Abstract
The aim of this study is to measure the parasite presence in treated and raw sewage by Bailenger method. All the samples were gathered from the influent and effluent of six wastewater treatment plants on a weekly basis in the period of 6 months. Totally, 180 samples were analyzed by microscopy using the modified Bailenger method. The results indicated that the average number of helminth egg and protozoan cysts per liter of raw sewage in Kermanshah, Sarpol-e-Zahab, Paveh, Ghasr-e-Shirin, Islam Abad-e-Gharb, and Gilan-e-Gharb treatment plants was 5.27, 53.41, 45.72, 35.85, 39.94, and 44.8 n/l as well as 30.1, 21.93, 25.42, 19.95, 18.1, and 15.61 n/l, respectively. Also, the highest number of helminth eggs including Ascaris lumbricoides and Hymenolepis nana was observed in the raw sewage of all the treatment plants. Thus, improvements in wastewater treatment, lack of reusing raw sewage for agricultural development, and encouraging the public to apply proper disinfection method for vegetables could be recommended for decreasing parasitic infection in the society.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Parasitic infection can be regarded as the most common disease worldwide (Beaver 1999; Sharafi et al. 2012a, b). So, more than 40 million people are affected by a parasitic infection and more than 10 % of world population are exposed to parasitic diseases (Abdussalam et al. 1995). Ascaris, Giardia, and Amoebia have been selected as the most common parasites (Sharafi et al. 2012a, b; Mintz et al. 1993). Previous studies have also revealed that about 5 million people, mainly residents of developing countries, suffer from parasitic infection (UNESCO 2003). According to the World Health Organization’s (WHO) reports, in 1981, morbidity out of amoebiasis was about 110,000 people during 1975–1981, and ascariasis infection involved about 700 million people (26 % of the world population) in 1975 (Marietta 1992). Ascariasis was recorded as the most prevalent and endemic disease in Africa, Latin America, and the Far East in Jimenez’s report (Jimenez 2007). Iran is also among the regions with predominant parasitic infection (Masoud 1997). Previous studies have revealed that the widespread parasitic infection in Kerman, Kermanshah, Mazandaran, Kashan, Urmia, Yazd, Semnan, Ghaemshahr, Bandar Abbas, and Ardabil is 2.47, 13.59, 21, 9.46, 5.22, 61, 7.13, 4.8, 4.48, and 7.27 %, respectively (Zia and Massoud 1996; Vojdani et al. 2002; Razavyan and Massoud 2002; Arbabi and Talari 2004; Hazrati et al. 2006; Dehghani-Firoozabadi and Azizi 2003; Atashnafas et al. 2005; Ranjbar-Bahadori et al. 2005; Sharifi-Sarasiabi et al. 2002; Daryani and Ettehad 2003). Parasites require some factors such as identifiable environmental conditions, availability of host, and manner of getting out of the host body. These conditions usually lead to the occurrence of parasitic infection in an area (Massod and Molavi 1995).
Parasitic diseases can be expressed through a variety of issues, including meat contamination and agricultural crops which are irrigated with raw sewage and contaminated water with domestic wastewater. Consequently, wastewater reused for agriculture is the most important source of spreading infective agents in the environment (Aimandel et al. 1997). About 600, 32, 1, 10, and 20 helminthes, hookworms, Schistosome sp, Taenia saginata, and Trichuris trichiura (whipworm) ova are found per 1 l of wastewater, respectively (Feachem et al. 1983). In addition, the average number of ova per liter of untreated sewage in different countries such as Brazil, Morocco, Jordan, Ukraine, America, France, Germany, and Pakistan has been 166–202, 214–840, 300, 60, 1–8, 9–10, 40, and 142–202, respectively (Schwartzbrod et al. 1989; Bennani et al. 1992; Strauss 1997; Ensink et al. 2007).
Based on the above-mentioned points, evaluating the type of parasitic contamination and its rate in the resources such as wastewater is very important due to the epidemiology of parasitic diseases. Furthermore, clinical applications have indicated a comprehensive example of wastewater parasitic pollution. Therefore, some studies are required to indentify the amount and type of certain and dominant contaminations in the society. The purpose of the present study is to determine the parasite presence in treated and raw sewage in Kermanshah Province.
Materials and Methods
This research was a descriptive-cross-sectional study. From each treatment plant, samples were collected from influent with the volume of 2 liter and effluent with the volume of 1 l (totally, 30 samples from each plant) using sampling plastic containers. All the samples were compounded and collected within 24 h on a random day of a week according to the standard method. Totally, 180 samples were gathered during 6 months from 5 treatment plants (five samples from each treatment plant in a month and, in sum, 30 samples during 6 months). As a result, the samples were analyzed using the modified Bailenger process with McMaster counting slide (the hole volume of 0.3 ml) (Ranjbar-Bahadori et al. 2005). In brief, all the samples were kept in the laboratory for 2 h at room temperature and then about 90 % of the supernatant was drained off and separated. The remaining residue was centrifuged at 1000 g for 15 min. Then, the entire sediment of all centrifuged tubes was transferred to a single centrifuge tube and centrifuged for the second time at 1000×g for 15 min. Afterward, the samples were suspended in an acetoacetic buffer with the formula CH3COCH2COOH (pH 4.5) with equal volume and acetylcholine was added (double volume). The samples were completely mixed by stirring and, consequently, centrifuged at 1000×g for 15 min. After removing the upper black and intermediate film, three layers were emerged in all the tubes. The deposited material was suspended in zinc sulfate 33 % (with 5 volumes and density of 1.18) and mixed up. The final product including precipitate and zinc sulfate solution was measured as an adequate volume. Finally, 0.3 ml of this solution was moved to the slide with 100 and 40 magnifications. The quantity of the cysts and helminthes eggs was recognized and counted. The equation was utilized to estimate the number of cysts and eggs in the samples (Eq. 1):
where N is the number of cysts or helminth eggs per liter of the sample, A is the average number of eggs or cysts counted on three slides under microscopic observation, X is the final volume of product in (ml), P is volume pot on the McMaster slide (0.3 ml), and V is original sample volume in (ml).
Mann–Whitney U test was used to compare the data of protozoan cysts and parasite eggs in raw sewage during spring and summer seasons at the significance level of α = 0.05. Also, levels of protozoan cysts and helminth eggs in the raw wastewater of different plants in Kermanshah Province were estimated using Kruskal–Wallis H test at the significance level of α = 0.05 (SPSS, ver. 16). The limitation of this study was in sampling period which was 6 months.
Results and Discussion
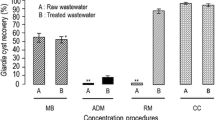
Tables 1 and 2 reveal the average mean, maximum, and minimum number of helminth eggs and protozoa cysts in the influent of all WWTP (n/l). It can be pointed out that the Enterobius vermicularis and Fasciola hepatica eggs were derived just in a sample in Kermanshah Treatment Plant. Statistical test results are represented in Table 3. Figures 1 and 2 indicate the average number of helminthe eggs and protozoan cysts in the influent raw wastewater of all the 6 WTPs during spring and summer seasons (Fig. 3).
According to the statistical test results (Kruskal–Wallis H), there was no significant difference between the average level of total protozoan cysts and helminth ova in raw sewage wastewater of all WTPs (P value > 0.05). However, the maximum and minimum of total helminth eggs per liter of raw wastewater were observed in Sarpol-e-Zahab and Ghasr-e-Shirin as well as the maximum and minimum of aggregate protozoan cysts were found in Kermanshah and Gilan-e-Gharb WTP, respectively. There were slight differences between all the investigated treatment plants in Kermanshah Province due to the presence of parasitic infection, which depends on some factors including health conditions and culture of people, climate condition, water consumption, etc. The average range number of helminth eggs of raw sewage in all the WTPs was 35.88–53.41 n/l. However, the average number of parasitic helminth ova in the raw sewage of Kermanshah WTP was higher than that of the unprocessed sewage of Tehran, Isfahan, and Shahr-e-Kurd WTP according to the previous studies (Miranzadeh and Mahmodi 2002; Arbabi and Zahedi 1998). Given that industrial places are more apread in Tehran and Isfahan than city of Kermanshah, the amount of produced wastewater without helminth eggs was further in terms of industrial places in the former; so, the average number of parasitic helminth eggs in raw wastewater of these cities was less than that of Kermanshah.
Comparison of the results of the present study with some other studies in different countries indicated that the average number of influent parasite egg per liter in all the investigated WTPs was less than that of the developing countries (70–300 n/l), Brazil (166–202 n/l), Morocco (214–840 n/l), Jordan (300 n/l), Pakistan (144 n/l), Russia (≤2000 n/l), and Ukraine (60 n/l), while the obtained results were more than the amount reported by developed countries including America (1–8 n/l), France (9–10 n/l), and Germany (≤40 n/l) (Jimenez 2007). Zamo et al. (2003) indicated that the average number of parasites including Ascaris, Toxocara, T. trichiura, Hymenolepis nana, T. saginata, and F. hepatica was 3, 17, 7, 2, 1, and 1 per liter, respectively (Zamo et al. 2003). Total average number of parasitic helminth eggs present in sewage was 31 per liter and positive test percentage in terms of the presence of Nematode, Cestodes, and Trematodes was 86, 10, and 4 %, respectively.
Maximum numbers of helminth eggs in the raw sewage wastewater of all WTPs were related to Ascaris Ascaris lumbricoides as well as H. nana eggs. Also, T. trichiura or whipworm eggs were found in the raw sewage wastewater of Gilan-e-Gharb and Sarpol-e-Zahab (even less than A. lumbricoides and H. nana), which could be due to the high resistance of A. lumbricoides than other helminth eggs such as Hookworms and T. trichiura under unsuitable environmental conditions (Arbabi and Zahedi 1998; Zamo et al. 2003; Mahvi and Kia 2006; Bitton 2005). These results indicated that the present levels of Ascaris contamination were higher than others, which was consistent with the results of similar studies. Miranzadeh and Mahmodi (2002) reported that the maximum and minimum numbers of nematode eggs in the influent raw wastewater of Shoosh Treatment Plant in Tehran were related to A. lumbricoides and T. trichiura, respectively (Miranzadeh and Mahmodi 2002). Moreover, in another study by Mahvi and Kia (2006), A. lumbricoides eggs were observed to have the greatest number in the influent sewage of 8 treatment plants in Tehran and 2 treatment plants in Isfahan (Mahvi and Kia 2006). Additionally, Arbabi and Zahedi (1998) found a similar result in their research (Arbabi and Zahedi 1998). Jimenez (2007) reviewed parasitic contamination in different countries including America, Germany, Pakistan, Egypt, Brazil, etc. and showed a similar result (Jimenez 2007). Accordingly, higher contamination with A. lumbricoides eggs than other parasites in most of the areas could be due to the high reproduction of eggs (approximately 200,000) with Ascaris and eating only a few infected eggs causes pneumonia diseases (Loffler Syndrome) (Bitton 2005). Easy prevailing of Ascaris causes an increase in parasitic infection in society, for which some parameters such as climate and geographical conditions, health situation, culture of people, etc. are very effective in terms of detecting the public contamination level. Also, Zamo et al. (2003) indicated the maximum number of Toxacara eggs in raw sewage wastewater among all the investigated helminths (Zamo et al. 2003), whereas in the present study the number of A. lumbricoides was less than that of T. trichiura. Since T. trichiura are found in soil or crops at a certain temperature (22–26 °C) and moisture, a similar condition might be found in Gilan-e-Gharb and Sarpol-e-Zahab due to the presence of T. trichiura egg. Previous studies have indicated that the maximum and minimum amount of T. trichiura infection is originated in the Caspian Sea, Sistan and Baluchistan Province, and western regions of Iran, respectively (Amirbaygi 2004).
Statistical test results showed that the parasitic infections in the raw sewage wastewater of all the investigated wastewater treatment plants were higher in spring than summer (Mann–Whitney U at the significance level of α = 0.05). Given that, in this study, all the analysis samples were taken on non-rainy days, since the amount of water consumption in the summer season is more than spring, hence, parasitic contamination of domestic wastewater in spring (low volume of wastewater production) was higher than that of the summer season.
Conclusion
According to the obtained results, the parasitic contamination status of domestic wastewater in Kermanshah Province needs to be specifically considered. Therefore, there is a pushing need for more educational programs, diagnosis, and treatment of infected individuals to reduce helminthic diseases in the society. Worms need a host to develope; therefore, they cannot live in wastewater. So, to control a part of helminthiasis, eggs are required to be either removed from wastewater or get inactivated in the wastewater. Thus, dynamic treatment plants with appropriate processes in all areas of the province might be considered. Eggs are not usually infective in wastewater, for which proper conditions including appropriate temperature and moisture are need to grow larva (26 °C and 1 month in laboratory environment). Generally, these conditions are available in soil or crops, in which eggs are dropped through irrigation with raw sewage (Atashnafas et al. 2005). Thus, improvements in wastewater treatment, lack of reusing raw sewage wastewater for agricultural development, public health knowledge, major changes in the attitude of people, and encouraging people to properly disinfect vegetables could be recommended for decreasing parasitic infection in the society.
References
Abdussalam M, Kaferstein FK, Mott KE (1995) Food safety measures for the control of food borne trematode infections. Food Control 6:71–79
Aimandel K, Mobedi A, Mesdaghinia A, Vaazi F (1997) Determination of minimal rate of ultra violet ray for kills of Ascaris egg in wastewater disinfection. J Med Fac 2:13–17
Amirbaygi H (2004) Health & treatment water, 1st edn. Andishe Rafiaa Danesh Publication, Tehran, pp 53–71
Arbabi M, Talari SA (2004) Intestinal parasites in student of Kashan university of medical sciences. J Ilam Univ Med Sci 44:24–33
Arbabi M, Zahedi MR (1998) Performance evaluation of stabilization ponds in urban wastewater treatment (in cooling climate), 2nd Congress of Environmental Health University of Medical Sciences, Kerman, Iran
Atashnafas E, Ghorbani R, Peyvandi S, Imani S (2005) Prevalence of oxyuriasis and some related factors in kindergarten and primary school children in urban areas of Semnan province. J Semnan Univ Med Sci 1:67–74
Beaver PC (1999) Clinical Parasitology, 9th edn. Lea & Febiger, Philadelphia, pp 1–16
Bennani A, Nrhari A, Razouki L, Bize J, Nivault N (1992) Wastewater treatment in greater Agadir (Morocco): An original solution for protecting the bay of Agadir by using the dune sands. Water management in coastal areas. CFRP-AGTM, Paris
Bitton G (2005) Wastewater microbiology, 3rd edn. Wiley, Hoboken
Daryani A, Ettehad GH (2003) Prevalence of intestinal infestation among primary school students in Ardabil. J Ardabil Univ Med Sci Health Serv 3:229–234
Dehghani-Firoozabadi AA, Azizi M (2003) Study of the rate of contamination of intestinal parasites among workers in fast food outlets of Yazd. J Shahid Sadoughi Univ Med Sci Health Serv 1:29–33
Ensink J, Hoek WV, Mara D, Cairncross S (2007) Waste stabilization pond performance in Pakistan and its implications for wastewater use in agriculture. Urban Water 4:261–267
Feachem RG, Bradley DJ, Garelick H, Mara DD (1983) Sanitation and disease, health aspects of excreta and wastewater management. Wiley, Chichester
Hazrati K, Mostaghim M, Khalkhali HR, Makooei A (2006) The prevalence of intestinal parasitic infection in the students of primary schools in Nazloo region in Urmia during 2004–2005. Urmia Med J 4:212–217
Jimenez B (2007) Helminth ova removal from wastewater for agriculture and aquaculture reuse. Water Sci Technol 55:485–493
Mahvi AH, Kia EB (2006) Helminnth eggs in raw and treated wastewater in the Islamic Republic of Iran. East Mediterr Health J 12:137–143
Marietta V (1992) Medical parasitology, 4th edn. Saunders Company Press, Philadelphia
Masoud J (1997) The importance of helminth diseases in Iran, 2nd congress of parasitic disease, 18–20 Oct, Tehran, Iran
Massod J, Molavi GR (1995) Survey of parasitic contamination in Isfahan hall city workers, Scientific publication of public health faculty of Tehran, pp 23–28
Mintz D, Hudson M, Mshar P, Catter M (1993) Food-born Jiardiasis in a corporate office setting. Infect Dis 167:250–253
Miranzadeh MB, Mahmodi S (2002) Investigation into the removal of nematodes eggs in influent and effluent of shoosh wastewater treatment plant. Water Wastewater J 42:32–36
Ranjbar-Bahadori S, Dastorian AR, Heidari B (2005) Prevalence of intestinal parasites in Ghaemshahr in 2004. Med Sci J Islam Azad Univ 3:151–155
Razavyan T, Massoud J (2002) Intestinal parasitic in Feraydoon Kenar, Mazandaran. J Sch Public Health Inst Public Health Res 1:39–49
Schwartzbrod J, Stien JL, Bouhoum K, Baleux B (1989) Impact of wastewater treatment on helminth eggs. Water Sci Technol 21:295–297
Sharafi K, Fazlzadehdavil M, Heidari M, Almasi A, Taheri H (2012a) Comparison of conventional activated sludge system and stabilization pond in removal of chemical and biological parameters. Int J Environ Health Eng 1(5):1–5
Sharafi K, Fazlzadehdavil M, Pirsaheb M, Derayat J, Hazrati S (2012b) The comparison of parasite eggs and protozoan cysts of urban raw wastewater and efficiency of various wastewater treatment systems to remove them. Ecol Eng 44:244–248
Sharifi-Sarasiabi K, Madani AH, Zare S (2002) Prevalence of intestinal parasites in primary school publish of Bandar Abbas. J Hormozgan Univ Med Sci 4:25–30
Strauss M (1997) Health (pathogen) considerations regarding the use of human waste in aquaculture. Environ Res Forum 5–6:83–98
UNESCO (2003) Water for people water for life, the United Nations. World Water Development Report, Barcelona
Vojdani M, Barzegar A, Shamsian A (2002) Frequency of parasitic infections in patients referred to special clinic of Kermanshah University of Medical Sciences in 1995–1999. Behbood 13:31–37
Zamo AC, Belghyti D, Lyagoubi M, Elkharrim K (2003) Parasitological analysis of the untreated wastewater of the “Ville Haute urban emissary (Maamora district, Kenitra, Morocco), US National Library of Medicine National Institutes of Health 13: 269–272
Zia A, Massoud J (1996) A survey of the prevalence of intestinal parasites in the city of Kerman. J Kerman Univ Med Sci 3:129–134
Acknowledgments
The authors wish to acknowledge the invaluable cooperating and supporting by Deputy of research committee of Kermanshah University of Medical Sciences, Health services of Kermanshah, Kermanshah wastewater company staff and officials of Kermanshah treatment plant for facilitating the issue of this project, with the No: 88091.
Compliance with Ethical Standards
All authors have read and approved the content of the manuscript and agreed with transfer of the copyright in case accepted for publication. The authors also certify that the manuscript has not been submitted or published elsewhere. All the authors contributed significantly to fit authorship criteria. No potential conflict of interest exists. Sources of funding for this study are covered by Kermanshah University of Medical Sciences. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharafi, K., Fazlzadeh, M., Pirsaheb, M. et al. Determining Parasite Presence in Raw Municipal Wastewater by Bailenger Method in Kermanshah, Iran. Water Qual Expo Health 7, 525–530 (2015). https://doi.org/10.1007/s12403-015-0168-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-015-0168-4