Abstract
Geochemical characteristics and potentially toxic element contents of groundwater, thermal springs, and cold springs of Taftan area in the southeast of Iran examined in two different seasons in order to assess their quality and possible contamination source. Groundwater and spring water samples were collected in May and September 2012 and analyzed for major parameters, anions, cations, and potentially toxic elements. Groundwater is the local source of drinking water and along with cold springs is used for agricultural irrigation. Thermal springs are mainly used for bathing and balneological purposes. For both wells and springs under study, boron was found to have the higher concentration than the specifications in WHO standards except for PF spring. Arsenic, Fe, Mn, Pb, and Ni in thermal springs and most of groundwater samples and cold springs indicate higher concentration than those of WHO standard. The low Na/Ca and Na/K ratios in STS and TTS thermal springs confirm these waters associated with up-flow zones, while higher Na/K ratios for cold springs reveal effects of lateral flows. Conservative elements indicate that thermal springs fall within the hydrothermal field, indicating magmatic affiliation of the thermal waters. High concentrations of trace elements and major ions in well water, thermal springs, and acidic cold springs provide evidences for water–rock interaction processes and the presence of active deep circulations. Saturation indices (SI) show that thermal waters are oversaturated with respect to quartz, chalcedony, alunite, gypsum, celestite, and barite, evident by precipitation of sulfate and siliceous minerals in the most recent precipitates of the geothermal system. The cold springs and groundwater are oversaturated with chalcedony, quartz, hematite (four water wells), and goethite (two water wells), slightly highlighting the mixing of the groundwater with thermal and acidic springs. The SI indicates that the Fe-phase minerals could control mobility of As in the groundwater of the Taftan area. The present study is addressing a significant risk of toxic elements in groundwater resource management, in the volcanic regions in the southeast of Iran, and suggests some preventive measures for controlling adverse effects of using such waters for drinking and irrigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Potentially toxic elements pollution is a serious problem associated with active volcanic areas and geothermal systems. The presence of potentially toxic elements, especially arsenic in geothermal and groundwater of these areas, and its environmental impact has long been recognized, e.g., Long Valle Caldera, USA (Wilkie and Hering 1998); Los Azufres, Mexico (Birkle and Merkel 2000); Los Humeros, Mexico (González et al. 2001); Kurdistan province, Iran (Mosaferi et al. 2003), west of Iran (Keshavarzi et al. 2011) and Latin America (Lopez et al. 2012). Natural contamination of groundwater with metals and metalloids due to the mixing of cold waters with geothermal fluids is often associated with a high total dissolved solids content and significant concentrations of As, B, Fe, Mn, and other trace elements (Smedley and Kinniburgh 2002; Brown and Simmons 2003; Brugger et al. 2005; Angelone et al. 2009; Landrun et al. 2009; Aksoy et al. 2009; Henke 2009). The release of potentially toxic elements into groundwater and water springs poses a health risk to the local population when these waters are the main source of water supply (Komatina 2004; Agusa et al. 2006).
Extensive areas of Iran are covered with the Tertiary volcanic rocks in a long belt from Turkey to Pakistan. According to the geological information, geothermal resources are available throughout Iran in a variety of forms and settings with or without surface exposure (thermal springs).
Taftan stratovolcano is one of the largest geothermal fields located in flysch zone of eastern Iran.
Despite visible health effects related to heavy metals and arsenic toxicity from other geothermal fields of Iran such as Kurdistan Province in west of Iran (Mosaferi et al. 2008; Barati et al. 2010; Keshavarzi et al. 2011), a few studies were carried out on water quality and its environmental aspect in Taftan area. The previous studies mainly deal with geological, petrological, geochemical, and hydrothermal aspects of the Taftan area (Gansser 1971; Boomeri 2004; Moore et al. 2005; Shakeri et al. 2008). The contemporary toxic elements crisis in thermal springs and the ongoing groundwater exploration scheme without proper attention to quality issues, and will certainly lead to a substantial threat in the Taftan volcano area in southeast of Iran, where several thousand people will be in a considerable risk of chronic trace elements poisoning.
The objectives of this study are (1) to study the selected trace element contamination in groundwater, thermal springs, and cold springs of Taftan area; (2) to study hydrogeochemical behavior of potentially toxic elements in groundwater and springs; and (3) to evaluate the possible sources of toxic elements concentration in groundwater of south of Taftan volcano.
Study Area
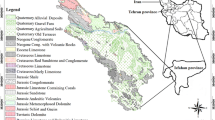
The study area lies within the Makran structural zone in southeastern Iran. The Taftan area is confined within \(28^{\circ }\,15'\) to \(28^{\circ }\,45'\) N latitudes, and \(61^{\circ }\,00'\) to \(61^{\circ }\,15'\) E longitude (Fig. 1). The oldest rocks in the Taftan area are the Upper Cretaceous pelagic limestone followed by Eocene flysch-type sediments (widespread in northern Taftan), Pleistocene, and Quaternary volcanic and volcanic-clastic rocks mainly consist of tuff and andesitic flows. The most distinct geological characteristic in the Taftan area is the exposure of thick Quaternary volcanic rocks.
Taftan volcano is a strongly eroded andesitic stratovolcano with two prominent summits. The higher one, 3940 m SE summit cone, is well preserved and highly active with sulfur-encrusted fumaroles. The deeply dissected NW cone is of the Pleistocene age (Moinvaziri and Aminsobhani 1978). Mount Taftan is now in an active, post-volcanic, and fumarolic stage.
Taftan’s volcanic rocks consist of sequence of pyroclastics and epiclastics, and lava flows of mainly porphyry andesites, dacites, and rhyolites. Andesites are dominant rocks containing plagioclase, hornblende, orthopyroxene, biotite, clinopyroxene, and quartz in association with magnetite, hematite, pyrite, chalcopyrite, titanomagnetite, and ilmenite (Boomeri 2004). Taftan mountain area is characterized by a steep topography with deep V- and U-shaped river valleys. The hottest months are from June to August with mean maximum temperature of about 30 \(^{\circ }\hbox {C}\). Average annual rainfall in the study area is about 150 mm, which mainly occurs between November and May. Snow generally falls between December and February, when the temperatures drop to a minimum of several degrees below freezing and rainfall in the region is scant.
The field studies show that waters in the Taftan area can be divided into three major groups of (1) thermal spring, (2) cold spring, and (3) groundwater. The existences of active volcanism, shallow magmatically heated rocks, and deep fracture and fault systems have created favorable conditions for the development of hydrothermal systems and permeable aquifer beds in the Taftan region (Shakeri et al. 2008).
Materials and Methods
Water samples were collected from groundwater (ten samples of Khash aquifer) and springs (five samples of Taftan volcano area; two thermal and three cold springs) in two periods i.e., May and September 2012 (a total of 30 samples in wet and dry seasons, respectively) (Tables 1, 2; Fig. 1). Prior to sampling of groundwater, water wells were pumped for 10 minute to discharge the standing volume of groundwater in wells to obtain representative formation water from the aquifer. Each sample was collected in a 1.5 l polythene bottle. The bottles were thoroughly washed with dilute hydrochloric acid, and then with distilled water in the laboratory. In the field, each bottle was filled and emptied twice with the water before final sampling. Water samples were filtered using a vacuum pump and 0.45 \(\upmu \)m pore-size filter papers in order to separate particulate matter. The filtered samples were then split into two bottles: one bottle was acidified with \(\hbox {HNO}_{3}\) for dissolved trace element measurement, and the second unacidified portion was used for the determination of dissolved anions. The samples were kept at \(4~^{\circ }\hbox {C}\) prior to analysis. Major parameters including temperature, pH, oxidation-reduction potential (ORP), and electrical conductivity (EC) were measured in the field during sampling using portable measuring devices (Eutech instruments, PCD650). The concentrations of calcium, potassium, magnesium, sodium, bicarbonate, sulfate, and chloride ions were measured in the laboratory using standard titration and ICP-OES methods. Toxic and trace elements were analyzed by ICP-MS in West laboratory, Australia. Certified reference materials were analyzed with each rack of samples to check for accuracy. In addition, a proportion of samples were analyzed in duplicate, to check for reproducibility, and reagent blanks were analyzed with each rack of samples, to check baseline contamination.
Results and Discussion
Chemistry of Springs and Groundwater
The major ion concentrations; selected toxic and trace elements; temperature; pH, Eh, TDS, and EC of thermal spring; cold spring; and groundwater samples are presented in Tables 1 and 2.
The collected water samples are characterized by the remarkable variety in their physicochemical parameters, ranging from dilute cold groundwater (samples Kh1, Kh2, Kh4, Kh7, Kh8, Kh9, Kh10, Kh11, Kh14, and Kh15) and cold springs (samples APS, PF, and FTS) to thermal springs (samples STS and TTS). Two thermal springs (STS and TTS in Table 1) have the highest measured temperatures (45–\(57~^{\circ }\hbox {C}\)), while other water samples have temperature less than \(16~^{\circ }\hbox {C}\).
Due to non-significant differences between wet and dry seasons, data processing was conducted on both seasons based on single database.
Thermal and Cold Springs
Electrical conductivity ranges from 354 to 2,240 and 25,090 to 33,560 \(\upmu \)s/cm in cold and thermal springs, respectively. According to WHO standard, thermal springs and APS cold spring display EC values above drinking water standard (Table 1). The thermal and cold springs have pH from 1.1 to 1.4 and 3.8 to 7.1, respectively. All samples except FTS cold spring sample show pH values less than 7 that reflecting acidic tendency. The Piper diagram shows that thermal and cold springs of the study area classified (Fig. 2) as type 1: Na-\(\hbox {SO}_{4}\)-Cl (STS, APS and FTS springs), type 2: Ca-\(\hbox {SO}_{4}\)-Cl (TTS Spring), and type 4: Na-\(\hbox {HCO}_{3}\) (PF spring). This suggests that Na is dominant cation in the thermal and cold springs except for TTS thermal spring, in which Ca is dominate cation. \(\hbox {SO}_{4}^{-2}\) and \(\hbox {Cl}^{-}\) are dominant anions in the thermal and cold springs, except PF spring, that \(\hbox {HCO}_{3}^{-}\) is dominate one.
Classification of groundwater, thermal springs, and cold springs of the study area according to Piper and Giggenbach (1991) diagrams. Water samples are classified into four types: Type-1: Na–\(\hbox {SO}_{4}\)–Cl, Type-2: Ca–\(\hbox {SO}_{4}\)–Cl Type-3: Na–\(\hbox {HCO}_{3}\)–Cl, and Type-4: Na–\(\hbox {HCO}_{3}\)
The mean concentrations of Na for thermal and cold springs are 699 and 169.10 mg/l, respectively (Table 1). PF and FTS cold springs display Na content lower than WHO standard value in both seasons. Calcium contents in thermal and cold springs are in the range of 740–1020 and 28–106 mg/l, respectively. It is notable that STS and TTS thermal springs display Ca content above the WHO standard value (Table 1) in both seasons. The mean concentrations of Mg in thermal and cold springs are 222.10 and 15.80 mg/l, respectively (Table 1). The concentration of Mg in STS and TTS thermal springs is higher than WHO drinking water standards. The ratio of Ca/Mg in water samples is higher than 1, probably reflecting the high solubility of altered calcic plagioclase. Chloride ranges from 6,112 to 6,160 and 32 to 522 mg/l in the thermal and cold springs, respectively. According to WHO standard, Cl content in the thermal springs and APS cold spring is above drinking water standard. The mean concentrations of sulfate in thermal and cold springs are 12,720 and 151.20 mg/l, respectively (Table 1). The concentration of sulfate in STS and TTS thermal springs is higher than WHO standard.
The STS, TTS and APS samples are classified as acid-sulfate waters (Fig. 2). The high mean concentrations of \(\hbox {SO}_{4}^{-2},\,\hbox {Cl}^{-}\), and Ca along with lower concentrations of \(\hbox {HCO}_{3}^{-}\) (Table 1) may suggest the deep circulation of the thermal springs. So STS and TTS springs may be considered as volcanic water inherent of absorbing sulfur gas phases. The \(\hbox {SO}_{4}^{-2}\) enrichment can be explained by the \(\hbox {O}_{2}\)-driven oxidation of \(\hbox {H}_{2}\hbox {S}\) to \(\hbox {H}_{2}\hbox {SO}_{4}\) in oxygenated near-surface groundwater (Henley and Stewart 1983; Tassi et al. 2010; Joseph et al. 2011).
Table 3 shows the molar ratios of some of the major components of thermal and cold springs in the study area.
Based on relatively low Na/K ratios (\(<15\), Table 3), water of thermal springs at the Taftan area have reached to the surface rapidly which is related to up-flow structures or permeable zones. The higher Na/K ratios (\(>15\), Table 3) are indicative of lateral flows for FTS, PF, and APS cold springs, which may undergo near-surface reactions and conductive cooling (Nicholson 1993; Cortecci et al. 2005; Di Napoli et al. 2009). Similarity, low Na/Ca in STS and TTS confirm thermal waters are associated with up-flow zones.
The behavior of conservative components is useful in the delineation of water formation processes. The Br/Cl and B/Cl ratios of thermal and cold spring’s samples are shown in Fig. 3 (Vengosh et al. 1991; Vengosh and Spivack 2000). In this diagram, TTS and STS thermal springs fit within the hydrothermal field, indicating their magmatic affiliation.
Ground Water
Electrical conductivity and pH range from 702 to 2,645 and 5.06 to 6.56 in water well samples, respectively. Electrical conductivity for 50 % of groundwater samples (Kh1, Kh2, Kh11, Kh14 and Kh15) is above WHO standard value. The Piper diagram shows that groundwater in the study area can be broadly divided into two types (Fig. 2); Type-1, Na–\(\hbox {SO}_{4}\)–Cl; (KH1, KH8 and KH11) and Type-3, Na–\(\hbox {HCO}_{3}\)–Cl (KH2, KH4, KH7, KH9, KH10, KH14 and KH15). Calcium and Mg contents of groundwater samples are in the range of 39–188 and 13–58.80 mg/l, respectively. Average sulfate content for water wells is 222.24 mg/l, whereby 30 % of samples (KH1, KH2 and KH11) are above WHO standard value (Table 2). Average sodium content in water wells is 318.55 mg/l, whereby 60 percent of samples are above WHO standard value (Table 2). Chloride ranges are between 94.5 to 602 mg/l in the groundwater samples. According to WHO standard, Cl content in 60 percent of water wells is above drinking water standard. The Na/Cl ratio is used for discriminating the origin of Na in groundwater. The ratios greater than 1 are typically interpreted as released Na from silicate weathering reactions, whereas ratio close to 1 is related to halite dissolution (Meyback 1987). The Na/Cl ratio in the majority of analyzed groundwater samples (60–70 %) is higher than 1, implying that sodium could be originated from silicate water–rock interaction. The Cl/\(\sum \) anion ratios of groundwater samples vary from 0.21 to 0.45 with an average value of 0.30 (Table 4). In contrast, the \(\hbox {HCO}_{3}/\sum \) anion ratios change from 0.26 to 0.58 with an average value of 0.47. These results suggest a principally silicate interaction with fluids or carbonate dissolution, which has been observed on the Piper plot (Edmunds et al. 1982). The occurrence of Na–\(\hbox {HCO}_{3}\)–Cl water also suggests the possibility of ion-exchange process.
Overall, the observed increase in the concentration of most soluble ions, especially in dry season, indicates the presence of higher proportion of deep water and closer resemblance of groundwater composition to acidic springs.
Quality Assessment
For protection of human health, guidelines for the presence of heavy metals and potentially toxic elements in drinking water have been set by different international organizations such as USEPA and WHO (Marcovecchio et al. 2007). Maximum contaminant level (MCL) is an enforceable standard set at a numerical value with an adequate margin of safety to ensure no adverse effect on human health. It is the highest level of a contaminant that is allowed in a water system. The trace elements studied in this research are B, Al, As, Fe, Pb, Ni, V, Cd, and Mn.
Boron for all water wells, cold and thermal springs has higher concentration than the WHO standard, except for PF cold spring. Aluminum is the most abundant element found in the earth’s crust (John De Zuane 1990). Aluminum contents in the water wells, thermal springs, and cold springs range from \(<0.001\) to 0.008, 2,146 to 2,420, and \(<0.001\) to 79 mg/l, respectively. The concentration of Al in thermal springs and cold springs (APS and PF) is higher than WHO standards, which may harm human health causing Alzheimer’s and parkinson’s disease (Buschmann et al. 2006).
The Concentrations of Arsenic in the thermal and cold springs ranged from 1816 to 3788 and 1 to 2.8 \(\upmu \)g/l, respectively, while in groundwater, it ranged from 3.7 to 14 \(\upmu \)g/l (Tables 1, 2). The concentration of As in thermal springs and Kh2, Kh14, and Kh15 water well samples is higher than the drinking water standard (WHO 2004). Long time exposure to arsenic may cause various diseases including skin disorders (Chen et al. 1996; Rahman et al. 2001; Wang et al. 2007).
The mean concentration of iron in two thermal springs and Kh2, Kh8, Kh14, and Kh15 groundwater samples is higher than the WHO standard (Tables 1, 2). Lead in STS and TTS thermal springs and Kh8 water well indicates higher concentration than the drinking water standard. There is still no evidence for an essential function of Pb in the human body; it seems that it can merely do harm after uptake from water and food, such as disruption of or damage to organ systems. It is a neurotoxin and is responsible for the most common type of human metal toxicosis (Berman 1980). Also, studies have linked lead exposures even at low levels with an increase in blood pressure (Zietz et al. 2007) as well as with reduced intelligence quotient in children (Needleman 1993) and with attention disorders (Yule and Rutter 1985). The concentration of nickel in drinking water is normally less than 0.02 mg/l, and higher concentration of nickel compounds is considered to be carcinogenic when related to pulmonary exposure (Ragunath 1982). The concentration of nickel in the thermal and cold springs ranges from 161 to 275.20 \(\upmu \)g/l and 1 to 40.6 \(\upmu \)g/l, respectively, while in groundwater, it ranged from 3.9 to 59.6 \(\upmu \)g/l (Tables 1, 2). Nickels in thermal springs, APS cold spring, and 50 % of water wells indicate higher concentration than the WHO standard, which may create problems to the human health. STS and TTS thermal springs have higher concentrations of V (1.57 and 2.78 mg/l) exceeding the maximum permissible limit set by the WHO, whereas, in other cold spring and groundwater samples, V is lower than WHO guideline. Our study reveals that two thermal spring samples have Cd concentrations higher than the WHO drinking water guidelines. The mean concentrations of Cd in STS and TTS thermal springs were 0.024 and 0.013 mg/l, respectively.
High concentration of Mn (WHO, 0.05 mg/l) in the water samples was found in STS and TTS thermal springs, APS cold spring, and Kh1, Kh2, Kh4, Kh14, and Kh15 groundwater (6.39, 8.81, 1.24, 0.584, 1.671, 1.281, 2.02, and 2.26 mg/l respectively). Although Mn is known as an essential element for human survival, high doses of Mn may cause lung embolisms, bronchitis, impotency, hallucinations, forgetfulness, and nerve damage, even to the point of Parkinsonism (Buschmann et al. 2006).
Mineral Saturation Index and Arsenic Geochemistry
Hydrogeochemical modeling of waters can be useful to explain the inter-relationship of the lithologies encountered by water and their chemical composition (Lopez-Chicano et al. 2001). Thus, saturation indices are used to evaluate the degree of equilibrium between minerals calculated by PHREEQC interactive 2.17.4799. Saturation index is defined as SI = log (PAI/KT), where PAI is the product of ionic activity of ions and KT is the equilibrium constant of mineral at the emergence temperature.
Table 5 shows the calculated saturation index (SI) values of various mineral phases in groundwater, thermal spring, and cold spring. A saturation index of zero indicates that ion activity product and the solubility product are equal, and liquid and solid are thermodynamically in equilibrium. A negative or a positive index indicates undersaturation or oversaturation (Gemici and Filiz 2001). For the rest of the minerals, thermal waters are generally oversaturated with respect to quartz, chalcedony, alunite, gypsum (TTS spring), barite, and celestite (Table 5; Fig. 4), explaining the precipitation of sulfate and siliceous minerals in the most recent precipitates of the geothermal system.
APS cold spring is oversaturated with alunite, barite, chalcedony, and quartz. PF and FTS cold springs are oversaturated with chalcedony, quartz, hematite, and goethite. The groundwater samples are saturated with quartz, chalcedony, barite, hematite (four water wells), and goethite (two water wells), somehow highlighting the mixing of the groundwater with thermal and cold springs (Table 5; Fig. 5).
However, groundwater samples are found to be undersaturated with respect to evaporated minerals (halite and gypsum) and calcite (Table 5). This can be explained by the lower input of Ca ions into groundwater by thermal springs. Furthermore, the absence of halite in the host rocks also plays an important role in this respect. The SI versus pH is shown in Fig. 6a, b.
The evolution of pH value in the water wells, thermal springs, and cold springs indicates that the pH played significant role in precipitation of various mineral phases, except for quartz and chalcedony. Saturation indices are less influenced by EC in the area (Figs. 4, 5). The SI of goethite and hematite versus As content is shown in Fig. 7. Generally, As can be adsorbed on to the surfaces of Fe hydroxides and oxides (Pal et al. 2002). Figure 7 reveals that the arsenic content decrease with increasing SI of Fe in water samples; hence, Fe content could play significant role to control mobility of As in the groundwater.
In Fig. 8, the concentration of As in groundwater samples is plotted against selected physicochemical parameters. Arsenic exhibited significant (\(\hbox {r}> 0.65\)) correlation with B, Cl, \(\hbox {HCO}_{3}\), Mn, K, and Na. The wells with high arsenic content exhibited high boron and chloride concentrations suggesting a common source for elements (Fig. 8a, b). Relationship between arsenic and boron in groundwater has also been reported by other investigators (Smedley et al. 2002; Bhattacharya et al. 2006).
Significant positive correlation between bicarbonate and arsenic (Fig. 8c) indicates that this ion can play an important role in the mobilization of arsenic through the competition for adsorption sites and by the formation of arseno–carbonate complexes (Kim et al. 2003; Bhattacharya et al. 2006). Moreover, positive correlation between arsenic and manganese is often observed in groundwater (Fig. 8 d) though under certain conditions, the presence of Mn oxyhydroxide could lead to desorption of arsenic and manganese (Kim et al. 2002; Smedley et al. 2002). The relatively good correlation of potassium with arsenic as well as with bicarbonate and sodium (Fig. 8c, e, f) could be the result of a hydrochemical process such as hydrolysis of K-feldspars and albite, which can lead to an increase in K, Na, and \(\hbox {HCO}_{3}\) of groundwater.
Conclusion
The present study revealed a high concentration of some selected potentially toxic elements in groundwater, thermal springs, and cold spring of Taftan area. The concentration of boron for all water samples (except for PF spring) and Fe (thermal springs and 40 % water wells) is higher than those of WHO standard. Mn in acidic springs (STS, TTS, and APS) and 50 % of water wells samples indicates higher concentration than those of WHO drinking water guidelines. The concentration of V, Cd, Ni, Pb, and As in STS and TTS thermal springs is higher than the WHO drinking water guidelines. As, Ni, and Pb for 30, 50, and 10 % of groundwater samples reveal higher concentration than those of WHO drinking water standard.
High concentrations of trace elements such as As, B, Mn, Fe, and Ni along with Ca, Na, Cl, and \(\hbox {SO}_{4}\) in thermal springs and water wells reveal the presence of water–rock interactions and active deep circulations in the study area. Conservative elements in TTS and STS thermal springs fall within the hydrothermal field. Low Na/Ca and Na/K ratios in STS and TTS confirm thermal waters associated with up-flow zones, while high Na/K ratios for FTS, PF, and APS cold springs indicate a lateral flow, which may undergo near-surface reactions and conductive cooling.
The PHREEQC was used to calculate the saturation state (SI) and to evaluate the speciation of dissolved constituent of the groundwater and springs waters. Thermal waters are generally oversaturated with respect to quartz, chalcedony, alunite, gypsum (TTS spring), barite, and celestite which is supported by precipitation of sulfate and siliceous minerals in the most recent precipitates of the geothermal system. PF and FTS cold springs are oversaturated with chalcedony, quartz, hematite, and goethite. The groundwater samples are saturated with quartz, chalcedony, barite, hematite (four water wells), and goethite (two water wells), slightly highlighting the mixing of the groundwater with thermal and acidic springs.
The comparison of SI with pH content reveals the significant role of pH for the precipitation of various mineral phases, except for quartz and chalcedony in the groundwater, thermal springs, and cold spring samples. The SI indicates that the Fe-phase minerals could control mobility of As in the groundwater of the Taftan area. Significant positive correlation between bicarbonate and arsenic reveals that this ion can play an important role in the mobilization of arsenic through the competition for adsorption sites and through the formation of arseno–carbonate complexes in groundwater samples. Speciation of toxic elements needs to be addressed in future investigation.
Overall, the obtained results suggest a significant risk of toxic elements for many peoples who live in the Taftan area considering that groundwater (hand-dug wells and bore holes) is the only sources of water supply in the area. Therefore, the following steps are suggested for the establishment of preventive measures on protection of cold groundwater resources: (1) control the discharge of geothermal and cold acidic waters in areas of diffuse discharge; (2) avoid the overexploitation of cold groundwater near the thermal and acidic cold springs discharges, as the excessive pumping of water may result in the spread of geothermal and cold acidic fluids; (3) avoid the construction of deep pumping wells in order to minimize the flow of hot and cold acidic fluids in their capture zones; and (4) control the extraction and use of geothermal fluids by mineral water plants.
References
Aksoy N, Simsek C, Gunduz O (2009) Groundwater contamination mechanism in a geothermal field: a case study of Balcova, Turkey. J Contam Hydrol 103:13–28
Agusa T, Kunito T, Fujihara J, Kubota R, Minh TBM, Trang PTK et al (2006) Contamination by arsenic and other trace elements in tube-well water and its risk assessment to humans in Hanoi, Vietnam. Environ Pollut 139:95–106
Angelone M, Cremisini C, Piscopo V, Proposito M, Spaziani F (2009) Influence of hydrostratigraphy and structural setting on the arsenic occurrence in groundwater of the Cimino-Vico volcanic area (central Italy). Hydrogeol J 17:901–914
Barati AH, Maleki A, Alasvand M (2010) Multi-trace elements level in drinking water and the prevalence of multi-chronic arsenical poisoning in residents in the west area of Iran. Sci Total Environ 408:1523–1529
Berman E (1980) Toxic metals and their analysis. Hayden and Sons, Philadelphia
Bhattacharya P, Claesson M, Bundschuh J, Sracek O, Fagerberg J, Jacks G, Martin RA, Storniolo A, Thir M (2006) Distribution and mobility of arsenic in the Rio Dulce alluvial aquifers in Santiago del Estero Province, Argentina. Sci Total Environ 358:97–120
Birkle P, Merkel B (2000) Environmental impact by spill of geothermal fluids at the geothermal field of Los Azufres, Michoacán, México. Water Air Soil Pollut 124:371–410
Boomeri M (2004) Geochemistry, petrography and formation style of Taftan Volcano. Research Project Report, University of Sistan & Baluchistan, p 118 (in Farsi).
Brown KL, Simmons SF (2003) Precious metals in high-temperature geothermal systems in New Zealand. Geothermics 32:619–625
Brugger J, Long N, McPhail DC, Plimer I (2005) An active magmatic hydrothermal system: the Paralana hot springs, Northern Flinders ranges, South Australia. Chem Geol 222:35–64
Buschmann J, Berg M, Stengel C, Sampson ML (2006) Arsenic and manganese contamination in Cambodia: relation to ‘micro-topography’?. In: Philadelphia Annual Meeting. 22–25 Oct.
Chen CJ, Chiou HY, Tai TY (1996) Dose response relationship between ischemic heart disease mortality and longterm arsenic exposure. Arterioscler Thromb Vasc Biol 16:504–510
Cortecci G, Boschetti T, Missi M, Lameli CH, Mucchino C, Barbieri M (2005) New chemical and original isotopic data on waters from El Tatio geothermal field, northern Chile. Geochem J 39:547–571
Di Napoli R, Aiuppa A, Bellomo S, Brusca L, D’Alessandro W, Candela EG, Longo M, Percoraino G, Valenza M (2009) A model for Ischia hydrothermal system: evidence from the chemistry of thermal groundwaters. J Volcanol Geotherm Res 186:133–159
Edmunds WM, Bath AH, Miles DL (1982) Hydrochemical evolution of the East Midlands Triassic sandstone aquifer, England. Geochim Cosmochim Acta 46:2069–2081
Gansser A (1971) The Taftan volcano (SE Iran). Eclogae Geol Helv 64:319–334
Gemici U, Filiz S (2001) Hydrogeochemistry of the Cesme geothermal field, western Turkey. J Volcanol Geotherm Res 110:171–187
Giggenbach WF (1991) Chemical techniques in geothermal exploration. Application of Geochemistry in Geothermal Reservoir Development (Co-ordinator D’Amore, F.). UNITAR/UNDP Centre on Small Energy Resources, Rome, pp 119–144.
González EP, Tello EH (2001) Interaction between geothermal water and springs at the Los Humeros geothemal field, Puebla, Mexico. Ing Hidraul Mex XV(2):185–194
Henke KH (2009) Arsenic: environmental chemistry., Health threats and waste treatmentWiley, Chichester, p 588
Henley RW, Stewart MK (1983) Chemical and isotopic changes in the hydrology of the Tauhara geothermal field due to exploitation at Wairakei. J Volcanol Geotherm Res 15(4):285–314
John De Zuane PE (1990) Chemical parameters-inorganics. In: Quality standards and constraints. Von Nostrand and Reinhold, New-York, pp 47–151.
Joseph EP, Fournier N, Lindsay JM, Fischer TP (2011) Gas and water geochemistry of geothermal systems in Dominica, Lesser Antilles island arc. J Volcanol Geotherm Res 206:1–14
Kim MJ, Nriagu J, Haack S (2002) Arsenic species and chemistry in groundwater of southeast Michigan. Environ Pollut 120:379–390
Kim MJ, Nriagu J, Haack S (2003) Arsenic behaviour in newly drilled wells. Chemosphere 52:623–633
Keshavarzi B, Moore F, Mosaferi M, Rahmani F (2011) The source of natural arsenic contamination in groundwater. Water Qual Expo Health, West of Iran. doi:10.1007/s12403-011-0051-x
Komatina MM (2004) Medical geology: effects of geological environments on human health, vol 2., Developments in earth and environmental sciencesElsevier, Amsterdam, p 500
Landrun JT, Bennet PC, Engel AS, Alsina MA, Pasten PA, Milliken K (2009) Partitioning geochemistry of arsenic and antimony, El Tatio Geyser Field, Chile. Appl Geochem 24:664–676
Lopez DL, Bundschuh J, Birkle P, Armienta MA et al (2012) Arsenic in volcanic geothermal fluids of Latin America. Sci Total Environ 429:57–75
Lopez-Chicano M, Ceron JC, Vallejos A, Pulido-Bosch A (2001) Geochemistry of thermal springs, Alhama de Granada (southern Spain). Appl Geochem 16:1153–1163
Marcovecchio JE, Botte SE, Freije RH (2007) Heavy metals, major metals, trace elements. In: Nollet LM (ed) Handbook of water analysis, 2nd edn. CRC Press, London, pp 275–311
Meyback M (1987) Global chemical weathering of surficial rocks estimated from river dissolved loads. Am J Sci 287:401–428
Moinvaziri H, Aminsobhani E (1978) Etudes Volcanologique du Taftan. Ecole normale superieure de Tehran, Tehran.
Moore F, Shakeri A, Kompani-Zare M, Raeisi A (2005) Geothermometry and hydrogeochemistry of Taftan thermal springs. In: Proceeding 9th symposium of geological society of Iran, Tehran. 30–31 Aug 2:22–36 (in Farsi)
Mosaferi M, Yunesian M, Dastgiri S, Mesdaghinia AR, Esmailnasab N (2008) Prevalence of skin lesions and exposure in drinking water in Iran. Sci Total Environ 392:69–76
Mosaferi M, Yunesion M, Mesdaghinia A, Naidu A, Nasseri S, Mahvi AH (2003) Arsenic occurrence in drinking water of IR of Iran: the case of Kurdistan Province. In: Ahmed MF, Ali MA, Adeel Z (eds.) Book: fate of arsenic in the environment- BUET-UNU international symposium, February. Tokyo, Japan 7 International Training Network Centre, Bangladesh University of Engineering and Technology, Dhaka, Bangladesh and the United Nations University, pp 1–6.
Needleman HL (1993) The current status of childhood low-level lead toxicity. Neurotoxicology 14:161–166
Nicholson K (1993) Geothermal fluids. Springer, Berlin
Pal T, Mukherjee PK, Sengupta S (2002) Nature of arsenic pollutants in groundwater of Bengal basin: a case study from Baruipur area, West Bengal, India. Curr Sci 82:554–561
Ragunath HM (1982) Ground water. Wiley Eastern, New Delhi
Rahman MM, Chowdhury UK, Mukherjee SC, Mondal BK, Paul K, Lodh D et al (2001) Chronic arsenic toxicity in Bangladesh and West Bengal, India—a review and commentary. Clin Toxicol 39(7):683–700
Shakeri A, Moore F, Kompani-zare M (2008) Geochemistry of the thermal springs of Mount Taftan, southeastern Iran. J Volcanol Geotherm Res 178:829–836
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Smedley PL, Nicolli HB, MacDonald DMJ, Baros AJ, Tullio JO (2002) Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Appl Geochem 17:259–284
Tassi F, Aguilera F, Darrah D, Vaselli O, Capaccioni B, Poreda RJ, Huertas AD (2010) Fluid geochemistry of hydrothermal systems in the Arica-Parinacota, Tarapacá and Antofagasta regions (northern Chile). J Volcanol Geotherm Res 192:1–15
Vengosh A, Chivas AR, McCulloch MT, Starinsky A, Kolodny Y (1991) Boron-isotope geochemistry of Australian salt lakes. Geochim Cosmochim Acta 55:2591–2606
Vengosh A, Spivack AJ (2000) Boron in ground water. In: Cook P, Herczeg A (eds) Environmental tracers in groundwater hydrology. Kluwer Academic, Boston, pp 479–485
Wang CH, Hsiao CK, Chen CL et al (2007) A review of the epidemiologic literature on the role of environmental arsenic exposure and cardiovascular diseases. Toxicol Appl Pharmacol 222:315– 326
WHO (World Health Organization) (2004) Guidelines for drinking water quality, vol 1, 3rd edn. WHO, Geneva, p 515
Wilkie JA, Hering JG (1998) Rapid oxidation of geothermal arsenic (III) in stream waters of the Eastern Sierra Nevada. Environ Sci Technol 32:657–662
Yule W, Rutter M (1985) Effect on children’s behavior and cognitive performance: a critical review. In: Mahaffey R (ed) Dietary and environmental lead (Pb): human health effects. Elsevier, New York, pp 211–251
Zietz BP, Lap J, Suchenwirth R (2007) Assessment and management of tap water Lead contamination in Lower Saxon, Germany. Int J Environ Health Res 17(6):407–418
Acknowledgments
The authors wish to express their gratitude to Sistan and Baluchestan regional water company of Iran for financial support. We would also like to extend our thanks to the research committee of Kharazmi University for logistic assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shakeri, A., Ghoreyshinia, S. & Mehrabi, B. Surface and Groundwater Quality in Taftan Geothermal Field, SE Iran. Water Qual Expo Health 7, 205–218 (2015). https://doi.org/10.1007/s12403-014-0141-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-014-0141-7