Abstract
The poor water quality, bacterial contamination, seasonal variation, uncertainty in monsoon, lack of sustainability etc. are some major challenges of the water sector in the Himalayan region of India. To tackle the major problem of water quality, River Bank Filtration (RBF) has been applied in Uttarakhand as a domestic water pre-treatment technology. This technique is found to be effective for removal of turbidity and bacterial contamination present in surface water of four rivers of Uttarakhand, namely Alaknanda, Mandakini, East Nayar, and Pinder. The present paper reveals the improved water quality of rivers produced through RBF in a sustainable manner as compared to surface river water being supplied for drinking purpose. The classification of water quality using Pearson correlation followed by Piper trilinear and Chadha’s diagrams further provide support to the better water quality through RBF. Moreover, the results of Water Quality Index (WQI) also reflect the excellent water quality with ‘A-Grade’ of all river water samples obtained after RBF process in comparison to normal river water samples having good water quality with ‘B-Grade’ except the Srinagar site, where the river water sample was found to be unsuitable for drinking purpose with ‘E-Grade’. Alluvial deposits of RBF sites along the banks of the four rivers show the potential of replication of RBF at large scale in the hill state of Uttarakhand.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is the most vital source for sustainability of life. Various geogenic activities (weathering of rocks, erosion, heavy rainfall etc.) and anthropogenic activities (urbanization, agriculture, industrialization, growth of population etc.) are responsible for water pollution, which make it unsuitable for potability (Oluyemi et al. 2010). In Uttarakhand, weathering of rocks and improper disposal of sewage waste due to the slope factor of mountains are mainly responsible for the pollution of water sources. The water sector of Uttarakhand is facing issues of poor water quality with respect to turbidity and bacterial contamination of available drinking water sources. A large portion of state lives in the hill area and about 90 % of the rural population depends upon the natural water sources for their daily water demand (Jain et al. 2010). Due to the lack of sufficient water pre-treatment systems throughout the state, mainly chlorination is done prior to supply of domestic water, which reduces the bacterial contamination but ineffective for turbidity and other chemical contaminants. Coliform contaminations, especially fecal coliform, are extensively found in the natural water sources of state due to mixing of untreated sewage and waste water owing to the slope factor (Rawat et al. 2012). The fecal contamination in water is still the pollutant that most seriously affects the human health and entails the major water borne diseases such as diarrhea, cholera, typhoid, schistosomiasis etc. especially in the hills of Uttarakhand as well as of other similar states like Himachal, Jammu, and Kashmir, the North East states (Joshi et al. 2009). Mixing of soil and rock etc. in fast flowing river and tributaries water and rain water (Semwal and Akolkar 2006) also lead to high turbidity, which render the existing pre-treatment systems ineffective.
River Bank Filtration (RBF) process has been widely proved as domestic water pre-treatment technology to overcome various water quality problems (Dash et al., 2008, 2010; Shankar et al., 2009; Sudhakaran et al., 2013; Varadi, 2013). The concept of RBF was developed in Germany and has widely been used in Europe for public and industrial water supply along Rhine, Elbe, and Danube rivers over 100 years (Abdel-Lah 2013). It is an efficient and well accepted technique for the treatment of surface water in many European countries such as Switzerland where 80 % of the drinking water comes from RBF wells, 50 % in France, 48 % in Finland, 40 % in Hungary, 16 % in Germany, and 7 % in the Netherlands (Grischek et al., 2002; Tufenkji et al., 2002; Vet de et al., 2009, 2010). In Germany, around 75 % of the city of Berlin depends on RBF, while in Düsseldorf, it is being used since 1870 as the main drinking water supply (Schubert 2002). Moreover, other countries like India (Sandhu et al. 2011; Ojha 2012; Singh et al. 2012), China, and South Korea (Ray 2008) have recently started implementing RBF for drinking water supply. Shamrukh and Abdel-Wahab (2011) also reported the improved water quality of Nile river in Egypt by using the RBF method. At a number of Indian cities including Delhi (Lorenzen et al. 2010), Haridwar (Dash et al. 2010), Mathura (Luckins et al. 2011), Ahmadabad, Medinipur, Kharagpur (Sandhu et al. 2011), Nainital (Dash et al. 2008), Patna (Prasad et al. 2009), RBF sites are operational to produce water of drinking quality.
In RBF, river water passes through the riverbed sediments or alluvial aquifer, which comprises layers of sand and gravel that also contain underground water (Fig. 1).
The riverbed serves as a natural filter and removes contaminants present in the surface water by overlapping physical, chemical, and biological processes. Physical processes such as hydrodynamics involves advection, dispersion, transport, and dilution, while mechanical processes include filtration i.e. improvement of water quality through the natural filtration of fine sediments by trapping of particles in pore spaces especially for particulate organic matters and pathogens (Abdel-Lah 2013). Physicochemical processes occur involving various processes such as filtration, sorption-desorption, solution-precipitation, redox reaction, complexation, acid-base reaction, hydrolysis, biochemical, microbial biodegradation reactions etc. and by the mixing with groundwater (dilution). These physicochemical processes are subjected to the porosity of the medium, the concentration and the behavior of metals and other inorganic compounds, the water residence time in the aquifer, temperature, and pH conditions of water and oxygen concentrations (Jaramillo 2012). However, biological processes involve the degradation of organic matter for metabolic processes of these organisms and mineralization of secondary substrates. Then, bank filtered water is extracted through production wells (RBF well) located near the bank of rivers (∼20 to 200 m away). Aquifer provides the slow rate filtration and extracted water is of higher and more consistent quality than the river water abstracted directly (Derx et al. 2013). During infiltration and travel through the soil and aquifer sediments, the concentration of water quality parameters change due to different natural attenuation processes (Sharma and Amy 2009).
The pumping action creates a pressure between the river and aquifer and induces the water from the river to flow downward through the porous media into the production well. The efficiency of RBF process is dependent on number of factors such as surface water quality, permeability of the riverbank, river level and sediment transport variability, the residence time of the water in the soil and the temperature (Sandhu et al. 2011). Riverbank filtration has been extensively used for drinking water pre-treatment for various organic pollutants such as pesticides, herbicides, odorous compounds, oil sub-products, and pharmaceuticals (Juttner 1995, 1999). The removal and the behavior of such organic compounds depends on the factors of hydrophobicity of the compound, the potential for biochemical degradation, the amount of organic matter in the aquifer, infiltration rate, microbial activity, biodegradability, etc. (Tufenkji et al. 2002). However, redox reactions, microbial degradation of organic matter, and dilution are the most common processes to control the transport and fate of inorganic compounds. In redox reactions, manganese and iron oxides are mobilized under reducing conditions and adsorbed, precipitated, or co-precipitated under oxidizing conditions. Microbial degradation of organic matter can alter the geochemical conditions and mobilize metals like copper and cadmium, which are usually associated with natural organic matter (NOM). It can remove the heavy metals like chromium, arsenic etc. up to 90 % (Sontheimer 1980). Dilution occurs when high quality ground water is mixed into river water and depletes the higher concentration of inorganic compounds in river water. Removal of microbial pathogens in RBF takes place through inactivation and adsorption to soil grains, and is primarily dependent on the detention or travel time in the bank, temperature, pH, and soil properties (Shamrukh and Abdel-Wahab 2008).
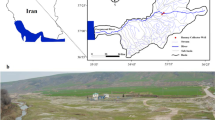
In view of the vast potential of reduction in certain water quality characteristics, RBF has been widely used for transforming river water into drinking water grade by improving water quality. The evaluation of water quality obtained through RBF has been done globally but very few efforts have been made in Uttarakhand so far. With the above backgrounds, water samples from four selected rivers and also from four RBF wells situated near the bank of the rivers have been analyzed. At all the four places, i.e. Srinagar, Augustmuni, Satpuli, and Karanprayag, in Uttarakhand at the banks of four rivers, namely Alaknanda, Mandakini, East Nayar and Pinder, the drinking water is being drawn through surface abstraction by state’s drinking water supplying agency and is then supplied after coagulation, filtration, and, mainly, chlorination.
However, the recurring cost of running and maintenance of pre-treatment system has its limitation along with limited capacity of treatment unit and very high demand of water daily for domestic supply for the local population of selected locations. Thus, the quality of water being supplied is sometimes does not fulfill the required criteria with the existing installations and existing pre-treatment methods. The situation gets worsened during and after the monsoon seasons when a very high turbidity is obtained. In order to search and evaluate the RBF as viable alternative pre-treatment technology, the water quality results of river water and RBF processed river water are compared and discussed in the present paper. Moreover, the study also demonstrates the water quality of these sources in a simple way with unique numerical expression by using Water Quality Index (WQI) in easily understandable form for the general public. Statistical analysis was performed for the computation of Pearson’s correlation matrix to represent the nature of correlation between physicochemical parameters and a quantitative independent approach of samples. In addition, the water quality has been classified through Piper trilinear and Chadha’s diagrams.
Methodology
Four water sampling sites, namely Srinagar, Augustmuni, Satpuli, and Karanprayag, were identified in Uttarakhand, India where RBF technology has been established on the bank of following rivers, as shown in Table 1.
The water samples from rivers and RBF production wells at each of the four selected sites were collected in July 2012 using garb sampling. The collection, preservation, transportation, and analysis of the samples were done in accordance to the standard procedures of the American Public Health Association (APHA 2005) for 19 water quality characteristics namely pH, turbidity, total dissolved solids (TDS), hardness, calcium, magnesium, nitrate, sulfate, iron, chloride, fluoride, arsenic, manganese, zinc, lead, phenol, sodium, potassium, and coliform bacteria. pH and turbidity were measured on-site. Other parameters and coliform bacteria were determined in the laboratory.
pH and turbidity were measured using pH meter (PC-II; Hach, USA) and nephelometer meter, respectively (PCcompact; Aqualytic, Germany). All spectrophotometric measurements were made using DR 5000 spectrophotometer (Hach, USA). Sodium, potassium, and other metal ions were analyzed using AA240 atomic absorption spectrophotometer (Varian, Australia). Coliform bacteria were determined by using the membrane filter technique as per APHA.
The water quality of selected river water and RBF well water sources have been discussed on the basis of Weight Arithmetic Water Quality Index method by using ten water quality variables viz. pH, turbidity, TDS, hardness, calcium, magnesium, nitrate, sulfate, iron, and chloride (Brown et al. 1970; Chauhan and Singh 2010; Akoteyon et al. 2011; Balan et al. 2012). Moreover, a statistical analysis has been made through MS Excel 2007 by calculating Pearson’s correlation coefficient between different pairs of selected ten parameters.
The weight arithmetic index method (Brown et al. 1970; Akoteyon et al. 2011; Tyagi et al. 2013) is widely used for calculation of WQI of water sources and calculated by using the standard values of BIS. The overall WQI is determined by using Eq. (1):
The unit weight (W i ) for each water quality parameter is calculated by using the following expression:
where K is a proportionality constant and is determined by using the following formula:
S i is the standard permissible value of the ith parameter.
The quality rating (Q i ) of Eq. (1) is calculated as under:
where V i is estimated concentration of the ith parameter in the analyzed water and V o is the ideal value of this parameter in pure water. All ideal values are taken as zero for drinking water, except pH=7.0.
The status of water quality according to the WQI was defined on the basis of criteria as given in Table 2.
Results and Discussion
The water quality monitoring data of analyzed river water samples (through surface abstraction) and RBF well water samples (obtained after RBF pre-treatment method) show the usefulness and efficiency of RBF technique in improving the water quality. The analytical data are summarized below in Table 3 and compared with the Indian Standard prescribed for drinking purpose (BIS 1991, 2004).
pH values in the analyzed river samples and RBF well water samples fluctuated in a limited range from 7.9 to 8.2 and from 7.4 to 8.1, respectively. All these pH values were well within the prescribed limit of 6.5 to 8.5 as per BIS.
The turbidity in all river water samples were found higher than the permissible limit (10 NTU) of BIS and varied from 13 to 240 NTU, where Srinagar sampling site has maximum turbidity as 240 NTU. While in RBF well water, its values ranged from 0.6 to 0.8 NTU. All values in RBF well water were below than the desirable limit of BIS, which showed the effective removal of turbidity from water making it suitable for drinking. The TDS values varied from 68 to 110 mg/l in river water and 104 to 650 mg/l in RBF well water. All sites have TDS content lower than the desirable limit of 500 mg/l except in RBF well water of Srinagar as 650 mg/l but lower than the permissible limit of 2000 mg/l.
The hardness values ranged from 39 to 82 mg/l in river water samples and from 75 to 270 mg/l in RBF well samples. No sample of the study area exceeds the desirable limit prescribed for hardness in both types of water samples. The calcium and magnesium concentrations in selected river water samples were observed between 14 to 20 mg/l and 1 to 9 mg/l, respectively, at all four sites of study area, whereas in RBF well water samples, these calcium and magnesium concentrations varied from 20 to 55 mg/l and 6 to 32 mg/l, respectively. RBF well water of the Srinagar sampling site has a slightly higher concentration of magnesium than the desirable limit of 30 mg/l but all values fall within the maximum permissible limit of 100 mg/l of BIS similar to calcium.
Nitrate determination in drinking water is considered important for its adverse health effects. A limit of 45 mg/l has been recommended as per BIS for nitrate in drinking water supplies. The nitrate content in river water samples varied from 4 to 8 mg/l and from 3 to 44 mg/l in the RBF samples. All the samples were far below the prescribed limit of BIS for nitrate. The values of the sulfate ion were found to be within 8 to 20 mg/l in river water samples while they were 10 to 88 mg/l in RBF well water samples. The sulfate values in RBF well water were slightly higher than the river water samples but fall well within the desirable limit of 200 mg/l.
The ranges of 0.3 mg/l and 1.0 mg/l have been suggested as desirable and permissible limits, respectively, for iron metal as per BIS. The concentration of iron in river water samples were found between 0.01 to 0.18 mg/l, whereas in RBF well water these concentrations varied from 0.03 to 0.06 mg/l during the monitoring course. All these values were much lower than the desirable as well as permissible limits for iron. Locations near the study area have been found to contain a higher amount of iron in different water supply schemes and handpumps and even samples have been reported to possess orange/red color. The effectiveness of RBF in iron content reduction may be used as a tool for an iron removal method at large scale without any disposal problem or associated disadvantages of existing iron treatment techniques.
The concentration of chloride in the study area is also quite low at all the four locations. The values of chloride were confined between 4 to 28 mg/l in river water and 10 to 75 mg/l in RBF well water. The RBF water contains slightly higher concentration of chloride as compared to river water but chloride has no adverse effect on human health from intake of water containing even higher chloride content. The fluoride content in all collected samples was quite low and varied from <0.1 to 0.3 mg/l in both river and RBF well water samples during the study. The arsenic values in all river and RBF well water samples were found to be less than 0.005 mg/l, which were lower than the prescribed maximum limit of 0.01 mg/l.
The concentrations of manganese were quite low and found either less than or equal to 0.01 mg/l in all river and RBF well water samples, which were much lower than the desirable limit of 0.1 mg/l. Similarly, zinc content was also quite lower in comparison to the desirable limit of 5 mg/l as per BIS. In river water samples, zinc content oscillated between 0.01 to 0.03 mg/l, while in RBF well water, its concentration ranged from 0.01 to 0.05 mg/l.
The occurrence of lead in all collected samples was not found in a considerable amount and all samples contained less than 0.01 mg/l, which were many fold lower than the maximum permissible limit of 0.05 mg/l. Similarly, phenols were also not found in appreciable amounts and the values were noted to be less than 0.001 mg/l, also lower than the desirable limit (0.001 mg/l) of phenol.
The concentration of sodium and potassium were measured from 3 to 13 mg/l and from 1 to 2 mg/l, respectively, in river water samples, whereas, in RBF well water samples, these concentrations varied from 3 to 45 mg/l and from 3 to 15 mg/l, respectively. BIS has no recommended limits for both ions but WHO has recommended sodium for drinking water supplies and both ions are required for total ionic balance.
The bacteriological analysis shows the presence of coliform contamination in river water samples, which varied from 141 to 900 colonies/100 ml. No coliform contamination was observed in any water sample of RBF wells and this reflects the high efficiency of the RBF technique over bacterial reduction.
To sum up, the water quality at the studied sites has improved significantly for turbidity, pathogens, pH, and iron. Removal of microbial pathogens and turbidity in RBF takes place by involving biodegradation, sorption, natural filtration, and dilution of ground water (LeChevallier and Au, 2004; Cady, 2011). In RBF, various factors, including pH, ionic strength, the redox conditions in groundwater, pore water velocity, detention or travel time in the bank, temperature, pH, and soil properties, are also involved in pathogen removal processes (Shamrukh and Abdel-Wahab 2008). The pH is decreased due to the degradation of organic matter. In the process, CO2 reacts with water and forms carbonic acid and hydrogen carbonate, which lowered the pH of the sample (Sprenger et al. 2012). The iron concentration in RBF water is reduced due to redox reactions, in which iron oxides are mobilized under reduced conditions, while they are adsorbed and precipitated under oxidized conditions. The transport and fate of inorganic compounds are controlled by various processes like redox reactions, microbial degradation of organic matter, and dilution with ground water.
Correlation Analysis
In river and RBF well water samples, Pearson’s correlation matrix shows positive close association among the selected parameters. In river water samples (Table 4), pH shows significant positive correlation with hardness. It indicates that with increase or decrease in the value of pH, the value of the hardness also increases or decreases. Turbidity has a significant positive correlation with nitrate and chloride. A significant positive correlation was also found between TDS and hardness with magnesium and of nitrate with chloride.
Similarly in RBF water samples (Table 5), the pH variable has significant negative correlation with magnesium. This shows that with increase or decrease in the value of pH, the value of magnesium content also decreases or increases. However, TDS reflects significant positive correlation with hardness, magnesium, sulfate, and chloride. The significant positive correlation was also observed between hardness and calcium as well as magnesium ions, whereas sulfate has a significant perfect positive correlation with chloride ions.
Turbidity, which is highly improved through RBF, has a positive correlation with TDS, hardness, magnesium, nitrate, sulfate, and chloride, and this indicates the negative association with calcium and iron in river water samples, whereas, in RBF water samples, it shows the positive association with TDS, hardness, calcium, magnesium, nitrate, sulfate, iron, and chloride.
Water Quality Classification
In the present attempt, the Piper trilinear (Piper 1944) and Chadha diagrams (Chadha 1999) are used to classify the type of water or hydrochemical facies. These diagrams reveal the chemical equilibrium between cations (metals or bases) and anions (acid radicles) in the water samples. They also express the presence of a main contributor of ions and chemical reactions taking place in the water.
Piper Diagram
The Piper (1944) diagram is the basis for the interpretations of the hydrochemical data. It graphically represents the composition (cations, anions and combined) of the water sample as a part of hydrochemical facies. The Piper trilinear diagram is composed of two lower triangles of cations (Ca2+, Mg2+, Na+ and K+) and anions (Cl−, SO4 2−, CO3 2− and HCO3 2−), respectively, and middle quadrilateral. The quadrilateral or diamond shape indicates the combined distribution of both ions (cations and anions) and the final water type of sources. It can be used to describe various hydrochemical processes, such as base cation exchange, cement pollution, mixing of natural waters, sulfate reduction, saline water (end-product water), and other related hydrochemical problems.
In the present study, Piper diagram is drawn for hydrochemical evaluation and classification of river water as well as RBF water (Fig. 2). The plotted Piper diagram shows that except Srinagar river water sample, all river water samples and all RBF water samples fall in Ca–Mg–HCO3 hydrochemical facies. The selected water sources exhibit the dominance of Ca–Mg and HCO3 ions and show the nature of carbonate hardness. The Srinagar river water sample lies in Ca–Mg–Cl–SO4 hydrochemical facies and shows the nature of non-carbonate hardness. At selected sites of the state, the occurrence of limestone/dolomite, magnesite, talc, steatite, soapstone etc. is very high. The ions are dissolved in water due to the chemical weathering of rock-forming minerals and increased the concentration of calcium, magnesium, carbonate, and other minerals as described above.
Chadha’s Diagram
In this diagram, the difference in milliequivalent percentage between alkaline earths (calcium plus magnesium) and alkali metals (sodium plus potassium) is plotted on the X axis and the difference in milliequivalent percentage between weak acidic anions (carbonate plus bicarbonate) and strong acidic anions (chloride plus sulfate) is plotted on the Y axis (Chadha 1999). The square or rectangular field expresses the overall character of the water. It has all the advantages as the Piper trilinear diagram and helps in the classification of water. In the present study, Chadha’s diagram is plotted by using the chemical analysis data of all the four rivers and RBF water samples collected from the four sampling sites (Fig. 3).
From the plotted diagram, it is also clearly evident that all river water samples and RBF well water samples fall in the Type-1 field and show the dominating presence of Ca–Mg–HCO3 ions except the Srinagar river water. Srinagar river water shows the dominating presence of Ca–Mg–Cl–SO4 ions and falls in Type-2 field.
Water Quality Index (WQI) Analysis
The WQI values (Table 6) have been calculated for all selected samples for determining their suitability for drinking purposes in simple, understandable, and usable format by users as well as communities.
The results of WQI show that the water quality of river water sample at Srinagar site was found ‘unsuitable’ with 207.44 WQI value and shows ‘E-grade’ water, whereas the river water quality at the rest of the three sites was observed as ‘good’ with ‘B-grade’ water and WQI values ranged from 26 to 50. However, the WQI values for RBF well water ranged from 8.21 to 12.54, which values were less than 25 and depict the ‘excellent’ water quality of RBF wells at all selected sites with ‘A-grade’ water. According to the WQI results (Fig. 4), the water quality of the selected rivers has been improved through the RBF process.
Conclusions
From the present studies, RBF is proved to be a natural, sustainable, and low cost pre-treatment process for drinking water supplies, which does not require any prior treatment like coagulation through alum or mixing of chlorine etc. Bank filtration technique at four selected sites, namely Srinagar, Augustmuni, Satpuli, and Karanprayag in Uttarakhand, has thus provided evidence of qualitative improvement in water quality for domestic water supply. The water quality classification through Piper trilinear and Chadha’s diagrams and statistical analysis using the Pearson correlation matrix also support the improvement in water quality in RBF water, reflected through WQI. The major outcome of the RBF studies includes significant improvement in drinking water quality by reduction of major problematic water quality parameters viz. turbidity and bacterial contamination along with pH and iron in the present case. Thus, RBF may be applied and used at other suitable locations at the banks of rivers of Uttarakhand for supply of good quality water in adequate quantities for its population, living in a difficult hill area.
References
Abdel-Lah AK (2013) Riverbank filtration for water supply in semi arid environment. J Eng Sci 41(3):840–850
Akoteyon IS, Omotayo AO, Soladoye O, Olaoye HO (2011) Determination of water quality index and suitability of urban river for municipal water supply in Lagos-Nigeria. Eur J Sci Res 54:263–271
APHA (2005) Standard methods for the examination of water and waste waters. American Public Health Association, Washington
Balan IN, Shivakumar M, Kumar PDM (2012) An assessment of ground water quality using water quality index in Chennai Tamil Nadu India. Chron Young Sci 3:146–150
BIS (1991) Specifications for drinking water IS, vol 10500. Bureau of Indian Standards, New Delhi, p 1991
BIS (2004) Specifications for drinking water IS: 10500:2004 2nd revision. Bureau of Indian Standards, New Delhi
Brown RM, McClelland NI, Deininger RA, Tozer RG (1970) Water quality index-do we dare? Water Sew Works 117:339–343
Cady P (2011) A riverbank filtration demonstration project on the Kali river. Master’s thesis, University of Rhode Island, Dandeli, Karnataka, India, p 66
Chadha DK (1999) A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrogeol J 7(5):431–439
Chauhan A, Singh S (2010) Evaluation of Ganga water for drinking purpose by water quality index at Rishikesh, Uttarakhand, India. Rep Opin 2:53–61
Dash RR, Mehrotra I, Kumar P, Grischek T (2008) Lake bank filtration at Nainital India: water-quality evaluation. Hydrogeol J 16:1089–1099
Dash RR, Prakash EVPB, Kumar P, Mehrotra I, Sandhu C, Grischek T (2010) River bank filtration in Haridwar India: removal of turbidity organics and bacteria. Hydrogeol J 18:973–983
de Vet WWJM, van Genuchten CCA, van Loosdrecht MCM, van Dijk JC (2009) Water quality and treatment of river bank filtrate. Drink Water Eng Sci Discuss 2:127–159
de Vet WWJM, van Genuchten CCA, van Loosdrecht MCM, van Dijk JC (2010) Water quality and treatment of river bank filtrate. Drink Water Eng Sci 3:79–90
Derx J, Blaschke AP, Farnleitner AH, Pang L, Bloschl G, Schijven JF (2013) Effects of fluctuations in river water level on virus removal by bank filtration and aquifer passage—a scenario analysis. J Contam Hydrol 147:34–44
Grischek T, Schoenheinz D, Ray C (2002) Siting and design issues for riverbank filtration schemes. In: Ray C, Melin G, Linsky RB (eds) Riverbank filtration: improving source-water quality. Kluwer Academic, Dordrecht, pp 291–302
Jain CK, Bandyopadhyay A, Bhadra A (2010) Assessment of ground water quality for drinking purpose, District Nainital, Uttarakhand, India. Environ Monit Assess 166:663–676
Jaramillo M (2012) Riverbank filtration: an efficient and economical drinking-water treatment technology. Dyna 171:148–157
Joshi DM, Kumar A, Agrawal N (2009) Studies on physicochemical parameters to assess the water quality of river Ganga for drinking purpose in Haridwar district. Ras J Chem 2:195–203
Juttner F (1995) Elimination of terpenoid odorous compounds by slow sand and river bank filtration of the Ruhr River, Germany. Water Sci Technol 31(11):211–217
Juttner F (1999) Efficacy of bank filtration for the removal of fragrance compounds and aromatic hydrocarbons. Water Sci Technol 40(6):123–128
LeChevallier MW, Au KK (2004) Water treatment and pathogen control: process efficiency in achieving safe drinking water. IWA, London. http://www.who.int/water_sanitation_health/dwq/en/watreatpathtoc.pdf. Accessed 15 Feb 2013
Lorenzen G, Sprenger C, Taute T, Pekdeger A, Mittal A, Massmann G (2010) Assessment of the potential for bank filtration in a water-stressed megacity (Delhi, India ). Environ Earth Sci 61(7):1419–1434
Luckins N, Mehrotra I, Kumar P, Grischek T (2011) Riverbank filtration as an alternative to pre-chlorination: a case study at Mathura. In: Dobhal R, Grischek T, Uniyal HP, Uniyal DP, Sandhu C (eds) Drinking water: source, treatment and distribution. Uttarakhand State Council for Science and Technology, Dehradun, pp 125–135
Ojha CSP (2012) Simulating turbidity removal at a river bank filtration site in India using SCS-CN approach. J Hydrol Eng 17(11):1240–1244
Oluyemi EA, Adekunle AS, Adenuga AA, Makinde WO (2010) Physico-chemical properties and heavy metal content of water sources in Ife North local government area of Osun state, Nigeria. African J Environ Sci Technol 4(10):691–697
Piper AM (1944) A graphical procedure in the geochemical interpretation of water analysis. Trans Am Geophys Union 25:914–923
Prasad T, Varma AK, Singh BP (2009) Water supply to Patna through river bank filtration: problems and prospects. In: Proc of the international conference of water, environment, energy and society WEES, New Delhi, 12–16 January 2009, pp 1348–1357
Rawat V, Jha SK, Bag A, Singhai M, Rawat CM (2012) The bacteriological quality of drinking water in Haldwani block of Nainital district, Uttarakhand, India. J Water Health 10(3):465–470
Ray C (2008) Worldwide potential of riverbank filtration. Clean Technol Environ Policy 10:223–225
Sandhu C, Grischek T, Kumar P, Ray C (2011) Potential for riverbank filtration in India. Clean Technol Environ Policy 13:295–316
Schubert J (2002) Hydraulic aspects of riverbank filtration—field studies. J Hydrol 266:145–161
Semwal N, Akolkar P (2006) Water quality assessment of sacred Himalayan rivers of Uttaranchal. Curr Sci 91:486–496
Shamrukh M, Abdel-Wahab A (2008) Riverbank filtration for sustainable water supply: application to a large-scale facility on the Nile River. Clean Technol Environ Policy 10(4):351–358
Shamrukh M, Abdel-Wahab A (2011) Water pollution and riverbank filtration for water supply along river Nile, Egypt. In: Ray C, Shamrukh M (eds) Riverbank filtration for water security in desert countries. Springer, The Netherlands, pp 5–28
Shankar V, Eckert P, Ojha C, Konig CM (2009) Transient three-dimensional modeling of riverbank filtration at grind well field Germany. Hydrogeol J 17:321–326
Sharma SK, Amy G (2009) Bank filtration: a sustainable water treatment technology for developing countries. In: Proceedings of the 34th WEDC international conference. Addis Ababa, Ethiopia
Singh AK, Shah G, Sharma V (2012) Revival of defunct radial collector wells for urban water supply using river bank filtration technique in India. J Indian Water Works Assoc 49(4):24–39
Sontheimer H (1980) Experience with river bank filtration along the Rhine river. J AWWA, 386–390
Sprenger C, Lorenzen G, Pekdeger A (2012) Environmental tracer application and purification capacity at a riverbank filtration well in Delhi (India). J Ind Water Works Assoc, 25–32
Sudhakaran S, Lattemann S, Amy GL (2013) Appropriate drinking water treatment processes for organic micropollutants removal based on experimental and model studies—a multi-criteria analysis study. Sci Total Environ 442:478–488
Tyagi S, Sharma B, Singh P, Dobhal R (2013) Water quality assessment in terms of water quality index. J Am Water Resour Assoc 1(3):34–38
Tufenkji N, Ryan JN, Elimelech M (2002) The promise of bank filtration. Environ Sci Technol 36(21):422A–428A
Varadi Z (2013) Analytical calculation methods of riverbank filtration. In: Proceedings of the 1st global virtual conference, vol 8–12, Goce Delchev University Macedonia & Thomson Ltd. Slovakia, 8–12 April 2013, pp 573–578
Acknowledgements
The authors are thankful to the Water Technology Initiative (WTI) Programme, Department of Science and Technology, Government of India, New Delhi and Uttarakhand State Council for Science and Technology (UCOST), Dehradun for financial assistance provided for this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tyagi, S., Dobhal, R., Kimothi, P.C. et al. Studies of River Water Quality Using River Bank Filtration in Uttarakhand, India. Water Qual Expo Health 5, 139–148 (2013). https://doi.org/10.1007/s12403-013-0097-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-013-0097-z