Abstract
To study the distribution, sources and enrichment factors of groundwater As in the Shihezi area in Xinjiang, China, 19 indexes in 23 groundwater samples were tested and analyzed with a control area of 1611 km2. Results showed that As concentrations ranged between non-detected and 49.1 μg/L with an average of 11.0 μg/L. Among all the samples, 34.8% of which had As concentrations greater than 10 μg/L, according to “Standards for Drinking Water Quality (GB5749-2006)”. Groundwater As showed a significant spatial distribution. High-As groundwaters (>10 μg/L) were mainly distributed in the confined groundwater in the northern part of the study area. Arsenic concentrations in the deep confined groundwater were higher than that in the shallow confined groundwater due to mixed exploitation of groundwater in confined groundwater region and overexploitation of deep groundwater in the Shihezi area. The hydrogeochemical type of groundwater changed from HCO3·SO4–Ca·Na in the southern piedmont zone to HCO3·SO4–Na in the northern fine soil plain. High-As groundwater generally occurred under weakly alkaline and reducing conditions with dominant hydrogeochemical type of groundwater of HCO3·SO4–Na. Groundwater As mainly derived from As-containing minerals in the coal seam of the southern mountain and the extensive use of As-containing pesticides in agricultural areas. Enrichment of groundwater As was mainly influenced by climate, geological settings and hydrogeochemical characteristic. In the transition zone of oasis and desert in the north of study area, intensive evaporation of groundwater promoted the enrichment of As in shallow groundwater. In the confined groundwater area in the northern part of the study area, relatively weak groundwater runoff and reducing environment may contribute to groundwater As enrichment in confined aquifers. In addition, high pH values and high F− concentrations in the groundwater may contribute to the enrichment of groundwater As, while TDS, total Fe and Mn concentrations of groundwater had little effect on As enrichment in the study area. Two confined groundwater flow paths were selected in the study area. Inverse geochemical modeling was performed using PHREEQC. The results showed that As-containing realgar dissolved in groundwater, indicating that realgar was the major mineral source of groundwater As. The dissolved amount of realgar along deep confined groundwater flow path was higher than that along shallow confined groundwater flow path, indicating that the concentrations of As dissolved in deep confined groundwater were higher than that in shallow confined groundwater. Meanwhile, dissolution of fluorite in groundwater caused the increase in groundwater F−, which further confirmed that groundwater As and F− had a positive correlation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater As contamination is harm to environment and human health, which attracted broad concern over public health (Polya 2010). As a common element, As is widely found in the air, soil and water, and As contents in the crust ranged between 2 and 5 mg/kg (Smedley and Kinniburgh 2002). Arsenic can bond to metallic or nonmetallic substances forming organic or inorganic arsenide (Wang and Mulligan 2006; Feng et al. 2009). Under natural conditions, As can release to the environment through oxidation, reduction and desorption of As-containing compounds. Once entering the groundwater, the released As can cause enrichment of As concentrations and deterioration of groundwater quality (Liu et al. 2013). Groundwater with As concentrations greater than 10 μg/L is considered as high-As groundwater, according to the World Health Organization (WHO) drinking water standards.
Arsenic is an essential trace element to human health which can boost metabolism, smooth skin and circulate blood with extremely low As concentration (Mayer et al. 1993). In the meantime, As is also toxic (Kar et al. 2011). When accumulated to a certain level in human body, As can be harmful to human health. Long-term intake of high-As groundwater will cause arseniasis, with the symptoms of skin depigmentation, tinting and keratosis, even anemia, jaundice, cirrhosis, skin cancer and respiratory tract cancer (Joseph et al. 2015a; Cozzarelli et al. 2016). High As in groundwater has been found in many parts of the world (Chauhan et al. 2011; Kim et al. 2012). China is one of the largest countries suffering from high-As groundwater in inland basin in arid and semiarid continental climate (Guo et al. 2008; Herath et al. 2016). Based on the risk evaluation model, there are about 20 million residents in China in risk of high-As groundwater (Rodríguez-Lado et al. 2013).
Natural As mainly comes from geological environment (soil, rocks, mineral deposit) (Herath et al. 2016). Influenced by natural processes and human activities, As can release to the environment (Smedley and Kinniburgh 2002). Arsenic in rocks derives from As-containing minerals (arsenic sulfide, arsenic trioxide, polymetallic arsenic compounds), while soil As mainly comes from rock weathering. Arsenic is widely distributed in all types of waters with generally low concentrations but fluctuate greatly depending on different geological settings. Weathering, biological effect and volcanic eruption are the main natural sources of As in water and soil (Zhao et al. 2010). Human activities, such as mining, consuming of fossil fuel, use of As-containing pesticides, wood anticorrosion and chemical processing, may lead to As contamination in water and soil (Wang and Mulligan 2006; Joseph et al. 2015b). However, massive groundwater As abnormality is usually considered to be a result of natural forces (Keshavarzi et al. 2011). Migration, transformation, release and enrichment of groundwater As are closely correlated with geological settings, sedimentary environment and hydrochemical characteristics (McArthur et al. 2004; Guo et al. 2013; Shah 2015). Governments and the public over the world have aroused great concerns on groundwater As abnormality (Zhang et al. 2013). Many countries and regions including China have made great efforts to study the pathology and toxicology of endemic arsenism, as well as the mechanism of high-As groundwater in affected areas (Krishna et al. 2001).
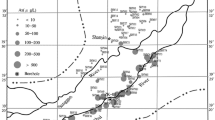
The Shihezi area is located in the center of the northern slope economic zone of Tianshan Mountains in Xinjiang Uygur Autonomous Region (referred to as “Xinjiang”). It is an important area on the Silk Road Economic Belt which connecting China with Central Asia, South Asia, Eastern Europe and other countries. It also plays an important role in the construction of Xinjiang and the Silk Road Economic Belt (Li et al. 2015a, b). Located in the inland arid region in the northwest China, there is a lack of water resources in the Shihezi area (Li 2016). Groundwater is an important part of water resources and essential to human life, and groundwater quality deterioration may inhibit the development of social economy (Li et al. 2013). Pang et al. (2010) found that environmental entropy of groundwater As in the Shihezi area increased annually, indicating a deterioration trend of groundwater quality with urbanization, which will further aggravate the imbalance between supply and demand of water resources. It is essential to carry out groundwater As research in order to ensure drinking water safety and rational exploitation of groundwater resources in Shihezi area. However, there are little previous studies on groundwater As in this area. Our research studied the distribution and enrichment factors of groundwater As in the Shihezi area. Two groundwater flow paths were selected to perform inverse geochemical modeling to reveal the migration and enrichment of groundwater As. It can provide theoretical basis for solving endemic arsenism, As removal technology and reasonable exploitation of groundwater resources, which is of great practical significance to promote social and economic development and ecological and environmental protection in the Shihezi area.
Study Area
Location and Climate
The Shihezi area (E85°45′ ~ E86°20′, N44°10′ ~ N45°00′) is located in the middle of northern piedmont of Tianshan Mountains and to the southern of Gurbantunggut Desert in Xinjiang, covering an area of 7529 km2. It has a continental arid climate with annual average temperature range between 7.5 and 8.2 °C, sunshine duration between 2318 and 2732 h, frostless period of 147–191 days. The annual evaporation (range between 1000 and 1500 mm) is about 6 times of annual precipitation (range between 180 and 270 mm). The precipitation decreases from south to north, while the evaporation increases from south to the north (Dong et al. 2013).
Hydrogeological Settings
The Shihezi area is composed of Tianshan Mountains, piedmont hilly area, piedmont inclined plain, alluvial-proluvial plain and eolian desert from south to north. The desert in the north is rich in petroleum, and the mountain area in the south has coal mines. The Shihezi area (high in the south and low in the north) has an average altitude of 451 m. The aquifer in the study area is a Quaternary gravel layer. Sedimentary thickness is controlled by the basement with thickness of around 1200 m near piedmont depression belt and decreased to about 400 m to the north with the uplifting of the base. According to the previous data, the Quaternary aquifer in the study area is comprehensively divided by lithological characteristics. The Lower Pleistocene (Q1) is mainly constituted of sand and gravel, sandwiched with silty sand and silt. The Middle Pleistocene (Q2) is the glaciofluvial deposit and alluvium, the piedmont is mainly constituted of sandy gravel and pebble gravel, while the plain is mainly constituted of silt, silty clay and sandy gravel. The Upper Pleistocene (Q3) is alluvial-pluvial deposit which is mainly consist of pebble and gravel with the upper part generally covered with silt layer. The Holocene Series (Q4) is alluvial-pluvial deposit mainly consist of loose pebble, gravel, sand and silt (Fig. 1) (Duan et al. 2007; Li et al. 2015a, b). The Shihezi area is demarcated by the Urumqi-Yili highway, the south of which is a piedmont plain with single unconfined aquifer (buried depth of unconfined groundwater range between 15 and 80 m), while the north is a multilayer confined aquifer with unconfined aquifer and shallow confined aquifer distributed at depth <100 m and deep confined aquifer mainly distributed at depth >100 m (where multilayer confined groundwater and artesian water are distributed) (Zeng et al. 2016).
Regional hydrogeological cross section from south to north in the Shihezi area (Zeng et al. 2016)
There are five rivers, namely the Manas River, the Ningjia River, the Jingou River, the Taxi River and the Hutubi River, located in the study area. These rivers are recharged by meltwater, precipitation and bedrock fissure water with small interannual variation. Originated from mountain area, rivers runoff and dissipate in the plain area and finally disappear in Gurbantunggut Desert.
The main recharge sources of groundwater in this area are the leakage of river water and canal water. Besides, the infiltration of irrigation water, plain reservoirs water and precipitation are the recharge sources. Groundwater runs off from south to north which is related with the lithology. From south to north, with decreased aquifer particles size and water permeability, the runoff is inhibited. The major discharge sources of groundwater are spring water overflow, evaporation and transpiration, artificial exploitation, spring water overflow and lateral outflow.
Materials and Methods
Groundwater Samples Collection and Preservation
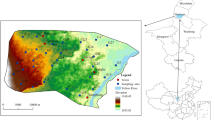
Twenty-three groundwater samples (five unconfined groundwater samples, eight shallow confined groundwater samples and 10 deep confined groundwater samples) were collected in August 2014 (Fig. 2), which mainly distributed in the plain of the Shihezi area with the control area of 1611 km2. The depths of the sampling wells ranged between 20 and 300 m. Samples collection, preservation and detection were performed in strict accordance with China’s Technical Specifications for Environmental Monitoring of Groundwater (HL/T164-2004).
Water samples were filtered with 0.45 μm disposable filter membranes to remove most colloids and particles. Water samples for major cations and trace elements analysis were collected in 100 mL polyethylene bottles washed by HNO3 and acidified with 1:1 HNO3 to pH < 2. Samples for anions analysis were filtered without addition of any reagents.
Quality Control and Groundwater Samples Analysis
Strict quality control was taken during field sample collection and laboratory determination. The purpose of quality control of field sample collection is tracking errors and distorted data, which can help to improve measurements when dealing with inacceptable sample collection and error analysis, confirm the data correctness, and provide uncertainties of sample collection and measurement in results analysis. The samples were preserved at low temperature (4 °C) using incubator and ice before transported to the laboratory for analysis. Besides, field blank samples, blank samples with addition of standard samples and duplicate samples were used for sampling reliability assessment.
Groundwater samples analysis was carried out according to Technical Specifications for Environmental Monitoring of Groundwater (HL/T164-2004) by the Mineral Water Testing Center of Institute of Hydrogeology and Environmental Geology, Chinese Academy of Geological Sciences. Four indexes including pH values, water temperature, oxidation–reduction potential (ORP) values and electrical conductivity (EC) were measured in the field; fifteen inorganic indexes (K+, Na+, Ca2+, Mg2+, Cl−, SO4 2−, HCO3 −, NH4 +, NO3 −, NO2 −, total dissolved solids (TDS), total Fe (TFe), Mn, As and F−) were measured. The pH values were measured using portable digital pH meter MT-8060 (Kady). Water temperature and ORP values were measured using pen-type ORP/temperature meter YSI ORP15A (Zhong Heng Ri Xin). EC was measured using pen-type EC meter 5021 (Ying Ao). As, F−, TFe and Mn were determined using Inductively Coupled Plasma Atomic Emission Spectrometer (ICP-AES) (iCAP 6300, Thermo Fisher Scientific) with As detection limit of 0.5 μg/L. NH4 +, NO3 −, and NO2 − were measured using Ultraviolet Spectrophotometer UV2550 (Shimadzu). Major cations (K+, Na+, Ca2+, Mg2+) and anions (SO4 2−, Cl−) were analyzed using Ion Chromatograph ICS1500 (Dionex). HCO3 − was detected with titration method.
The anions and cations balance test method was used to test the reliability of the groundwater samples data by calculating percent charge balance errors (%CBE) according to formula (1) (Li et al. 2014a, b):
where all cations and anions are expressed in meq/L. Physiochemical analysis with % CBE < ±5% is reliable. The %CBE of the anions and cations of water samples in our study area were −3.12 ~ 3.16%, respectively, indicating that all the data were reliable.
Groundwater As concentrations are shown in Table 1.
Results and Discussion
Hydrochemical Characteristics
Groundwater As is closely related to hydrochemical environment represented by major ions, and fifteen main hydrogeochemical parameters were analyzed statistically (Table 2).
The pH values ranged between 7.27 and 9.30, with an average of 8.41, which indicated a weak alkaline environment. Total dissolved solids (TDS) values ranged between 130 and 1006 mg/L with an average of 365 mg/L. Total hardness (TH) values ranged between 8.6 and 692.8 mg/L with an average of 161.2 mg/L. Oxidation–reduction potential (ORP) values ranged between −0.4 and 194.1 mV with an average of 72.3 mV.
The concentrations of cations in groundwater ranged from 16.3 to 182.4 mg/L (Na+ + K+), from 2.3 to 197.3 mg/L (Ca2+) and from 0.5 to 48.2 mg/L (Mg2+), respectively, with the average concentrations in descending order as Na+ + K+ > Ca2+ > Mg2+. As for anions, the concentrations of Cl−, SO4 2− and HCO3 − ranged from 5.5 to 153.4, 21.8 to 409.6 and 90.7 to 360.0 mg/L, respectively, with the average concentrations in descending order as HCO3 − > SO4 2− > Cl−.
As common trace elements in groundwater which are important for human health, the upper limit for As and F− in groundwater are 10 and 1.0 mg/L, respectively, according to the “Standards for Drinking Water Quality (GB5749-2006)”. Results showed that As concentrations ranged between non-detected and 49.1 μg/L with an average of 11.0 μg/L and that F− concentrations ranged between 0.1 and 1.5 mg/L with an average of 0.6 mg/L.
Hydrochemistry Types of Groundwater
Groundwater hydrochemistry types in the study were classified with the Shug Kalev classification method. According to major ion types (six major types in groundwater, with K+ combined to Na+, and CO3 2− combined to HCO3 −) and TDS, anions and cations with concentrations over 25% mmol are combined to classify water types. Each water type represented the natural water formed in specific environment (Li et al. 2013; Li et al. 2014a, b).
Leaching was the dominant hydrogeochemical process in southern piedmont zone with HCO3·SO4–Ca·Na type as the main hydrogeochemical type (Fig. 3). In the northern plain area, the dominant hydrogeochemical processes were evaporation and concentration with HCO3·SO4–Na type as the major hydrogeochemical type. High-As groundwater (>10 μg/L) were mainly distributed in confined aquifers with the major hydrogeochemical type of HCO3·SO4–Na type (Fig. 3).
Distribution of Groundwater As
The average As concentration in 23 groundwater samples was 11.0 μg/L with the maximum of 49.1 μg/L. High-As groundwater accounted for 34.8% of the total samples.
-
(1)
Horizontal distribution of groundwater As
Groundwater samples with As concentrations <10 μg/L were distributed in the southern piedmont gobi zone. Groundwater had relatively higher As concentrations in the discharge area (near the northern desert area) than that in the recharge area (in the piedmont gobi zone). The concentrations of groundwater As increased gradually with reduced runoff and leaching and intensive evaporation concentration.
-
(2)
Vertical distribution of groundwater As
The depths of the sampling wells ranged between 20 and 300 m bls. There were eight samples with As concentrations >10 μg/L, which were distributed in confined groundwater (25.0% distributed in shallow confined groundwater and 75.0% in deep confined groundwater). Vertically, As concentrations in deep confined groundwater (ranged between 14.4 and 49.1 μg/L with an average of 31.5 μg/L) were higher than that in shallow confined groundwater (11.5 ~ 13.6 μg/L, average 12.6 μg/L) (Fig. 4). Based on the confined groundwater quality data determined in May 2016 from the multilayer monitoring well located in the northern study area of the 149th Regiment, eighth division of Xinjiang Production and Construction Corps (refer to XJ23 in Fig. 2), As concentrations were <10 μg/L in shallow confined groundwater at depths between 60 and 100 m, while that was 16 μg/L in deep confined groundwater at depth of 280 m. It further indicated that As concentrations in deep confined groundwater were higher than that in shallow confined groundwater (Smedley and Kinniburgh 2002).
There were two causes account for the As concentrations in deep confined groundwater higher than that in shallow confined groundwater in the Shihezi area. One was that less human activities in the single unconfined aquifer in the gravel belt in southern piedmont gobi contributed to less effect on groundwater As contamination. The other was that mixed exploitation of groundwater (confined and unconfined groundwater at different depths were exploited simultaneously) may lead to the recharge of inferior groundwater from unconfined aquifers to confined aquifers through side-wall infiltration. It may cause the transfer of groundwater As into deeper aquifers and the increase in groundwater As in confined aquifers (Luo 2008). Meanwhile, due to overexploitation of deep groundwater in some areas in Shihezi, there was a deep groundwater falling funnel caused by significant drop of deep groundwater level (Wu 2007). It would increase the difference of water level between deep and shallow groundwater, causing the leakage recharge from contaminated sallow groundwater to deep groundwater (Tamea and Butera 2014). The recharge of As contaminated groundwater transferred to deeper aquifers and caused the intensified As contamination in deep confined groundwater.
Sources of As
Groundwater As in the Shihezi area derived from natural or anthropogenic sources. Honggou coal mine, Xiaogou coal mine, etc., were distributed in the southern part of the mountain area. Arsenic occurred as sulfide-binding species in As-riched coal seam (Luo et al. 2005; Kang 2014). Under natural conditions, As adsorbed on arsenide may be released into the groundwater due to the change of redox conditions (Camm et al. 2004). Therefore, the coal seam in the south of the Shihezi Area was one of groundwater As sources.
Besides, as the Shihezi area is an important agricultural production base in Xinjiang, extensive use of As-containing pesticides can lead to As accumulation in soil (Wang et al. 2016). It was recognized that As-containing pesticides including lead arsenate, copper arsenate, ferric arsenate and calcium arsenate (Pelley 2005) were the major arsenical compounds used in agriculture (Delistraty and Yokel 2014; Zhou et al. 2016). Long-term excessive use of As-containing pesticides would lead to migration and accumulation of most As into soil (Solgi et al. 2012). Although government issued documents on phasing out As-containing pesticides in China in 1970s, large agricultural areas contaminated by years of As-containing pesticide application still exist (Li et al. 2016). Wen et al. (2015) analyzed 200 arable soil samples in Shihezi reclamation area in 2013 and found that arable soils had As contents between 3.41 and 31.1 mg/kg, with an average of 13.5 mg/kg, standard deviation of 5.79 mg/kg and variation coefficient of 0.43. The average As content was comparable to the background value of soil As in Xinjiang (11.2 mg/kg). It demonstrated that high-As soil was the source of groundwater As in this area. Arsenic-containing pesticides accumulated in soil may enter the unconfined groundwater through rainfall and leaching, resulting in a rise of As concentrations in aquifers. It can be inferred that it may cause severe damage on human health by long-term use of groundwater contaminated by As-containing pesticides (Naujokas et al. 2013).
Enrichment Factors of As
-
(1)
Climate conditions
The evaporation increased from south to the north in the Shihezi area. High-As groundwater samples mostly distributed to the north (near the desert area). Increasingly intensive groundwater evaporation facilitated groundwater As enrichment (Nicolli et al. 2010). Due to shallow buried depth of the shallow groundwater, arid climate conditions may lead to groundwater As migration and enrichment in shallow aquifers. As for deep confined groundwater, climate had little effect on As enrichment (Tian and Zhang 2010).
-
(2)
Runoff conditions
High-As groundwater samples were mainly distributed in northern confined groundwater. Among all the samples with As > 10 μg/L, 75.0% of which were deep confined groundwater. The study area tilts from south to north. It is not easy for groundwater As enrichment due to advantageous runoff conditions in the unconfined aquifer in the southern piedmont zone. Arsenic concentrations in this area were <2 μg/L. In the northern fine soil plain area, the deep confined aquifer was composed of fine particles under poor runoff conditions in enclosed environment, which benefited the accumulation for various elements and was conducive to As enrichment (Bian et al. 2009; Zhang et al. 2013).
-
(3)
pH values
The pH value of groundwater was an important factor of groundwater As enrichment (Boyle et al. 1998; Ryan et al. 2013). The groundwater was weakly alkaline in the study area. The pH values ranged between 7.27 and 9.30, with an average of 8.41. Alkaline conditions may contribute to the desorption of As and the enrichment of groundwater As. Arsenate and arsenite were two main species in groundwater under pH values ranged between 6 and 9 (Barbieri et al. 2014). These As species can be easily adsorbed by positively charged minerals, such as Fe and Al oxides. With the increase in pH values, positive charges carried by colloids and clay minerals decreased. Consequently, the desorption of arsenate or arsenite was reduced, leading to an increase in As concentrations in groundwater (Pierce and Moore 1982; Bundschuh et al. 2004; Zhang et al. 2014). High-As groundwater had high pH values ranged between 8.54 and 9.30 (Fig. 5a). Groundwater As was significantly positive correlated with pH values with the correlation coefficient of r = 0.772 (n = 23, r α=0.05 = 0.413, r α=0.01 = 0.526). Groundwater As increased with the increasing pH values.
-
(4)
Redox environment
It indicated a redox environment of groundwater with ORP values ranged between −0.4 and 194.1 mV (average of 72.3 mV). High-As groundwater generally had low ORP values (ranged between −0.4 and 31.7 mV), indicating a reducing environment. Fe/Mn oxides and hydroxides were reduced and dissolved, causing the release and enrichment of groundwater As (Pei et al. 2005; Root et al. 2010; Guo et al. 2011). There was a significantly negative correlation between groundwater As and ORP values on the whole with a correlation coefficient between As concentrations and ORP values of r = −0.692 (n = 23, r α=0.05 = 0.413, r α=0.01 = 0.526) (Fig. 5b). Once exceeded 30 μg/L, however, groundwater As increased with ORP values.
Meanwhile, NO3 − concentrations in high-As groundwater were generally lower than 0.20 mg/L and SO4 2− concentrations were less than 100 mg/L (Fig. 5c, d). Low concentrations of NO3 − and SO4 2− indicated a reducing environment, which may contribute to groundwater As enrichment.
-
(5)
TDS
TDS ranged between 130 and 1006 mg/L with an average of 365 mg/L. There was no significant correlation between As concentrations and TDS (Fig. 5e). Moreover, high-As groundwater had low TDS values ranged between 182 and 604 mg/L, which indicated that TDS had little impact on groundwater As enrichment.
-
(6)
F− concentrations
Of eight groundwater samples with As > 10 μg/L, there were 50% of which had F− concentrations greater than the upper limit value (1.0 mg/L). These samples were mainly distributed in the desert in the north of the study area (groundwater discharge area). Due to intense evaporation and concentration, F− was greatly enriched in groundwater. Groundwater with high F− concentration (ranged between 0.47 and 1.54 mg/L with an average of 0.95 mg/L) generally had As > 10 μg/L (Fig. 5f). There was a significant positive correlation between As concentrations and F− concentrations with correlation coefficient of r = 0.765 (n = 23, r α=0.05 = 0.413, r α=0.01 = 0.526).
-
(7)
Other hydrogeochemical parameters
There was no significant correlation between As concentrations and TFe/Mn concentrations which were low (Fig. 5g, h) in the study area. This was different from the results in other parts of the world which indicated a significant correlation between As concentrations and TFe/Mn concentrations (Islam et al. 2009; Nicolli et al. 2010; Gibbon-Walsh et al. 2011). This inconsistency may be due to the alkaline reducing environment of high-As groundwater. Under reducing conditions, SO4 2− was reduced to S2− which may precipitate with dissolvable Fe and Mn, forming Fe/Mn sulfides. The concentrations of groundwater Fe and Mn may reduce (Zhang et al. 2014) during the process.
Inverse Geochemical Modeling
Simulated Paths and Possible Mineral Phases
Inverse geochemical modeling can be used to determine water–rock interaction in a system with determined hydrochemical data. On the basis of mass conservation model, determined chemical compositions in groundwater in initial and final states were used to calculate mass transfer in groundwater system. The results showed that high-As groundwater was distributed in confined groundwater in the Shihezi area. Therefore, two flow paths, namely A–A’ (W16S-S03-W66) for shallow confined groundwater and B–B’ (W16D-W28D-W33) for deep confined groundwater (Fig. 2), were selected to reveal groundwater As migration and enrichment in the study area with inverse geochemical modeling method using PHREEQC (Parkhurst and Appelo 1999; Polizzotto et al. 2006).
Identification of possible mineral phases was an important part in inverse geochemical modeling. Determination of possible mineral phases, including uncertain minerals and gases, was a key to establish mass reaction balance equation as the most likely reactants/products in groundwater system. The stratum in the study area was mainly constituted of conglomerate, sandstone, shale, cobble and gravel, sandy gravel, medium-fine sand, silt and silty clay; and the aquifer was mainly constituted of cobble and gravel, sandy gravel and medium-fine sand from south to north (Wu 2007). The minerals in the southern mountain stratums were mainly constituted of quartz, feldspars, calcite, mica, dolomite, gypsum and clay minerals, according to previous geological studies (Liu et al. 2006; Chen et al. 2009; Li 2014). Gold was often coexisted or associated with As-containing minerals (Bullen et al. 2003). In Baogutu gold deposits in the western Shihezi, As-riched minerals were mainly orpiment and realgar (An and Zhu 2009; Zheng et al. 2015). In addition, as the shallow confined aquifers (path A–A’) was in semi-closed environment, CO2 was included in the possible mineral phase, while the deep confined aquifers (path B–B’) was in closed environment, CO2 was not chosen as the possible mineral phase in the calculation of calcium and magnesium salts of hydrogeochemical reaction (Li et al. 2010). The ion exchanges between Ca2+/Mg2+, Ca2+/Na+, as well as Mg2+/Na+, were significant processes in the chemical evolution of groundwater. With the combination of chemical compositions of groundwater and aquifer minerals, dolomite (CaMg(CO3)2), calcite (CaCO3), gypsum (CaSO4·2H2O), fluorite (CaF2), halite (NaCl), orpiment (As2S3), realgar (AsS), CO2 and cation exchange were used to carry out inverse hydrogeochemical modeling in shallow confined groundwater (path A–A’), all possible mineral phases above except CO2 were used to carry out inverse hydrogeochemical modeling in deep confined groundwater (path B–B’).
Mass Balance Simulation
There were multiple solutions or no solution in inverse geochemical modeling. The controlled mineral phases affecting chemical compositions of groundwater can be found by moderately adjusting the mineral phases and the uncertainty. Ten mineral phases were determined by multiple simulations in the Shihezi area. Mass exchanges between different samples in the paths A–A’ and B–B’ are shown in Table 3.
On simulated path A–A’, halite accounted for the largest amount of dissolution (2.78 × 10−3 mmol/L), followed by the dissolution of calcite, fluorite and realgar with the amounts of 2.82 × 10−4, 3.63 × 10−5 and 7.00 × 10−7 mmol/L, respectively. CO2 accounted for the largest amount of precipitation (2.14 × 10−3 mmol/L), followed by the precipitation of gypsum, dolomite and orpiment with the amounts of 1.37 × 10−3, 9.81 × 10−4 and 2.75 × 10−7 mmol/L, respectively. At the same time, cation exchange between Na+ and Ca2+ occurred. Calcite and fluorite dissolved in groundwater should have caused an increase in Ca2+ along groundwater flow path, whereas the results showed that the Ca2+ concentration decreased. It may be exposited that cation exchange reaction and the precipitation of gypsum and dolomite were stronger than the dissolution of calcite and fluorite. The concentrations of Na+ and Cl− gradually increased because of increasing dissolution effect of halite, while the reduction of Mg2+ and SO4 2− could be attributed to the precipitation of gypsum and dolomite.
On simulated path B–B’, halite accounted for the largest amount of dissolution (1.60 × 10−3 mmol/L), followed by the dissolution of calcite, gypsum, fluorite and realgar with the amounts of 9.53 × 10−4, 3.98 × 10−4, 2.53 × 10−5 and 2.92 × 10−6 mmol/L, respectively. Dolomite accounted for the largest amount of precipitation (1.47 × 10−4 mmol/L), followed by the precipitation of orpiment (1.15 × 10−6 mmol/L). Cation exchange reactions between Na+ and Ca2+ also occurred along simulated path B–B’. Similar to the case of simulated path A–A’, the concentration of Ca2+ decreased because cation exchange reaction and precipitation of dolomite were stronger than the dissolution of calcite, gypsum and fluorite. The concentrations of Na+, Cl− and SO4 2− gradually increased because of the increasing dissolution of halite and gypsum, while reduction of Mg2+ could be attributed to the precipitation of dolomite.
Mass exchanges in different groundwater samples from As-containing orpiment along paths A–A’ and B–B’ were −2.75 × 10−7 and −1.15 × 10−6 mmol/L, respectively, which indicated that orpiment was separated out from groundwater; the mass exchanges in different groundwater samples from realgar along paths A–A’ and B–B’ were 7.00 × 10−7 and 2.92 × 10−6 mmol/L, respectively, indicating realgar dissolution and release into groundwater which caused groundwater As enrichment. Therefore, realgar was believed to be the main mineral source of groundwater As in the Shihezi area, which was consistent with previous research results (Myoungjin et al. 2000; Wu et al. 2016). Meanwhile, the dissolved amount of realgar in path B–B′ was higher than that in path A–A′, indicating that As concentrations dissolved in deep confined groundwater were higher than that in shallow confined groundwater. It further confirmed that As concentrations in deep confined groundwater were higher than that in shallow confined groundwater. Mass exchanges between different groundwater samples from fluorine-containing fluorine in the paths A–A’ and B–B’ were 3.65 × 10−5 and 2.53 × 10−5 mmol/L, respectively, indicating that fluorite dissolution into groundwater caused the increase in groundwater F− concentrations. A positive correlation between groundwater As and F− was further confirmed by the simulation results.
Conclusions
-
(1)
High-As groundwater occurred under weakly alkaline reducing environment with groundwater of HCO3·SO4–Na type. Groundwater As showed significant spatial distribution characteristics. Horizontally, groundwater As increased from gravel belt in piedmont gobi in the south to desert area in the north. High-As groundwater samples were mainly distributed in confined aquifers. Vertically, groundwater As in deep confined aquifers was higher than that in shallow confined aquifers.
-
(2)
Groundwater As mainly came from As-containing minerals in the coal seam in the southern mountain. Arsenic-containing minerals were dissolved and adsorbed As released under reducing conditions. In addition, extensive use of As-containing pesticides in the Shihezi area may lead to As accumulation in soil, which was another source of groundwater As.
-
(3)
Enrichment of groundwater As was mainly dominated by climate, geological settings and hydrogeochemical environment. High-As groundwaters were mainly distributed in confined aquifers in the north, where the runoff condition was disadvantageous in enclosed environment. Under the reducing conditions, Fe/Mn oxides and hydroxides were reduced and adsorbed As released, which contributed to the groundwater As enrichment in confined aquifers. In the oasis and desert transition zone in the northern part of the study area, intensive evaporation of groundwater facilitated groundwater As enrichment in shallow aquifers. Moreover, high pH values and high F− concentrations in groundwater contributed to groundwater As enrichment while TDS, TFe and Mn concentrations in groundwater had little effect on As enrichment.
-
(4)
Two inverse geochemical modeling paths were set up along groundwater flow paths. Simulation results showed that orpiment separated out from groundwater and realgar dissolved into groundwater. The amount of dissolved realgar in path B–B′ was higher than that in path A–A′, indicating that groundwater As dissolved in deep confined aquifers were higher than that in shallow confined aquifers. Meanwhile, the dissolution of fluorite into groundwater caused the increase in groundwater F− concentrations. It further confirmed that there was a positive correlation between groundwater As and F−.
References
An F, Zhu YF (2009) Significance of native arsenic in the Baogutu gold deposit, western Junggar, Xinjiang, NW China. Chin Sci Bull 54(10):1744–1749
Barbieri M, Nigro A, Sappa G (2014) Arsenic contamination in groundwater system of Viterbo area (Central Italy). Sens Sci 1(3):101–106
Bian JM, Tang J, Feng L, Cha ES (2009) Hydrogeochemical characteristics in the arsenic poisoning area in western Jilin Province. Hydrogeol Eng Geol (Chinese) 36(5):80–83
Boyle D, Turner R, Hall G (1998) Anomalous arsenic concentrations in groundwaters of an island community, Bowen Island, British Columbia. Environ Geochem Health 20(4):199–212
Bullen HA, Dorko MJ, Oman JK, Garrett SJ (2003) Valence and core-level binding energy shifts in realgar (As4S4) and pararealgar (As4S4) arsenic sulfides. Surf Sci 531(3):319–328
Bundschuh J, Farias B, Martin R, Storniolo A, Bhattacharya P, Cortes J, Bonorino G, Albouy R (2004) Groundwater arsenic in the Chaco-Pampean Plain, Argentina: case study from Robles county, Santiago del Estero Province. Appl Geochem 19(2):231–243
Camm GS, Glass HJ, Bryce DW, Butcher AR (2004) Characterisation of a mining-related arsenic-contaminated site, Cornwall, UK. J Geochem Explor 82(1):1–15
Chauhan VS, Yunus M, Sankararamakrishnan N (2011) Geochemistry and mobilization of arsenic in Shuklaganj area of Kanpur-Unnao district, Uttar Pradesh, India. Environ Monit Assess 184(8):4889–4901
Chen X, Zhong JH, Yuan J, Nie KK, Yang YP (2009) Development and formation of Paleogene kaolinite, Bonan subsag. Petrol Explor Dev (Chinese) 36(4):456–462
Cozzarelli IM, Schreiber ME, Erickson ML, Ziegler BA (2016) Arsenic cycling in hydrocarbon plumes: secondary effects of natural attenuation. Groundwater 54(1):35–45
Delistraty D, Yokel J (2014) Ecotoxicological study of arsenic and lead contaminated soils in former orchards at the Hanford site, USA. Environ Toxicol 29(1):10–20
Dong HG, Wang D, Wang YT, Tong J, Liu T (2013) Spatial and temporal distribution characteristics of mulch residues in cotton field in Shihezi, Xinjiang. J Arid Land Resour Environ (Chinese) 27(9):182–186
Duan L, Wang WK, Cao YQ, Wang LJ, Liu B (2007) Hydrochemical characteristics and formation mechanics of groundwater in the middle of Northern Slope of Tianshan Mountains. J Arid Land Resour Environ (Chinese) 21(9):29–34
Feng XD, Dang Z, Huang WL, Yang C (2009) Chemical speciation of fine particle bound trace metals. Int J Environ Sci Technol 6(3):337–346
Gibbon-Walsh K, Salaün P, Uroic MK, Feldmann J, McArthur JM, Berg CMG (2011) Voltammetric determination of arsenic in high iron and manganese groundwaters. Talanta 85(3):1404–1411
Guo HM, Yang SZ, Tang XH, Li Y, Shen ZL (2008) Groundwater geochemistry and its implications for arsenic mobilization in shallow aquifers of the Hetao Basin, Inner Mongolia. Sci Total Environ 393:131–144
Guo HM, Zhang B, Zhang Y (2011) Control of organic and iron colloids on arsenic partition and transport in high arsenic groundwaters in the Hetao basin, Inner Mongolia. Appl Geochem 26(3):360–370
Guo HM, Liu C, Lu H, Wanty RB, Wang J, Zhou YZ (2013) Pathways of coupled arsenic and iron cycling in high arsenic groundwater of the Hetao basin, Inner Mongolia, China: an iron isotope approach. Geochim Cosmochim Acta 112(3):130–145
Herath I, Vithanage M, Bundschuh J, Maity JP, Bhattacharya P (2016) Natural arsenic in global groundwaters: distribution and geochemical triggers for mobilization. Curr Pollut Rep 2:68–89
Islam MA, Safiullah S, Islam MS, Islam MM (2009) Analysis of organic matter, iron and manganese in soil of arsenic affected Singair area, Bangladesh. Res J Environ Toxicol 3(1):31–35
Joseph T, Dubey B, Mcbean EA (2015a) Human health risk assessment from arsenic exposures in Bangladesh. Sci Total Environ 527–528:552–560
Joseph T, Dubey B, Mcbean EA (2015b) A critical review of arsenic exposures for Bangladeshi adults. Sci Total Environ 527–528:540–551
Kang Y (2014) Environmental biogeochemistry of arsenic in coal mining area. Doctoral dissertation, University of Science and Technology of China, Chinese
Kar S, Maity JP, Jean JS, Liu CC, Liu CW, Bundschuh J, Lu HY (2011) Health risks for human intake of aquacultural fish: arsenic bioaccumulation and contamination. J Environ Sci Health 46(11):1266–1273
Keshavarzi B, Moore F, Mosaferi M, Rahmani F (2011) The source of natural arsenic contamination in groundwater, west of Iran. Exposure Health 3(3–4):135–147
Kim SH, Kim K, Ko KS, Kim Y, Lee KS (2012) Co-contamination of arsenic and fluoride in the groundwater of unconsolidated aquifers under reducing environments. Chemosphere 87(8):851–856
Krishna MV, Chandrasekaran K, Karunasagar D, Arunachalam J (2001) A combined treatment approach using Fenton’s reagent and zero valent iron for the removal of arsenic from drinking water. J Hazard Mater 84(2–3):229–240
Li Q (2014) Spatial and temporal evolution of groundwater quality in the plain area of Jungar Basin. Doctoral dissertation, Xinjiang Agricultural University, Chinese
Li PY (2016) Groundwater quality in western China: challenges and paths forward for groundwater quality research in western China. Exposure Health 8(3):305–310
Li PY, Qian H, Wu JH, Ding J (2010) Geochemical modeling of groundwater in southern plain area of Pengyang County, Ningxia, China. Water Sci Eng 3(3):282–291
Li PY, Wu JH, Qian H (2013) Assessment of groundwater quality for irrigation purposes and identification of hydrogeochemical evolution mechanisms in Pengyang County, China. Environ Earth Sci 69(7):2211–2225
Li PY, Qian H, Wu JH, Chen J, Zhang YQ, Zhang HB (2014a) Occurrence and hydrogeochemistry of fluoride in shallow alluvial aquifer of Weihe River, China. Environ Earth Sci 71(7):3133–3145
Li Q, Zhou JL, Zhou YZ, Bai CY, Tao HF, Jia RL, Ji YY, Yang GY (2014b) Variation of groundwater hydrochemical characteristics in the plain area of the Tarim Basin, Xinjiang Region, China. Environ Earth Sci 72(11):4249–4263
Li PY, Qian H, Howard KWF, Wu JH (2015a) Building a new and sustainable “Silk Road economic belt”. Environ Earth Sci 74(10):7267–7270
Li Q, Zhou JL, Gao YX, Cheng F, Li FX, Meng Q (2015b) Groundwater hydro-geochemistry in plain of Manasi River Basin, Xinjiang. Geoscience (Chinese) 29(2):238–244
Li YF, Ye F, Wang AW, Wang D, Yang BY, Zheng QM, Sun GF, Gao XH (2016) Chronic arsenic poisoning probably caused by arsenic-based pesticides: findings from an investigation study of a household. Int J Environ Res Public Health 13(133):1–14
Liu Z, Liu SY, Wang GL (2006) Water-rock interaction simulation of groundwater in the plain of Manasi River Basin, Xinjiang. Acta Geol Sin (Chinese) 80(6):885–892
Liu CC, Kar S, Jean JS, Wang CH, Lee YC, Sracek O, Li ZH, Bundschuh J, Yang HJ, Chen CY (2013) Linking geochemical processes in mud volcanoes with arsenic mobilization driven by organic matter. J Hazard Mater 262(22):980–988
Luo L (2008) Research on groundwater pollution and its prevention-control policy in China. J China Univ Geosci (Social Sciences Edition) (Chinese) 8(2):72–75
Luo FZ, Hu WH, Song LJ (2005) Analysing of disaster relief factors after earthquakes with Ms 5.0 and Ms 5.4 in south of Shihezi of Xinjiang on Feb. 14, 2003. Inland Earthq 19(1):86–89
Mayer DR, Kosmus W, Pogglitsch H, Mayer D, Beyer W (1993) Essential trace elements in humans. Biol Trace Elem Res 37(1):27–38
Mcarthur JM, Banerjee DM, Hudson-Edwards KA, Mishra R, Purohit R, Ravenscroft P, Cronin A, Howarth RJ, Chatterjee A, Talukder T, Lowry D, Houghton S, Chadha DK (2004) Natural organic matter in sedimentary basins and its relation to arsenic in anoxic ground water: the example of West Bengal and its worldwide implications. Appl Geochem 19:1255–1293
Myoungjin K, Nriagu J, Haack S (2000) Carbonate ions and arsenic dissolution by groundwater. Environ Sci Technol 34(15):3094–3100
Naujokas MF, Anderson B, Ahsan H, Vasken Aposhian H, Graziano GH, Thompson C, Suk WA (2013) The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect 121(3):295–302
Nicolli HB, Bundschuh J, García JW, Falcón CM, Jean JS (2010) Sources and controls for the mobility of arsenic in oxidizing groundwaters from loess-type sediments in arid/semi-arid dry climates-Evidence from the Chaco-Pampean plain (Argentina). Water Res 44(19):5589–5604
Pang W, Wang KY, Yang L (2010) Evaluation of ground water environmental quality in the process of urbanization of Shihezi City by entropy model. Hubei Agric Sci 49(5):1092–1095
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC (Version 2)-a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. Water Resources Investigations Report 99-4259
Pei HH, Liang SX, Ning LY (2005) A discussion of the enrichment and formation of arsenic in groundwater in Datong Basin. Hydrogeol Eng Geol (Chinese) 32(4):65–69
Pelley J (2005) Common arsenical pesticide under scrutiny. Environ Sci Technol 39(6):122–123
Pierce ML, Moore CB (1982) Adsorption of arsenic and arsenate on amorphous iron hydroxide. Water Res 16(7):1247–1253
Polizzotto ML, Harvey CF, Li GC, Badruzzman B, Ali A, Newville M, Sutton S, Fendorf S (2006) Solid-phases and desorption processes of arsenic within Bangladesh sediments. Chem Geol 228(1–3):97–111
Polya DA (2010) Arsenic pollution: a global synthesis. Miner Mag 74(1):183–185
Rodríguez-Lado L, Sun GF, Berg M, Zhang Q, Xue HB, Zheng QM, Johnson A (2013) Groundwater arsenic contamination throughout China. Science 341:866–868
Root TL, Gotkowitz MB, Bahr JM, Attig JW (2010) Arsenic geochemistry and hydrostratigraphy in Midwestern U.S. Glacial deposits. Groundwater 48(6):903–912
Ryan PC, Kim JJ, Mango H, Hattori K, Thompson A (2013) Arsenic in a fractured slate aquifer system, New England, USA: influence of bedrock geochemistry, groundwater flow paths, redox and ion exchange. Appl Geochem 39:181–192
Shah BA (2015) Status of groundwater arsenic pollution of Mirzapur district in Holocene aquifers from parts of the Middle Ganga Plain, India. Environ Earth Sci 73(4):1505–1514
Smedley PL, Kinniburgh DG (2002) A review of the source, behavior and distribution of As in natural waters. Appl Geochem 17(5):517–568
Solgi E, Esmaili-Sari A, Riyahi-Bakhtiari A, Hadipour M (2012) Soil contamination of metals in the three industrial estates, Arak, Iran. Bull Environ Contam Toxicol 88(4):634–638
Tamea S, Butera I (2014) Stochastic description of infiltration between aquifers. J Hydrol 510:541–550
Tian CY, Zhang FC (2010) Distribution and enrichment of As in groundwater in Northern Yinbei plain. Saf Environ Eng (Chinese) 17(2):22–25
Wang S, Mulligan CN (2006) Occurrence of arsenic contamination in Canada: sources, behavior and distribution. Sci Total Environ 366(2–3):701–721
Wang QY, Zhang JB, Xin XL, Zhao BZ, Ma DH, Zhang HL (2016) The accumulation and transfer of arsenic and mercury in the soil under a long-term fertilization treatment. J Soils Sediments 16(2):1–11
Wen W, Hou ZA, Min W, Zhang XS, Li Q (2015) Investigation and evaluation of heavy metals in farmland soil in Shihezi reclamation area. Xinjiang Agric Sci (Chinese) 52(1):137–144
Wu B (2007) Study on groundwater system evolvement law and water environment effect of Shihezi City. Doctoral dissertation, Xinjiang Agricultural University, Chinese
Wu Y, Zhou XY, Lei M, Yang J, Ma J, Qiao PW, Chen TB (2016) Migration and transformation of arsenic: contamination control and remediation in realgar mining areas. Appl Geochem. doi:10.1016/j.apgeochem.2016.05.012
Zeng YY, Zhou JL, Zhou YZ, Jia RL (2016) Assessment and causes of groundwater organic pollution in typical plain areas in Xinjiang, China. Exposure Health 8:401–417
Zhang YL, Cao WG, Wang WZ, Dong QY (2013) Distribution of groundwater arsenic and hydraulic gradient along the shallow groundwater flow-path in Hetao Plain, Northern China. J Geochem Explor 135:31–39
Zhang LP, Xie XJ, Li JX, Wang YX (2014) Spatial variation, speciation and enrichment of arsenic in groundwater from the Datong Basin, Northern China. Geol Sci Technol Inf (Chinese) 33(1):178–184
Zhao FJ, Mcgrath SP, Meharg AA (2010) Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol 61(4):535–559
Zheng B, Zhu YF, An F, Huang QY, Qiu T (2015) As–Sb–Bi–Au mineralization in the Baogutu gold deposit, Xinjiang, NW China. Ore Geol Rev 69:17–32
Zhou YZ, Zeng YY, Zhou JL, Guo HM, Li Q, Jia RL, Chen YF, Zhao JT (2016) Distribution of groundwater arsenic in Xinjiang, P.R. China. Appl Geochem. doi:10.1016/j.apgeochem.2016.09.005
Acknowledgements
The study has been financially supported by NSFC-Xinjiang project “Transport of typical organic pollutants in the groundwater flow system of arid region and its risk assessment” (U1503282); China Geological Survey Bureau project “Groundwater pollution survey of main cities in north western China” (1212011220982); peak discipline project of hydraulic engineering of Xinjiang Uyghur Autonomous Region (xjslgcgfxk20161103).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeng, Y., Zhou, Y., Zhou, J. et al. Distribution and Enrichment Factors of High-Arsenic Groundwater in Inland Arid Area of P. R. China: A Case Study of the Shihezi Area, Xinjiang. Expo Health 10, 1–13 (2018). https://doi.org/10.1007/s12403-016-0241-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-016-0241-7