Abstract
The activity level of natural uranium pollutant in surface-based water around Princess Gold Mine in Roodepoort, South Africa was measured using inductively coupled plasma mass spectrometry. The highest activity level of 6.39E+04 mBq/L is reported in the reddish brown ochre surface water from tailing (SWA-RB) close to the houses, whereas the lowest value of 1.92E+03 mBq/L is reported in the flowing surface water (SRWA-5) 1 km away from the dump. Along the path high values of 1.56E+04, 1.07E+04, 1.57E+04 and 8.46E+03 mBq/L were reported at SRWA-2, SRWA-3 and SRWA-4, respectively. The inhabitants living around the tailings use the surface water for daily consumption. Based on the annual limit guideline for drinking water recommended by World Health Organization (731 L/year), this study revealed that, the community around this vicinity receives 2.10 mSv as the highest annual collective effective dose due to 238U in the drinking surface water. The radiological-health risks of 238U in the water samples analysed revealed the highest cancer mortality and morbidity values of 2.40E+03 and 3.67E+03, respectively. The mean chemical toxicity risk for the natural uranium over the lifetime consumption is 5.31E+05 μg/kg/day which shows that the main human risk may likely be due to the chemical toxicity of natural uranium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gold mining impacts on the quality of water may cause moderate or severe changes in the physical and chemical characteristic properties which might affect our environment directly or indirectly. The interactions between water and tailing can lead to contamination of the water due to acid mine drainage (AMD) production, resulting in the flow of contaminated water into the mine voids which eventually leach into the aquifers (Scott 1995). According to Reimann and Banks (2004), a surface water source can potentially contain several naturally occurring chemical elements, many of which are not tested routinely as indices of water. The guidance on all aspects of protection against ionizing radiations and recommended limit are provided by the International Commission on Radiological Protection (ICRP) published in their commission’s own scientific journal (The Annals of the ICRP 1993). The process of exposure starts through ingestion of surface water that contains radionuclide which eventually accumulates in the skeleton, liver, kidney and soft tissues. Ingested radionuclides are not entirely retained in the human body. During the decay of Uranium isotopes into other radioactive elements, the emission of alpha, beta and gamma radiations occurs (Bleise et al. 2003). The uranium concentration in surface water may depend on the mine tailing types and other anthropogenic activities of an area (Bhatt and Saklani 1996; Nisi et al. 2008; Schot and Van Der Wal 1992). Recent investigations have shown that the concentration of radium in water is correlated with its salinity.
All levels of radiological exposure may pose some probability linear response even at low doses which can be used for direct carcinogenic evaluations in relation to chemical changes (mutations) to DNA. Uranium is more harmful because of its toxic nature rather than its radioactivity (Kurttio et al. 2005) level and has been identified as a nephrotoxin by the World Health Organization (WHO) (Kurttio et al. 2002; 2005).
During the last few years, a number of studies have been carried out to investigate the anomalous behaviour of uranium isotopes in surface water (Rodriguez and Sanchez 1995). Though there is enough proof about the levels of surface water pollution (as a result of mining activities), little effort is directed into the types of chemical and health assessment dose due to natural radionuclides of the polluted sites. The motivation for this study is based on the fact that—water is becoming a scarce resource in South Africa and the available water resources are being polluted by industrial and domestic effluents, mine drainage, agricultural runoff and litter. Secondly, the impacts associated with polluted surface water are fast affecting the users in the vicinity of the site and downstream. The runoff water from the dump site goes down to meet the stream, rivers and surrounding hand dug wells. Thirdly, the presence of contaminated surface water creates a health hazard both to human life and aquatic health.
In Roodepoort, less information is available on activity and toxicity levels of uranium on the surface water, since the majority of the inhabitants rely on surface water due to inadequate public water supply by Rand water, which plays a key role in South African water sector such as bulk water supply infrastructure. The government-owned water board known as the Rand water supply is inadequate to meet with the public demand, and the inhabitants in Roodepoort area either use the flowing (dripped) water from the Princess Gold Mine contaminated with the AMD along the flowing path directly or through hand dug shallow wells. The study seeks to determine the concentrations, radiological and chemical toxicity risks of 238U on the inhabitants that may rely on the surface water for survival and to draw a comprehensive baseline. In order to evaluate concisely the effect of the abandoned Princess gold mine tailings and the environment, the water samples were collected from the seeping surface water from the mine dump and along the flowing path from the dump before it joined the down town river. Finally, this study is important because the outcomes can be applied to other sites which have similar pollution problems in South Africa and elsewhere.
Materials and Methodology
Study Site Description
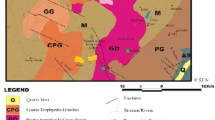
The Princess gold dump site is an old and abandoned gold tailings dam located in Davidsonville, Roodepoort, West of Johannesburg at Longitude 27°55′00″E and Latitude 26°09′30″S. The dump is approximately 3 km from the North-West of Durban Roodepoort. The area of the tailing site falls within the upper Klip River sub-catchments region, a tributary to the Vaal River. The general location of the study area is marked as point “A” in Fig. 1. The top of the mine dump is generally devoid of any vegetation, but there are plenty of trees, grasses and waters around it (Tshivhase et al. 2015).
The site has not been closed or rehabilitated as required by the South African legislation which is intended to protect the environment and human health from pollution risks that source from the tailings. The mine dump provides an example of the environmental and health risks as the surfaces are exposed to little or no vegetation. There are easy access to the site from the existing roads and a man-made wetland that has developed to the west of the tailing as a result of the previous mining activities, seepage and municipal discharge. The tailings sediments have contaminated the artificial surrounding surface water, streams, man-made wetland and other riparian zones around the site as shown in Figs. 2 and 3. The quality of surface runoff has become increasingly compromised, rendering it a threat to the environment and a potential human health risk. The impact of the contaminated water on the surface water system is striking. The colour of the water in the wetland and the waterway is reddish brown ochre due to the tailing site which is located close to the densely populated settlement. Some of the houses within this settlement have been erected at the foot of the tailing and children move and play around the surface water which creates a potential risk to their health (Figs. 2, 3).
Geology and Soils of the Study Site
The geology of the study site comprises a thick sequence of sediments overlain by Achaean sedimentary rocks. The surface geology is dominated by quartzite and conglomerates of the Central Rand Group, a sub-division of the Witwatersrand Super group. There are fresh hard rock dolomites at the depths ranging from 0 to 50 m below ground (DWAF 2004).
The soils are mostly sandy loams with low to medium base status derived from the weathering of the underlying dolomite and quartzite (SRK 2013). The site is highly susceptible to erosion which in turn results in a high sediment load that end up in the surrounding surface water, streams and rivers increasing the water course siltation, water texture and reducing the water quality around the environments (McMillan et al. 2012).
Surface Water Hydrology
The surface water flow pattern from the dump indicates that it hydrological system can no longer be regarded as a natural system but rather as a man-made system. This is because the natural features such as surface running waters, streams and wetlands have been impacted upon by human activities such as the gold mining, creation of retention dams, roads and bridges.
Sample Collection and Preparation
This study investigates the quality of surface-based surrounding water used possibly for human consumption without treatments around the Princess gold dump environs. The data on the chemical radiological of radionuclides and other toxic metals in surface drinking water supply in the study locations are scanty. Surface water samples were collected from the study area at SWA-RB (20 m away from the tailing) and SRWA-1 to SRWA-5 (which represented five surface water samples that were collected between 20 m and 1 km away from the mine dump) and were labelled for easy identifications (see Table 1).
High-density polyethylene containers were used for the collection of the water samples at the study sites as indicated in Table 1. 10 % nitric acid solution was used to wash the containers for 15 min, rinsed repeatedly with distilled water and finally rinsed with ultrapure water with resistivity of about 18 MΩ/cm. Before the sampling of the water samples, the prepared collection containers were kept in sealed polyethylene bags.
5 mL of nitric acid was used to stabilize the surface water samples in each 1 kg of water collected. In order to obtain accurate elemental compositions in the surface water samples, solution analytical method was used; Elmer Pure Plus NexION Dual Detector Calibration Solution as the Atomic Spectrometric Standard with the specifications: 200 μg per Litre of Al, Ba, Ce, Co, Cu, In, Li, Mg, Mn, Ni, Pb, Tb, U and Zn was used.
Sample Analysis Using NexION 300Q ICP-MS Instrument and Technique
The measurement was performed using the NexION 300Q ICP-MS instrument available at the Centre for Applied Radiation Science and Technology (CARST), North-West University, Mafikeng Campus. The NexION 300Q ICP-MS instrument performs analyses at the parts-per-trillion level and is ideal for surface water analysis, since the vast majority of target compounds can be detected below 0.1 mg/L.
The determination methods employed in this study have been accredited according to ISO standard 17025 (European Standard EN ISO/IEC 17025:2000). In order to guard against data tampering, all the raw data including methods and parameters used were stored in an encrypted checksum-protected dataset. The audit trails, system and security-related events offer traceability for the soft-ware applications and the quality-control system allows the setting of limits, parameters and standards based on Environmental Protection Agency (USEPA 1999).
Results and Discussion
The activity concentrations of 238U were determined in the surface water samples taken from the study area and are presented in Table 1. Using the WHO (2003), the data of elemental concentrations of 238U in the surface water samples in μg/L were converted into activity concentration using the conversion factors recommended by the IAEA Technical Report No 1363 (i.e. 15 μg/L of 238U is equal to 0.19 Bq/L of 238U).
The activity concentrations of 238U in the water supplies for drinking and domestic purposes were found to be higher at SWA-RB with a value of 6.39E+04 mBq/L, whereas lower value of 1.92E+03 mBq/L was reported at SRWA-5. The activity concentration of 1.57E+04 mBq/L was noted for the surface running water at 20 m away from dump (tailing), whereas 1.92E+03 mBq/L was reported in the surface flowing water at 1 km away from the slimes. It was observed that the activity concentrations of 238U radionuclide in surface water-based drinking water at 20, 50 and 100 m were higher than that of 500 m and 1 km away. Comparing the activity level in the reddish brown ochre surface water (SWA-RB) with the other locations, SWA-RB was higher by an average factor of approximately 1.10E+01. Interestingly, the flowing surface water turns to reduce further way from the dump. The activity concentration of 238U in SWA-RB was higher than all the values obtained in other samples; probably, the observed colouration in the water source is an indication that the water has come into contact with pyrite. This location is the closest to the houses in the background as seen in Figs. 2 and 3 at approximately 150 m from the polluted man-made wetland. In addition, the surface water analysed in this tailing sites which is the public water source in the region registered higher values. This may be attributed to solubility and high content of heavy metals and also the variation of the activity of 222Rn in the water. Another reason for higher solubility of 238U in SWA-RB may be the chemical composition of the tailing (slimes).
The Accumulation of 238U in Humans and Maximum Permissible Limit
The estimation of radionuclide accumulation was to know the level of exposure to the inhabitants of the study area that depend on the surface water around Princess mine dump environs, Roodepoort, by determining the annual effective dose and the life average daily dose. The ICRP provides recommendations and guidance on all aspects of protection against ionizing radiation, which we followed in developing this work. Basically, the process of exposure starts through ingestion of surface water that contains radionuclides; after entering the human body, radionuclides are typically accumulated in the skeleton, liver, kidney and soft tissues. The dose coefficients enable us to determine the effective dose which is associated with radiation exposure and assessment of health risk to humans. The dose coefficient is defined as the effective dose equivalent per unit water activity concentration (Sv/Bq) of the radionuclide.
The annual effective dose is calculated taking into account—the activity concentration of the nuclide (Bq/L) in the water samples, the dose coefficient (=4.5 × 10−8) (WHO 2003; ICRP 1995) and the annual water consumption (=731 L/year) from the following equation:
where, AED is the annual effective dose, AC the activity concentration, DC the dose coefficient and AWC the annual water consumption.
In this study, Eq. (1) was used to determine the annual effective dose of the surfaces water samples for 238U radionuclide as shown in Table 2.
The WHO and Environmental Protection Agency (EPA-USA) used the quantity of 2 L/day water consumption for adults (WHO 2004). The annual effective dose reported higher in SWA-RB with a value of 2.10E+00 mSv/year and lower value of 6.32E−02 mSv/year noted at SRWA-5 as shown in Table 2. Even though the dose reduces at increasing distances from the tailing, the values between the flowing path SRWA-1 and SRWA-5 were averagely high with the value of 3.45E−01 mSv/year and was 6.10E+00 in magnitude lower than the value obtained at SWA-RB with a value of 2.10 mSv/year. In disparity with the previous report of the international standard (Council Directive 98/83/EY/1996), 0.1 mSv/year, the value of 6.38E−01 was very high in all the surface water samples obtained in the study area, SRWA-1, SRWA-2 and SRWA-3, with values 5.16E−01, 5.13E−01 and 3.52E−01 mSv/year, which were far above the recommended limit.
Table 2 summarizes the concentrations of 238U in the surface water samples from the study area when compared to the results obtained in the daily consumption of both treated (TWGM) and fissure (FWGM) underground water samples from the mine in the border of Gauteng. It was noted that the concentrations of 238U in this present work are higher than the results of the underground water samples from the mine in the border of Gauteng with TWGM having a value of 9.22E+03 mBq/L and FWGM having a value of 4.46E+03 mBq/L. Even though these values in TWGM and FWGM were lower in comparison to the present study, they were far above the WHO (2003, 2006) proposed guideline value of 1.90E+02 mBq/L.
According to Internal Commission on Radiological Protection (ICRP 1995), uranium gets into the soft tissues and gets excreted in urine. It can be excreted in a few months, whereas in parents it could be retained for years. The WHO in 2003, proposed a guideline of 15 μg/L (0.19 Bq/L) and the result of this present study is far above the recommended limit and the range for the standard guideline for drinking water.
Health Risk Assessment of 238U in the Surface Water
The lifetime cancer risk associated with the intake of a given radionuclide in the surface water was determined as the health risk assessment focusing on the radiological risk assessment. The lifetime cancer risks LCR, associated with the intake of 238U, was evaluated by multiplying the applicable risk coefficient Rc and the per capita activity intake PCAI as follows:
The average life expectancy at birth total in 2013 in South Africa is 62.2 years (SAMRC 2015) and an annual consumption of water for an individual is about 731 L. This brings the lifetime intake of water to 45,468.2 L. The cancer risk coefficients of uranium, 1.13 × 10−9/Bq for mortality and 1.73 × 10−9/Bq for morbidity, respectively, were obtained from the literature (USEPA 1999; UNSCEAR 2000). With the aid of Eq. (2) and these coefficients, the cancer mortality and morbidity risks of uranium over lifetime consumption of water were calculated and the results are presented in Fig. 4.
In Fig. 4, the cancer mortality risk in the study site, ranged from 3.28E+03 to 9.86E+01, while the morbidity risk ranges from 5.03E+03 to 1.51E+02. The highest cancer mortality value was found at SWA-RB surface water with the highest value and SRWA-5 reported the lowest value. The highest cancer morbidity of 15.03E+03 was also noted at SWA-RB, whereas a lower value of 1.51E+02 reported at SRWA-5 (Fig. 4). Comparing the value of cancer mortality risk reported at SWA-RB water sample with a value of 3.28E+03 to 4.74E+02 and 2.29E+02 values of cancer mortality risks for TWGM and FWGM, respectively, SWA-RB was distinctly higher than the two values with a factor of 9.34E+00. In contrast with a study reported by Amakom and Jibiri (2010) in Ogun State, Nigeria, SWA-RB water sample of 3.28E+03 was far higher (Fig. 4). When compared the cancer morbidity of 5.03E+03 obtained from SWA-RB water sample was higher than 7.25E+02 and 3.51E+02 values obtained for cancer morbidity for TWGM and FWGM water samples, respectively. In the surface flowing water samples, SRWA-1 which was 20 m away from the tailing recorded a high value of 1.23E+03 while SRWA-1 sample obtained 1 km away recorded a value of 1.51E+02 for cancer morbidity. This value reduces further away from the tailing (slimes) as the water joined the downstream rivers. When compared with the value of 3.39E−04 for cancer morbidity reported in Ogun State, value of 1.05E−07, the value obtained in the present study is by far very high. The cancer risk at 10+2 is high compared to the acceptable level of 10−3 for the radiological risk (USEPA 1999) by a factor of 10+5. It can be observed that cancer mortality and morbidity risks reported higher at SWA-RB could be attributed to the closeness of this source to the mine dumps.
Chemical Toxicity Risk and Hazard Quotient of 238U in the Surface Water
The chemical toxicity was the main critical focus of this study and was determined in this study. This parameter was used to determine the effect of the carcinogenic and non-carcinogenic risks associated with 238U in the water sample selected for this study. The parameter (chemical toxicity risk) was evaluated using the lifetime average daily dose (LADD) of 238U through drinking water intake, and compared with the reference dose (RFD) of 0.6 μg/kg/day (USEPA 1999). These reference values are used as standard criteria for uranium in drinking water in several foreign organizations, and thereby producing an LADD and hazard quotient as follows:
where LADD is the lifetime average daily dose, EPC is the exposure point concentration (μg/L), IR is the water ingestion rate (L/day), EF is the exposure frequency (days/year), ED is the total exposure duration (years), AT is the average time (days) and BW is the body weight (kg).
Consequently, using IR = 2 L/day, EF = 350 days and ED = 62.2 years, AT = 22,703 (obtained from 62.2 × 365) and BW = 70 kg weight of a standard value for man according to USEPA (1999), the chemical toxicity risk and hazard quotient for 238U over a lifetime consumption was estimated and is presented in Fig. 5.
As seen in Fig. 5, the exposure dose in the study area ranged from 1.75E+06 to 5.26E+04 μg/kg/day. The LADD values were observed to be higher in the SWA-RB and SRWA-1 (20 m away from the tailing) compared to the SRWA-5 (1 km away from the tailing). This could be due to the presence of high concentration of radionuclides and heavy metals in the tailing. Comparing the LADD from SWA-RB to SRWA-5 and SRWA-4, it can be observed that the value of 1.75E+06 μg/kg/day for SWA-RB was higher than 5.26E+04 and 2.32E+05 μg/kg/day values for SRWA-5 and SRWA-4, respectively, with factors of 33.28 and 7.03 days, respectively. However, SWA-RB reported higher value of LADD of 238U than TWGM and FWGM which may have formed soluble complexes in aqueous phase in deeper underground water. Comparing the LADD obtained in this study and the RFD (0.6 μg/kg/day) that is an acceptable level (Yeshin et al. 2004), the chemical toxicity risk due to 238U in the surface water samples were all above the RFD. This shows that there may be health risks associated with 238U in the surface water samples, which are mainly due to the chemical toxicity risk of 238U.
Hazard Quotient of 238U in the Water Samples
The hazard quotient was obtained by dividing the LADD of 238U through drinking water intake, by the RFD of 0.6 μg/kg/day (USEPA 1999). In a clear context, the hazard quotient parameter which is the ratio of the potential exposure to a substance and the level at which no adverse effects are expected was determined in this study. It should be noted that if this parameter is calculated to be <1, then no adverse health effects would be expected as a result of the exposure. But, if the calculated parameter is >1, then adverse health effects may be possible (USEPA 2000). However, the hazard quotient cannot be translated to a probability that an adverse health effect will occur or would be proportional to risk. The hazard quotient of the measured water samples in the study area is presented in Fig. 5 with the values reported to be far above 1 in all the water samples. In contrast with the international organization standard, the values obtained in this present work are not in agreement with (USEPA 2000) a value less than one (<1).
Conclusion
In Princess Gold Mine tailing sites and vicinities in South Africa, the mean annual effective dose from the natural radionuclide (238U) for the users of the surface water was estimated to be 6.38E−01 mSv of the annual collective dose. The highest activity concentration of radionuclide was noted in SWA-RB with a value of 6.39E+04 mBq/L. The lowest value was reported at º which was 1 km away from the tailing site. The radiological risks of 238U in the water samples were found to be high, typically in magnitude of 10+2. It could be that the human risk due to 238U content in the surface flowing and the reddish brown ochre surface water from the tailing site may likely be linked to the chemical toxicity of 238U as a heavy metal rather than radiological risk. Taking the whole analysis of the surface water into consideration, it is recommended that carcinogenic pollution may be the primary pollutant that pose the health risk in the region.
References
Amakom CM, Jibiri NN (2010) Chemical and radiological risk assessment of uranium in borehole and well waters in the Odeda Area, Ogun State, Nigeria. Int J Phys Sci 5(7):1009–1014
Bhatt KB, Saklani S (1996) Hydro-geochemistry of the upper Ganges river. J Geol Soc India 3(48):171–182
Bleise A, Danesi PR, Burkart W (2003) Properties use and health effects of depleted uranium (DU): a general overview. J Environ Radiol 64(2–3):93–112
Council Directive 98/83/EY/ (1996) On the quality of water intended for human consumption. Off J Eur Commun L330:32–54
Department of Water Affairs and Forestry (DWAF) (2004) Free state region river systems: 2003. State of Rivers Report 6. Accessed March 2004
Internal Commission on Radiological Protection (1993) Age-dependent doses to members of the public from intake of radionuclides. Part 2: ingestion dose coefficients. Pergamon Press, Oxford (Annals on the ICRP. ICRP publication 67)
Internal Commission on Radiological Protection (1995) Age-dependent doses to members of the public from intake of radionuclides, Part 3: ingestion dose coefficients. Pergamon Press, Oxford (Annals of the ICRP, ICRP publication 69)
Kurttio P, Auvinen A, Salonen L, Saha H, Pekkanen J, Makelainen I, Komulainen H (2002) Renal effects of uranium in drinking water. Environ Health Perspect 110(4):337–342
Kurttio P, Komulainen H, Leino A, Salonen L, Auvinen A, Saha H (2005) Bone as a possible target of chemical toxicity of natural uranium in drinking water. Environ Health Perspect 113(1):68–72
McMillan H, Krueger T, Freer J (2012) Benchmarking observational uncertainties for hydrology: rainfall, river discharge and water quality. Hydrol Process 26:4078–4111
Nisi B, Buccianti A, Vaselli O, Perini G, Tassi F, Minissale A (2008) Hydro-geochemistry and strontium isotopes in the Arno river basin (Tuscany, Italy): constraints on natural controls by statistical modeling. J Hydrol 360:166–183
Reimann C, Banks D (2004) Setting action levels for drinking water: are we protecting our health or our economy (or our backs!)? Sci Tot Environ 332:13–21
Rodriguez MJ, Sanchez F (1995) Behavior of uranium along Jucar River (Eastern Spain): determination of 234U/238U and 235U/238U ratios. J Radioanal Nucl Chem 190:113–120
Schot PP, Van Der Wal J (1992) Human impact on regional groundwater composition through intervention in natural flow patterns and changes in land use. J Hydrol 134:297–313
Scott R (1995) Flooding of the central and east rand gold mines, an investigation into controls over the inflow rate, water quality and the predicted impacts of flooded mines. WRC Report No 486/1/95
SRK (2013) Final scoping report for the environmental impact assessment and environmental management programme for BHP Billiton Energy Coal South Africa (Pty) Ltd (BECSA)’s Vandyksdrift Central (VDDC) Project; BHP Billiton Energy Coal South Africa (Pty) Limited (BECSA). SRK Project Number 449019
SAMRC (2015) South African Medical Research Council (MRC); Rapid mortality surveillance report 2013, issued by the council’s Burden of Disease Research Unit; Media release, 27 January 2015. www.mrc.ac.za/bod/reports.htm
Tshivhase VM, Njinga RL, Mathuthu M, Dlamini TC (2015) Transfer rates of 238U and 232Th for E. globulus, A. mearnsii, H. filipendula and hazardous effects of the usage of medicinal plants from around gold mine dump Environs. Int J Environ Res Public Health 12:15782–15793
United Nations Scientific Committee on the Effects of Atomic Radiation, UNSCEAR (2000) Sources, effect and risks of ionising radiation. UNSCEAR, New York (Report to the General Assembly with Scientific Annexes)
United State Environmental Protection Agency, USEPA (1999) Cancer risk coefficients for environmental exposure to radionuclides. U.S. Environ. Protec. Agen. Feder. Guida. Repo. 13 (EPA. 402 R-99-001)
United State Environmental Protection Agency, USEPA (2000) Methodology for deriving ambient water quality criteria for the protection of human health. EPA-822-B-00-004, pp 2–3
World Health Organization (2003) Guidelines for drinking water quality health criteria and other supporting information, vol 2, 2nd edn. World Health Organization, Geneva, pp 367–370
World Health Organization (2004) Guidelines on drinking water quality, 3rd edn. World Health Organization, Geneva
World Health Organization (2006) Meeting the MDG drinking water and sanitation target: the urban and rural challenge of the decade. World Health Organization, New York, pp 1–47
Yeshin K, Hoasung P, Jinyong K, Sun-ku P, Byongwook C, Ighwan S, Dong Chun S (2004) Health risk assessment for uranium in Korean groundwater. J Environ Radioact 77:77–85
Acknowledgments
We gratefully acknowledge the financial support from the Council for Geoscience and the School of Agriculture science for providing the equipment used in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Njinga, R.L., Tshivhase, V.M. & Mathuthu, M. Chemical Toxicity of Surface-Based Drinking Water Sources Due to Natural Uranium Pollutant Around Princess Gold Mine Environs in Roodepoort, South Africa. Expo Health 8, 457–464 (2016). https://doi.org/10.1007/s12403-016-0203-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-016-0203-0