e2/8321ae506e646f32ce59d87b7589851aa7e4.pdf. Accessed 26 Aug 2018
Ordens CM (2007) Estudo da contaminação do aquífero superior na região de Estarreja. Unpublished M.Sc. thesis. Coimbra University. http://www.lneg.pt/download/3268/carlos_ordens.pdf. Accessed 11 Mar 2015
Ordens CM, Condesso de Melo MT, Grangeia C, Marques da Silva MA (2007) Groundwater–surface water interactions near a Chemical Complex (Estarreja, Portugal)—implications on groundwater quality. In Proceedings 35th congress of international association of hydrogeologists, Lisbon, Portugal, 17–21 Sept
Panaullah GM, Alam T, Hossain MB, Loeppert RH, Lauren JG, Meisner CA, Duxbury JM (2009) Arsenic toxicity to rice (Oryza sativa L.) in Bangladesh. Plant and Soil 317(1–2):31
Park JD, Choi SJ, Choi BS, Lee CR, Kim H, Kim YD, Chung JH (2016) Arsenic levels in the groundwater of Korea and the urinary excretion among contaminated area. J Expo Sci Environ Epidemiol 26(5):458
Patinha C, Reis AP, Dias AC, Abduljelil AA, Noack Y, Robert S, Ferreira Cave M, da Silva EF (2015) The mobility and human oral bioaccessibility of Zn and Pb in urban dusts of Estarreja (N Portugal). Environ Geochem Health 37(1):115–131
Pereira ME, Duarte AC, Millward GE, Vale C, Abreu SN (1998) Tidal export of particulate mercury from the most contaminated area of Aveiro’s Lagoon, Portugal. Sci Total Environ 213:157–163
Pereira ME, Lillebø AI, Pato P, Válega M, Coelho JP, Lopes C et al (2009) Mercury pollution in Ria de Aveiro(Portugal): a review of the system assessment. Environ Monit Assess 155:39–49
Petersen R, Thomsen JF, Jørgensen NK, Mikkelsen S (2000) Half life of chromium in serum and urine in a former plasma cutter of stainless steel. Occup Environ Med 57(2):140–142
Pham LH, Nguyen HT, Van Tran C, Nguyen HM, Nguyen TH, Tu MB (2017) Arsenic and other trace elements in groundwater and human urine in Ha Nam province, the Northern Vietnam: contamination characteristics and risk assessment. Environ Geochem Health 39(3):517–529
Phan K, Sthiannopkao S, Kim KW, Wong MH, Sao V, Hashim JH, Aljunid SM (2010) Health risk assessment of inorganic arsenic intake of Cambodia residents through groundwater drinking pathway. Water Res 44(19):5777–5788
Portuguese Decree 236 (1998) Portuguese legislation on water quality. Diário da República IA, pp 3676–3722. https://dre.pt/application/conteudo/430457. Accessed 15 Oct 2017
Portuguese Decree Law 152 (2017) Portuguese legislation on water quality. Diário da República IA, pp 5747–5765. https://dre.pt/application/conteudo/114315242. Accessed 10 May 2017
Putzrath RM (2000) Reducing uncertainty of risk estimates for mixtures of chemicals within regulatory constraints. Regul Toxicol Pharmacol 31:44–52
Rahman MF, Wang J, Patterson TA, Saini UT, Robinson BL, Newport GD, Ali SF (2009) Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicol Lett 187(1):15–21
Rapant S, Cvečková V, Fajčíková K, Dietzová Z, Stehlíková B (2017) Chemical composition of groundwater/drinking water and oncological disease mortality in Slovak Republic. Environ Geochem Health 39(1):191–208
Re V (2015) Incorporating the social dimension into hydrogeochemical investigations for rural development: the Bir Al-Nas approach for socio-hydrogeology. Hydrogeol J 23:1293–1304
Re V, Sacchi E, Kammoun S, Tringali C, Trabelsi R, Zouari K, Daniele S (2017) Integrated socio-hydrogeological approach to tackle nitrate contamination in groundwater resources. The case of Grombalia Basin (Tunisia). Sci Total Environ 593:664–676
Reis AP, Costa S, Santos I, Patinha C, Noack Y, Wragg J et al (2015) Investigating relationships between biomarkers of exposure and environmental copper and manganese levels in house dusts from a Portuguese industrial city. Environ Geochem Health 37(4):725–744
Rodushkin I, Axelsson MD (2000) Application of double focusing sector field ICP-MS for multielemental characterization of human hair and nails. Part II. A study of the inhabitants of Northern Sweden. Sci Total Environ 262(1–2):21–36
Rodushkin I, Axelsson MD (2003) Application of double focusing sector field ICP-MS for multielemental characterization of human hair and nails. Part III. Direct analysis by laser ablation. Sci Total Environ 305(1–3):23–39
Ryker SJ, Small MJ (2008) Combining occurrence and toxicity information to identify priorities for drinking water mixture research. Risk Anal Int J 28(3):653–666
Sällsten G, Barregård L, Schütz A (1994) Clearance half life of mercury in urine after the cessation of long term occupational exposure: influence of a chelating agent (DMPS) on excretion of mercury in urine. Occup Environ Med 51(5):337–342
Santamaria AB, Cushing CA, Antonini JM, Finley BL, Mowat FS (2007) State-of-the-science review: does manganese exposure during welding pose a neurological risk? J Toxicol Environ Health B 10(6):417–465
Schneider LS, Tariot PN, Dagerman KS, Davis SM, Hsiao JK, Ismail MS, Sultzer DL (2006) Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 355(15):1525–1538
Schneider P, Löser R, Biali G (2013) Water management at the former copper mining site Medet (Bulgaria). Environ Eng Manag J 12(4):835–841
Slotnick MJ, Nriagu JO (2006) Validity of human nails as a biomarker of arsenic and selenium exposure: a review. Environ Res 102(1):125–139
Sthiannopkao S, Kim KW, Cho KH, Wantala K, Sotham S, Sokuntheara C, Kim JH (2010) Arsenic levels in human hair, Kandal Province, Cambodia: the influences of groundwater arsenic, consumption period, age and gender. Appl Geochem 25(1):81–90
Sun JB, Czerkinsky C, Holmgren J (2010) Mucosally induced immunological tolerance, regulatory T cells and the adjuvant effect by cholera toxin B subunit. Scand J Immunol 71(1):1–11
Tartaglione AM, Venerosi A, Calamandrei G (2016) Early-life toxic insults and onset of sporadic neurodegenerative diseases—an overview of experimental studies. In Neurotoxin modeling of brain disorders—life-long outcomes in behavioral teratology, pp 231–264. Springer, New York
Tchounwou PB, Ayensu WK, Ninashvili N, Sutton D (2003) Environmental exposure to mercury and its toxicopathologic implications for public health. Environ Toxicol 18(3):149–175
USDE US (2013) Department of Energy. The risk assessment information system (RAIS). U.S. Department of Energy’s Oak ridge operations office: Oak Ridge, TN, USA, 2013. https://rais.ornl.gov/. Accessed 4 Sept 2017
USEPA (1989) United States Environmental Protection Agency. Risk assessment guidance for superfund, volume i: human health evaluation manual; EPA 540-1-89-002; U.S. Environmental Protection Agency: Washington, DC, USA
USEPA (1992) United States Environmental Protection Agency. Guidelines for exposure assessment, risk assessment forum; [EPA/600/Z-92/001]; United States Environmental Protection Agency: Washington, DC, USA, 1992
USEPA (2001) United States Environmental Protection Agency. Risk assessment guidance for superfund: volume III–part A, process for conducting probabilistic risk assessment; EPA 540-R-02-002. 2001. https://www.epa.gov/sites/production/files/2015-09/documents/rags3adt_complete.pdf. Accessed 4 Sept 2017
USEPA (2009) United States Environmental Protection Agency. National primary drinking water regulations. http://esdat.net/Environmental%20Standards/US/Federal/US%20Federal%20MLCs.pdf. Accessed 4 Sept 2017
USEPA (2010) (United States Environmental Protection Agency. Toxicological review of inorganic arsenic. Washington, DC: U.S. Environmental Protection Agency. https://www.epa.gov/sites/production/files/2016-03/documents/10004a.pdf. Accessed 15 Jan 2019
USEPA (2011) United States Environmental Protection Agency. exposure factors handbook 2011 edition (Final). https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=236252. Accessed Apr 2016
Van der Weijden C, Pacheco FAL (2006) Hydrogeochemistry in the Vouga River basin (central Portuhal): pollution and chemical weathering. Appl Geochem 21:580–613
Villanueva CM, Cantor KP, Grimalt JO, Malats N, Silverman D, Tardon A, Marcos R (2006) Bladder cancer and exposure to water disinfection by-products through ingestion, bathing, showering, and swimming in pools. Am J Epidemiol 165(2):148–156
WHO (2011) World Health Organisation—guidelines for drinkingwater quality (4th ed). http://who.int/en/. Accessed 28 Apr 2016
WHO (2015) Human biomonitoring: facts and figures. http://www.euro.who.int/__data/assets/pdf_file/0020/276311/Human–biomonitoring-facts-figures-en.pdf. Accessed 1 Sept 2017
WHO (2017) World Health Statistics 2017: monitoring health for the SDGs. http://www.who.int/gho/publications/worldhealth_statistics/2017/en/
Wongsasuluk P, Chotpantarat S, Siriwong W, Robson M (2014) Heavy metal contamination and human health risk assessment in drinking water from shallow groundwater wells in an agricultural area in Ubon Ratchathani province, Thailand. Environ Geochem Health 36(1):169–182
Wright JW, Davies KF, Lau JA, McCall AC, McKay JK (2006) Experimental verification of ecological niche modeling in a heterogeneous environment. Ecology 87(10):2433–2439
Wu B, Chen T (2010) Changes in hair arsenic concentration in a population exposed to heavy pollution: Follow-up investigation in Chenzhou City, Hunan Province, Southern China. Elsevier, New York
Wu S, Powers S, Zhu W, Hannun YA (2016) Substantial contribution of extrinsic risk factors to cancer development. Nature 529(7584):43
Xie T, Liu X, Sun T (2011) The effects of groundwater table and flood irrigation strategies on soil water and salt dynamics and reed water use in the Yellow River Delta, China. Ecol Model 222(2):241–252
Yan D, Zhang Y, Liu L, Yan H (2016) Pesticide exposure and risk of Alzheimer’s disease: a systematic review and meta-analysis. Sci Rep. https://doi.org/10.1038/srep32222
Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Tomassetti P (2011) Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 364(6):514–523
Zatta P, Lucchini R, Van Rensburg SJ, Taylor A (2003) The role of metals in neurodegenerative processes: aluminum, manganese, and zinc. Brain Res Bull 62:15–28
Acknowledgements
This research was partly funded by SOIL PRECAIRE—an EU co-funded project by FEDER (Interreg V Sudoe Program). Funding for this research was provided by the Labex DRIIHM, Réseau des Observatoires Hommes-Millieux–Centre National de la Recherche Scientifique (ROHM–CNRS) and OHMI-Estarreja, and by the Foundation for Science and the Technology—SFRH/BPD/71030/2010 and the Projects (SFRH/BPD/71030/2010, UID/MAR/04292/2013, and UI/D/GEO/04035/2013). The authors thank also the participants for taking part in this research and the local private institutions of social solidarity for the collaboration (Santa Casa Misericórdia de Estarreja, Associação Humanitária de Salreu, Centro Paroquial Social São Tomé de Canelas, Centro Paroquial Social Avanca, Fundação Cónego Filipe Figueiredo Beduído and Centro Paroquial de Pardilhó). The authors would further like to thank three anonymous reviewers for their comments that greatly helped improve the quality of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cabral Pinto, M.M.S., Ordens, C.M., Condesso de Melo, M.T. et al. An Inter-disciplinary Approach to Evaluate Human Health Risks Due to Long-Term Exposure to Contaminated Groundwater Near a Chemical Complex. Expo Health 12, 199–214 (2020). https://doi.org/10.1007/s12403-019-00305-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-019-00305-z