Abstract
The contamination of freshwater resources with various organic and inorganic contaminants is still a major problem in many parts of the world, especially in developing countries in which the poor water quality continues to pose a serious threat to human health. In this study, the assessment of groundwater quality was performed in the municipality of Bumbu (Kinshasa, Democratic Republic of the Congo) according to the seasonal variation. Water physicochemical parameters [pH, electrical conductivity (EC), dissolved oxygen and soluble ions (Na+, K+, PO4 3−, SO4 2−, NO3 −, NO2 −)] and faecal indicator bacteria (FIB) including Escherichia coli (E. coli), Enterococcus (ENT) and Total coliforms (TC) were analysed. Except for EC and NO3 −, the results revealed low concentration of other water physicochemical parameters, which are below the recommended limits, according to World Health Organization guideline for drinking water. Additionally, the result showed high concentration of FIB reaching the values of 1.6 × 104, 1.5 × 104 and 9.0 × 105 CFU 100 mL−1 for E. coli, ENT and TC, respectively. The pollution was substantial in wet season compared to dry season. PCR amplification for human-Bacteroides indicated that more than 90 % of bacteria were from human origin. Our results highlight the potential human risk associated with the exposure to water contamination from wells due to the high level of NO3 −, EC, E. coli and ENT in both dry and wet seasons. The approach developed in this study helps provide a better understanding of the physicochemical and microbiological pollution of wells in large cities characterized with lack of wastewater and sanitation facilities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The contamination of freshwater resources with micropollutants and pathogenic organisms is still unsolved problem in many parts of the world, especially in developing countries, such as the Democratic Republic of Congo (DRC), in which there are lack of adequate sanitation facilities and where people practice open defecation (WHO 2004; Devarajan et al. 2015a, b; Mwanamoki et al. 2015). Data concerning the occurrence of biological contaminants such as faecal indicator bacteria (FIB) in aquatic environments in Sub-Saharan African countries is limited. Diarrhoeal diseases, mainly due to the consumption of microbiologically contaminated drinking water, cause about one billion illnesses and 2.2 million deaths per year (Montgomery and Elimelech 2007; WHO 2011). The majority of cases are in Sub-Saharan Africa and south Asia. In some of these regions, according to the economic situation and lack of effective infrastructure, a large proportion of the population uses highly contaminated surface water, shallow wells, boreholes, springs and streams for irrigation, domestic and drinking purposes (Amanial 2015; Mwanamoki et al. 2015; Rochelle-Newall et al. 2015). Although most infections occur in developing countries, waterborne diseases are a worldwide problem that also concerns industrialized societies where highly populated centres draw their supply of drinking water (Haller et al. 2009; WHO 2011; Thevenon et al. 2012). In Democratic Republic of the Congo (DRC, with estimated population of 65.7 million inhabitants), despite the potential of its rich fresh water network, more than 75 % of the population have no access to safe water (UNEP 2011). The most common sources of domestic and drinking water for suburban and rural people are streams, shallow wells and springs. These water resources are in many cases polluted by micropollutants and pathogenic organisms which can represent significant potential impacts for human health.
At Kinshasa (capital of the DRC with more than 10 million inhabitants), waterborne diseases, including Entamoeba and Shigella and other diarrhoeal diseases, are great public health concerns. The prevalence of diarrhoeal in the province for the 15 last years affected more than 20.9 % of the population (EIES 2012). The city itself has experienced cholera outbreaks in years 1996, 2010 and 2011 and typhoid fever has affected nearly 1 % of the population (EIES 2012). To our knowledge, there have been no regular water quality monitoring and/or research programmes conducted to assess the microbial and physicochemical qualities of water resources. Our previous researches have been conducted to assess the quality of urban rivers surrounding the City of Kinshasa. The results of these research works indicated the deterioration of the quality of these water resources, especially rivers receiving urban and hospital effluent waters. The pollution can be explained with several aspects including domestic wastes, human open defecation and runoff of wastewater from septic tanks, and hospital wastewaters discharge (Mubedi et al. 2013; Mwanamoki et al. 2014). Our previous data showed also that sediments of urban river-reservoirs (such as Lake Ma Vallée and N’djili River) constitute important reservoirs of FIB (Tshibanda et al. 2014; Mwanamoki et al. 2015). To date, no programmes or researches have been conducted to assess the water quality from shallow wells according to the seasonal variation. Consequently, there is no quantitative information regarding the quantification of FIB in streams, rivers, shallow wells and other water sources in suburban municipalities of Kinshasa, other great cities as well as rural districts of DRC.
The main objective of the present study is to assess the seasonal variation of the water quality from shallow wells of the municipality of Bumbu, a suburban municipality located in south of the Capital City of Kinshasa. The assessment is based on (1) quantification of water physicochemical parameters including pH, electrical conductivity (EC), dissolved oxygen (O2) and soluble ions (Na+, K+, PO4 3−, SO4 2− NO3 −, NO2 −), and (2) quantification of FIB including E. coli, ENT and TC and (3) to characterize the isolated FIB by molecular approach in order to identify the possible cause of water contamination. The analysis of water samples was performed during both the dry and wet seasons in order to identify the eventual changes in water quality with season. Molecular analysis was performed not only to confirm the FIB but also to identify the eventual human source of faecal pollution in wells. The approach used for this research is very important as it allows determining whether shallow wells can provide safe drinking water according to the seasonal variation or whether there is a need to develop ways to improve the water quality from shallow wells (Pritchard et al. 2008).
Materials and Methods
Study Site Description
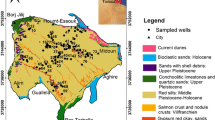
The study was conducted in the commune of Bumbu, a suburban municipality located in the Funa district of Kinshasa (the capital city of the DRC), with total area of 5.3 km2 and an estimated population of about 329.324 habitants. Seven shallow wells from four neighbourhoods (named Ubangi; Mfimi; Dipiya and Mongala) of the municipality were selected (Fig. 1; Table 1). The study area was chosen based on some criteria including, waterborne disease epidemiology, economic situation of local people as well as their access to safe water provided by national society (Regideso), the presence of several shallow wells which constitute the main water resource of more than 70 % of people for domestic use (drinking, cooking and washing) and sanitary risks such as the presence of uncontrolled landfills, pit latrines and septic tanks and how the wells are protected.
Sampling Procedure
Water samples from seven shallow wells were collected in June and July (dry season)/2015, and in October and November (wet season)/2015. The shallow wells (sampling sites) are labelled P1, P2, P3, P4, P5, P6 and P7 (Fig. 2). The frequency of water sampling from each shallow well was two times per month/season, that is, at the beginning (first week) and during the last week of each month. Water from wells was taken by a craft device made of one litre (L) clean polyethylene bottle attached to a rope. For physicochemical analysis, water samples (500 mL) were filled into clean plastic bottles. For bacteriological analysis, water samples (500 mL) were filled into sterile (autoclaved) plastic bottles.
After sampling, the samples were stored in an icebox at 4 °C and immediately transported to the laboratory for analysis within 24 h.
Water Physicochemical Parameter Analysis
Physicochemical parameters of water including temperature (T), pH, dissolved oxygen (O2) and electrical conductivity (EC) were measured in situ using a Multi parameter 350i (WTW, Germany). The concentration of dissolved major ions (Na+, K+, PO4 3−, SO4 2− NO3 −, NO2 −) was measured using the spectrophotometer HACH LANGER (DR-3800, Germany) according to the manufacture’s recommendations. The reference material (certified water CRM, Ontario-99) from the National Water Research Institute, Canada was used to calibrate and verify the accuracy of the instrument. All CRM results were within the acceptance range on the CRM certificate.
Faecal Indicator Bacteria (FIB) Analysis in Water
The FIB (including E. coli, ENT and TC) were quantified in water samples according to the international standard methods for water quality determination using the membrane filtration method (APHA 2005). Briefly, for each sample, triplicates of 100 mL of water was passed through a 0.45-mm filter (Sartorius stedim, biotech, Germany), and placed on different selective culture media (Biolife, Italiana), using the following incubation conditions: E. coli: Tryptone Soy Agar (TSA) medium, incubated at 37 °C for 4 h and transferred to Tryptone Bile X-Gluc Agar (TBX) medium at 44 °C for 24 h; ENT: Slanetz Bartley Agar (SBA) medium, incubated at 44 °C for 48 h and transferred into Bile Aesculin Agar (BAA) medium at 44 °C for 4 h; and TC: Endo agar medium, incubated at 35 °C for 24 h. The results are expressed as colony forming units per 100 mL of water (CFU 100 mL−1). The reproducibility of the whole experimental procedure was tested by means of triplicates.
Characterization of FIB Strains
The PCR amplification was performed directly on the colonies picked from selective-media plates (resuspended in 20 µL of sterile water) using human-specific Bacteroides primers HF183F- (5′-ATCATGAGTTCACATGTCCG-3′) and Bac708R- (5′-CAATCGGAGTTCTTCGTG-3′) (Bernhard and Field 2000; Ahmed et al. 2007), with the PCR conditions as described by Thevenon et al. (2012). PCR reactions of 25 µL total volume consisted of 0.025 U/µL Takara Ex Taq HS DNA polymerase (Takara Bio Europe, Saint-Germain-en-Laye, France), 1× PCR buffer (Takara, containing 2 mM of Mg2+), 800 µM of dNTP, 0.2 µM of each forward and reverse primers (Invitrogen), 50 ng/µL of BSA. PCR was performed in a Biometra thermocycler (BIOLABO) using the following conditions: 15 min for 95 °C initial denaturation; 35 cycles of denaturation (94 °C for 30 s), of annealing (60 °C for 1 min) and of extension (72 °C for 1 min), followed by a final extension at 72 °C for 10 min. PCR products were visualized after electrophoresis on 0.8 % agarose gels containing 1× SYBR Safe DNA gel stain (Invitrogen) in 1× TAE buffer. The experiment was conducted in triplicate in each set of conditions. The negative (without DNA) and positive controls (e.g. the expected 520 bp length) (for HF183/Bac708) from sewage (Poté et al. 2009) were used for each PCR essays.
Data Analysis
All analyses were conducted in triplicate for each set of conditions. Statistical processing of data was performed using SigmaStat 11.0 (Systat Software, Inc., USA). The data were subjected to a Spearman Rank Correlation test to investigate possible relationship using R statistical software, version 3.2.2 (R Core Team 2015). Data were subsequently subjected to a principle component analysis (PCA) using a correlation matrix and the ade4 package, to investigate potential effect of season and sampling site.
Results and Discussion
Physicochemical Quality of Water
The water physicochemical parameters including r, pH, EC, O2 are presented in Table 2. Due to the absence of DRC’s regulation, water quality was compared with the recommendations of the World Health Organization (WHO 2011). The monthly average temperature values varied from 25.0 to 26.8 °C in dry season and from 26.5 to 30.7 °C during the wet season. The highest value was observed in November and the lowest during June/July. These results are comparable with other published data obtained under tropical conditions (Pritchard et al. 2008; Nola et al. 2013). The pH values varied significantly according to the seasonal variation (p < 0.05) for some wells such as P1 (5.4–6.6), P3 (5.4–6.2) and P4 (4.9–6.8). The water from these wells can be considered as slightly acid water especially during dry season. There was no significant difference in pH levels according to the seasonal variation (p > 0.05) for other wells: P2 (6.2–7.2), P5 (6.2–6.9), P6 (6.8–7.4) and P7 (6.9–7.0). Except for P1, P3 and P4 during dry season, the values of pH from investigated wells comply with WHO recommendation for drinking water. All wells show high values of conductivity, ranging from 618 to 1547 and from 605 to 1121 µS cm−1 during the dry and wet season, respectively. According to our discussion with people in charge of P7, the commercial NaCl (put directly into the well) was used to eventually kill present pathogen. The concentration of the dissolved oxygen for each well was not significantly varied with the season variation, ranging the values from 0.9 to 3.5 mg L−1.
The concentrations of soluble ions (Na+, K+, PO4 3−, SO4 2− NO3 −, NO2 −) in water samples are reported in Table 3. The maximum average values were 94.5, 54.3, 1.9, 33.9 and 0.32 mg L−1, for Na+, K+, PO4 3−, SO4 2− and NO2 −, respectively. High values were obtained during the dry period. The lowest level of these dissolved ions can be explained by the dilution effect during the wet season. These results indicate that these soluble ions are within the WHO guideline values for domestically users in both dry and wet seasons. In contrary, NO3 − presents the highest values (which did not meet the WHO guideline of 50 mg L−1) ranging between 393.5–775.6 mg L−1 (dry season) and 189.2–302.0 mg L−1 (wet season). As observed for other soluble ions, the values of NO3 − were higher during dry season than wet season. This seasonal variation tendency of nitrate concentration in wells is in agreement with other studies performed under tropical conditions (Boutin 1987; Bricha et al. 2007; Mkandawire 2008; Pritchard et al. 2008).
It is very difficult to explain well the fluctuation of these parameters according to the wells and season. Some recent studies performed in the similar environment demonstrated that the various attributes of watersheds, such as morphological and geological factors, as well as the local socio-economic conditions, lack of adequate sanitation and uncontrolled landfills can influence the status of surface and ground water bodies through physical, chemical, biological or bacteriological parameters (e.g. Tshibanda et al. 2014; Devarajan et al. 2015a; Tallar and Suen 2015). The presence of high concentration of soluble ions such NO3 − in study area can probably be explained by water infiltration from crops (urban agriculture) which use fertilizers near wells (Ndembo Longo 2009; Ngelinkoto et al. 2014), mineralization of organic matter of septic tanks and uncontrolled landfills. It has been demonstrated that the concentration of NO3 − and K+ can be considered as the indicators of pollution from a range of organic sources, including latrines, landfills, leaking sewers, excrement from livestock or fertilizers or manures used on agricultural land (Banks et al. 2002).
Microbiological Quality of Water
Microbiological analysis of water showed that 100 % of analysed wells are substantially polluted with faecal matters in both dry and wet seasons (Fig. 3) and did not meet the WHO guideline for drinking/domestic using water. During the dry season, the FIB values ranged from (0.9–3.5) ×102, (1.1–5.8) ×102 and (1.9–4.2) ×103 CFU 100 mL−1 for E. coli, ENT and TC, respectively. FIB concentrations for the wet season increased about 2 to 3 orders of magnitude than those observed for the dry season, with the values ranged from 8.6 × 102 to 1.6 × 104, 0.8 × 102 to 1.5 × 104 and 2.5 × 102 to 9.0 × 105 100 mL−1 for E. coli, ENT and TC, respectively. These results indicate that water samples from tested shallow wells are heavily polluted in FIB. The contamination of wells in FIB can be attributed by several sources and mechanisms, including urban runoff, percolation from surface water, the absence of toilet facilities, septic systems’ leaky sewer lines, direct injection of wastewater effluent and direct contamination by users (John and Rose 2005; Kelly et al. 2009; Nwachukwu et al. 2010; Nola et al. 2013). In our studied site, the deterioration of the quality of water from wells can probably explained by several aspects including the lack of adequate sanitation, protection of wells (there are many open wells), the presence of pit latrines located in the proximity of wells, open defecation and uncontrolled landfills (Fig. 2).
The surface recreation water as well as groundwater generally contains indigenous microorganisms, pathogenic and non-pathogenic microbes. The choice of bacterial indicators is thus very important for the management of aquatic environmental quality (Haller et al. 2009). The U.S. Environmental Protection Agency and the European Union recommend the use of Escherichia coli (E. coli), a subset of the faecal coliform group, and members of the genus Enterococcus, the Enterococci (ENT), to assess the hygienic safety of recreational waters (US EPA 2000; EU 2006). In this study, E. coli, ENT and TC were chosen to monitor the microbiological quality for the studied site under tropical conditions (Mwanamoki et al. 2014; Rochelle-Newall et al. 2015). Exposure to waters with high concentrations of E. coli and ENT is documented in epidemiological studies as being associated with an increased risk of contracting gastrointestinal and respiratory illnesses. The occurrence of these indicators in the studied site can therefore indicates water contamination in faecal material, and consequently, the possible presence of pathogenic organisms responsible for water-related diseases such as gastrointestinal, typhoid, cholera and other diarrhoeal diseases (US EPA 1984; Kay et al. 1994; Haile et al. 1999; An et al. 2002; Noble et al. 2004).
Characterization of FIB
Qualitative PCR assays were applied for large-scale screening of human-specific Bacteroides, in order to estimate as potential alternative indicators of human faecal material. Human material in water is generally considered to be a greater risk to human health risk (Scott et al. 2005; Converse et al. 2009). A total of 106 isolated colonies of E. coli from each wells were selected for PCR amplification to detect the human-specific Bacteroides. The PCR amplification performed for faecal human pollution showed high specific-human strains with the values ranging from 70 to 95 % (average for all test wells). Surprisingly, during both dry and wet seasons, PCR amplification demonstrated that 100 % of bacteria were from human origin for P2, P5, P6 and P7. These wells are characterized by highly values of physicochemical parameters during both dry and wet seasons (Table 4).
Statistical Correlation
FIB and physicochemical parameters were, for the most part, poorly correlated in sampled wells whether during dry season than during the wet season. In June, few correlations were observed between (Table 5); E. coli and TC (r = 0.97, p = 0.001); K+, Na+ and NO2 − (0.86 < r<0.89, p < 0.01); Na+ and NO3 − (0.76 < r<0.86, p < 0.01); NO2 − and SO4 2− (0.82 < r<0.85, p < 0.02); NO3 − and PO4 3−(r = 0.86, p < 0.01); and SO4 − and TC (r = 0.81, p < 0.05). Likewise, in October (Table 6) some sporadic correlations were observed between E. coli and ENT (0.85 < r < 0.93, p < 0.05), Na+ and PO4 3− (r = 0.90, p < 0.01); and O2 and TC (r = 0.72, p < 0.05). These results indicate that, in general, FIB as well as soluble ion contamination wells could be considered to originate from different sources as explained above.
Inspection of ACP (Fig. 4) clearly shows the impact of seasonal variation on the wells contamination in FIB and dissolved ions, mainly NO3 −. Data show that the months of June and October are oppositely and separately clustered on separate axes of the first-principle component. During the rainy season, urban runoff, which is charged in contaminant, is continuously drained to wells leading to a large increase of bacterial contamination. On the contrary, during the wet season, there is no urban runoff to the well. FIB (mainly E. coli), which are indicator of recent contamination, showed a decrease and probably the evaporation of water can lead to a concentration of soluble ions in the wells. These hypotheses can explain higher concentrations of FIB during the wet season (less during the dry season) and higher concentrations of dissolved ions during the dry season (less during the wet season) (Mkandawire 2008; Pritchard et al. 2008; Msilimba and Wanda 2013). Additionally, domestic livestock and household activities as well as waste disposal could also be major contributory factors of wells contamination during both dry and wet seasons (Banks et al. 2002).
Conclusion
The results of this study revealed that water from shallow wells of the selected neighbourhoods of the municipality of Bumbu is heavily polluted in faecal material, in both dry and wet seasons. The situation is more alarming during the wet season. Physicochemical parameters, especially NO3 − and EC in water samples, are unacceptable for the human consumption for both dry and wet seasons. Consequently, water from the studied zone is not appropriate for drinking or other domestic uses.
To our knowledge, this is the first study, which assessed the FIB concentration in the shallow wells of a suburban municipality of Kinshasa. Their presence in water suggests that viable human pathogens may also be present. Shallow wells represent the majority of domestic water supply in suburban municipalities of Kinshasa. More than 70 % of domestic water supply in this study site originated from shallow wells. Our results highlight on the human risk associated with exposure to water contamination from wells according to the high level of NO3 −, EC, E. coli and ENT in both dry and wet seasons. The results of this study will help a better understanding of the microbiological pollution problematic in shallow wells under tropical conditions and will guide future municipality decisions on improving the water quality. Therefore, we recommend (1) the monitoring programme of water quality in shallow wells of other municipalities of Kinshasa, (2) implementation of wells protective measure (or construction of appropriate wells) and methods of water supply from shallow wells against to the risks of pollution, (3) education programme of population for sanitation tools and (4) the urgent development of appropriate and local treatment for purification of water such as the use of Moringa oleifera (Poumaye et al. 2012). The methods, scenarios and recommendations from this research can be performed in similar environment under tropical conditions. Nevertheless, further investigation is recommended for the assessment of emerging contaminants such as antibiotics, antibiotic resistant bacteria and antibiotic resistance genes in wells.
References
Ahmed W, Stewart J, Gardner T, Powell D, Brooks P, Sullivan D, Tindale N (2007) Sourcing faecal pollution: a combination of library-dependent and library-independent methods to identify human faecal pollution in non-sewered catchments. Water Res 41(16):3771–3779. doi:10.1016/j.watres.2007.02.051
Amanial HR (2015) Assessment of physicochemical quality of spring water in Arbaminch, Ethiopia. J Environ Anal Chem 2:157. doi:10.4172/2380
An Y-J, Kampbell DH, Breidenbach GP (2002) Escherichia coli and total coliforms in water and sediments at lake marinas. Environ Pollut 120(3):771–778
APHA (2005) Standard methods for the examination of water and wastewater. Washigton, DC
Banks D, Karnachuk OV, Parnachev VP, Holden W, Frengstad B (2002) Groundwater contamination from rural pit latrines: examples from Siberia and Kosova. Water Environ J 16(2):147–152
Bernhard AE, Field KG (2000) A PCR assay to discriminate human and ruminant feces on the basis of host differences in Bacteroides-Prevotella genes encoding 16S rRNA. Appl Environ Microbiol 66(10):4571–4574. doi:10.1128/aem.66.10.4571-4574.2000
Boutin C (1987) L’eau des nappes phréatiques superficielles, une richesse naturelle vitale mais vulnérable. L’exemple des zones rurales du Maroc. Sci Eau 6(3):357–365
Bricha S, Ounine K, Oulkheir S, El Haloui N, Attarassi B (2007) Etude de la qualité physicochimique et bactériologique de la nappe phréatique M’nasra (Maroc). Afr Sci 03(3):397
Converse RR, Blackwood AD, Kirs M, Griffith JF, Noble RT (2009) Rapid QPCR-based assay for fecal Bacteroides spp. as a tool for assessing fecal contamination in recreational waters. Water Res 43(19):4828–4837. doi:10.1016/j.watres.2009.06.036
Devarajan N, Laffite A, Graham ND, Meijer M, Prabakar K, Mubedi JI, Elongo V, Mpiana PT, Ibelings BW, Wildi W, Poté J (2015a) Accumulation of clinically relevant antibiotic-resistance genes, bacterial load, and metals in freshwater lake sediments in Central Europe. Environ Sci Technol 49(11):6528–6537. doi:10.1021/acs.est.5b01031
Devarajan N, Laffite A, Ngelikoto P, Elongo V, Prabakar K, Mubedi J, Piana PM, Wildi W, Poté J (2015b) Hospital and urban effluent waters as a source of accumulation of toxic metals in the sediment receiving system of the Cauvery River, Tiruchirappalli, Tamil Nadu, India. Env Sci Pollut Res 22(17):12941–12950. doi:10.1007/s11356-015-4457-z
EIES (Etudes d’impact environnemental et social) (2012) Projet d’alimentation en eau potable de la ville de Kinshasa, vol 74
EU (2006) European Directive 2006/7/CE of the European Parliament and of the Council of 15 February 2006 concerning the management of bathing water quality and repealing Directive 76/160/EEC
Haile RW, Witte JS, Gold M, Cressey R, McGee C, Millikan RC, Glasser A, Harawa N, Ervin C, Harmon P, Harper J, Dermand J, Alamillo J, Barrett K, Nides M, G-y Wang (1999) The Health effects of swimming in ocean water contaminated by storm drain runoff. Epidemiology 10(4):355–363
Haller L, Amedegnato E, Pote J, Wildi W (2009) Influence of freshwater sediment characteristics on persistence of fecal indicator bacteria. Water Air Soil Pollut 203:217–227. doi:10.1007/s11270-009-0005-0
John DE, Rose JB (2005) Review of factors affecting microbial survival in groundwater. Environ Sci Technol 39(19):7345–7356
Kay D, Jones F, Wyer MD, Fleisher JM, Salmon RL, Godfree AF, Zelenauch-Jacquotte A, Shore R (1994) Predicting likelihood of gastroenteritis from sea bathing: results from randomised exposure. Lancet 344(8927):905–909. doi:10.1016/S0140-6736(94)92267-5
Kelly WR, Panno SV, Hackley KC, Martinsek AT, Krapac IG, Weibel CP, Storment EC (2009) Bacteria contamination of groundwater in a mixed land-use Karst region. Water Qual Expo Health 1(2):69–78
Mkandawire T (2008) Quality of groundwater from shallow wells of selected villages in Blantyre District, Malawi. Phys Chem Earth Part B 33(8–13):807–811. doi:10.1016/j.pce.2008.06.023
Montgomery MA, Elimelech M (2007) Water and sanitation in developing countries: including health in the equation. Environ Sci Technol 41(1):17–24. doi:10.1021/es072435t
Msilimba G, Wanda EMM (2013) Microbial and geochemical quality of shallow well water in high-density areas in Mzuzu City in Malawi. Phys Chem Earth Part B 66:173–180
Mubedi JI, Devarajan N, Le Faucheur S, Mputu JK, Atibu EK, Sivalingam P, Prabakar K, Mpiana PT, Wildi W, Poté J (2013) Effects of untreated hospital effluents on the accumulation of toxic metals in sediments of receiving system under tropical conditions: case of South India and Democratic Republic of Congo. Chemosphere 93(6):1070–1076. doi:10.1016/j.chemosphere.2013.05.080
Mwanamoki PM, Devarajan N, Thevenon F, Atibu EK, Tshibanda JB, Ngelinkoto P, Mpiana PT, Prabakar K, Mubedi JI, Kabele CG, Wildi W, Pote J (2014) Assessment of pathogenic bacteria in water and sediment from a water reservoir under tropical conditions (Lake Ma Vallée), Kinshasa Democratic Republic of Congo. Environ Monit Assess 186(10):6821–6830. doi:10.1007/s10661-014-3891-6
Mwanamoki PM, Devarajan N, Niane B, Ngelinkoto P, Thevenon F, Nlandu JW, Mpiana PT, Prabakar K, Mubedi JI, Kabele CG, Wildi W, Poté J (2015) Trace metal distributions in the sediments from river-reservoir systems: case of the Congo River and Lake Ma Vallée, Kinshasa (Democratic Republic of Congo). Environ Sci Pollut Res 22(1):586–597. doi:10.1007/s11356-014-3381-y
Ndembo Longo J (2009) Apport des outils hydrographiques et isotopiques à la gestion de l’aquifère du Mont Amba Thesis. Université d’Avignon, France
Ngelinkoto P, Thevenon F, Devarajan N, Birane N, Maliani J, Buluku A, Musibono D, Mubedi JI, Poté J (2014) Trace metal pollution in aquatic sediments and some fish species from the Kwilu-Ngongo River, Democratic Republic of Congo (Bas-Congo). Toxicol Environ Chem 96(1):48–57. doi:10.1080/02772248.2014.910211
Noble RT, Leecaster MK, McGee CD, Weisberg SB, Ritter K (2004) Comparison of bacterial indicator analysis methods in stormwater-affected coastal waters. Water Res 38(5):1183–1188. doi:10.1016/j.watres.2003.11.038
Nola M, Nougang ME, Noah Ewoti OV, Moungang LM, Krier F, Chihib N-E (2013) Detection of pathogenic Escherichia coli strains in groundwater in the Yaoundé region (Cameroon, Central Africa). Water Environ J 27(3):328–337. doi:10.1111/j.1747-6593.2012.00349.x
Nwachukwu MAM, Feng HH, Amadi MIM, Umunna FUF (2010) The causes and the control of selective pollution of shallow wells by coliform bacteria, Imo River Basin Nigeria. Water Qual Expo Health 2(2):75–84
Poté J, Haller L, Kottelat R, Sastre V, Arpagaus P, Wildi W (2009) Persistence and growth of faecal culturable bacterial indicators in water column and sediments of Vidy Bay, Lake Geneva, Switzerland. J Environ Sci 21(1):62–69. doi:10.1016/S1001-0742(09)60012-7
Poumaye N, Mabingui J, Lutgen P, Bigan M (2012) Contribution to the clarification of surface water from the Moringa oleifera: case M’Poko River to Bangui, Central African Republic. Chem Eng Res Des 90:2346–2352. doi:10.1016/j.cherd.2012.05.017
Pritchard M, Mkandawire T, O’Neill JG (2008) Assessment of groundwater quality in shallow wells within the southern districts of Malawi. Phys Chem Earth Part B 33:812–823. doi:10.1016/j.pce.2008.06.036
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienne
Rochelle-Newall E, Nguyen TMH, Le TPQ, Sengtaheuanghoung O, Ribolzi O (2015) A short review of fecal indicator bacteria in tropical aquatic ecosystems: knowledge gaps and future directions. Front Microbiol 6:308. doi:10.3389/fmicb.2015.00308
Scott TM, Jenkins TM, Lukasik J, Rose JB (2005) Potential use of a host associated molecular marker in enterococcus faecium as an index of human fecal pollution. Environ Sci Technol 39(1):283–287. doi:10.1021/es035267n
Tallar RY, Suen J-P (2015) Identification of waterbody status in Indonesia by using predictive index assessment tool. Int Soil Water Conserv Res 3(3):224–238
Thevenon F, Regier N, Benagli C, Tonolla M, Adatte T, Wildi W, Poté J (2012) Characterization of fecal indicator bacteria in sediments cores from the largest freshwater lake of Western Europe (Lake Geneva, Switzerland). Ecotox Environ Saf 78:50–56. doi:10.1016/j.ecoenv.2011.11.005
Tshibanda JB, Devarajan N, Birane N, Mwanamoki PM, Atibu EK, Mpiana PT, Prabakar K, Mubedi Ilunga J, Wildi W, Poté J (2014) Microbiological and physicochemical characterization of water and sediment of an urban river: N’Djili River, Kinshasa, Democratic Republic of the Congo. Sustain Water Qual Ecol 3–4:47–54. doi:10.1016/j.swaqe.2014.07.001
UNEP (2011) Emerging issues in our global environment: post-conflict environmental assessment in democratic Republic of the Congo. Synthesis for policy makers (United Nations Environment Programme)
US EPA (1984) Health effects criteria for fresh recreational waters. Environmental Protection Agency, U.S
US EPA (2000) Improved enumeration methods for the recreational water quality indicators: enterococci and Escherichia coli EPA-821/R-97/004 (U.S. Environmental Protection Agency). Washington, DC
WHO (World Health Organisation) (2004) Guidelines for drinking-water quality, recommendations Geneva
WHO (World Health Organisation) (2011) Guidelines for drinking-water quality
Acknowledgments
We are grateful to the financial sources: the Swiss National Science Foundation (Grant No. 31003A_150163/1) and Swiss embassy in DRC via M. Christian Gobet. This result presents the collaboration between the University of Geneva (Institute F. A. Forel), University of Kinshasa and Pedagogic National University of Congo (Democratic Republic of Congo).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Standards
We confirm that the field studies and sampling did not involve misunderstanding. The funder had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Rights and permissions
About this article
Cite this article
Kapembo, M.L., Laffite, A., Bokolo, M.K. et al. Evaluation of Water Quality from Suburban Shallow Wells Under Tropical Conditions According to the Seasonal Variation, Bumbu, Kinshasa, Democratic Republic of the Congo. Expo Health 8, 487–496 (2016). https://doi.org/10.1007/s12403-016-0213-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-016-0213-y