Abstract
Nanofiltration (NF) membranes are the globally recognized membrane technology, having potential use in food industries from a consistent, economical and standard operation point of view. NF has also attracted industries due to the need for lower pressure–driven membranes compared to reverse osmosis (RO) membranes. NF membranes are used in various applications for concentrating, fractionating and purifying various edible products from the dilute streams. Food processing industries are countlessly utilizing the NF membranes for beverage, dairy, vegetable oils and other food items for separation, concentration/purification, deacidification, demineralization, microbial reduction, etc. However, the increasing challenge in membrane science and technology is to develop low-cost, highly efficient, long-lasting membranes. The permeance-selectivity trade-off relationship, physical ageing and fouling are the main disputes in developing a promising membrane. This review provides a broad view of the current advancement of NF membranes in diverse fields related to the food industry. In this review article, the noteworthy growth of NF membrane in the food industries has been discussed. Various methods for the development of efficient NF membranes along with fouling control measures and research opportunities have been discussed. It is anticipated that this inclusive review may inspire a new research platform for developing next-generation NF membrane processes for diverse applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
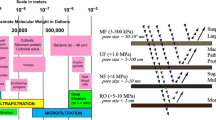
The introduction of nanofiltration (NF) membranes in the food industry was first acknowledged in the year 1976 when a Florida-based equipment producer thought of using an altered reverse osmosis membrane in the place of lime softening to treat water that contains dissolved solids [1]. In the year 1977, a membrane producer from California developed a special softening membrane with a rejection rate of about 47% for monovalent ions. The full commercialization of the softening membrane happened in 1984 with the help of a 3-month pilot study done for Brevard County using ultrafiltration (UF) membranes [2]. This softening membrane was named nanofiltration membrane due to its pore sizes. Properties of nanofiltration membranes are between those of ultrafiltration (UF) membrane and reverse osmosis (RO) membrane owning pore size ~ 1 nm and 300–500 Da molecular weight cut-off (MWCO). Polymeric NF membranes are slightly charged; they can be anionic or cationic depending on their surface charge. Mostly prepared polymeric nanofiltration membranes have polyamide layers on the surface, and the zeta potential result shows their anionic behaviour at neutral pH due to the strong carboxylic acid functionality; for instance, the polymeric NF membranes containing carboxylic groups as well as sulfonic acid groups give rise to a charged surface with the application of the feed solution [3]. NF membranes are capable of separating inorganic ions and organic compounds, just like RO membranes. Higher flux, lower operation pressure, lower monovalent ion rejection, strong divalent ion rejection and comparatively inexpensive investment and maintenance expenses distinguish NF membranes from RO membranes. Because of these properties, NF membranes have found applications in many fields particularly in wastewater treatment [4], desalination [5, 6] biotechnological and pharmaceutical applications [7, 8], food industries [9, 10], diary [11], and vegetable oil processing industries [12], beverage industries [13,14,15,16], plant extracts [17, 18], etc. The growing demand for healthy, fresh and nutritional foods has encouraged an appropriate separation process in the food industry. Obtaining valuable products and having longer shelf life with fewer waste products are some major requirements for the industry. NF membrane can be an ideal choice for the food industry as it provides a better separation process without compromising the nutritional properties of the substance. In recent years, there has been a remarkable increase in the number of research articles published on NF membranes in a variety of domains. The pie chart presented in Fig. 1 indicates the percentage of research that has been done on several sectors of the food industry using NF membranes.
Current scenario of published articles on NF membranes for food industries. Data source: Web of Science (https://mjl.clarivate.com/home), date of data collection 27 April 2022
The transportation models involved in the separation through NF membrane also rely on the properties of the solute particles. Depending on the solutes, the transport models are categorized into two main types which consist: (I) firstly, the solutions containing electrolytic solutes, where the separation through the NF membrane takes place by charge interactions between the membrane surface and electrolyte. Typical models representing this transportation include the space charge model and fixed charge model [19]. (II) Secondly, for non-electrolytic solutes, the appropriate separation models considered are based on the steric hindrance model and the solution-diffusion model. The steric hindrance model has based on the dimensions of the membrane pores by assuming that all pores are uniform, whereas in the solution diffusion model, it is assumed that the solute and the solvent dissolve through the membranes and are driven by the means of concentration gradient or pressure difference. In NF membranes, the steric hindrance and charge interaction control the selectivity performance of the membrane [20]. Narrowing the pores can be an excellent route for solute-selective separation while altering the surface charge of the membrane may increase the selectivity of the charged solutes via the Donnan effect and dielectric exclusion [21]. There is an estimation done by BCC Research, that the world market will witness 20% annual growth for NF membrane by 2024 [22]. A major hindrance to the application of NF membranes on a large scale is the trade-off between permeance and selectivity because the modification in the pore size of the membrane to improve the selectivity will result in a decline in permeability [23]. Therefore, work has been done to improve both the permeability and selectivity of the membrane by maintaining the thickness of the thin film layer, enhancing hydrophilicity and increasing the effective area of the membrane by adjusting the surface morphology [24,25,26]. In this review article, the growth in the field of the food industry using modified NF membranes has been discussed. Several significant and new methods applied by researchers to generate efficient NF membranes for applications in different sectors of food including dairy, vegetable oils, beverages and plant extracts have been tried to present in this review article. The technologies employed, and the effect of different physiochemical conditions on the results and the fouling problems that arise with the application of NF membrane along with its mitigation solutions have been discussed. Lastly, the obstacles and future challenges related to the NF membrane are also presented in this review article.
Nanofiltration (NF) Membranes for Food Industry Application
The market for “fresh foods” and products that are free from harmful chemical additives and high-quality grades is one of the most significant developments in the food industry today. New processes based on the principle of “green food processing” have been launched to ensure food safety and efficiency. “Green food processing” is focused on the development and discovery of technical methods that are energy-efficient and water-saving, while still enabling the by-products to be recycled by bio-refinery as well as maintaining a sustainable and superior-quality product [27, 28]. Membrane methods have many benefits over traditional methodologies, including moderate temperature and pressure operating conditions, which preserve the functional features of food items, and also the capacity to prevent the employment of harmful solvents or reagents. They also feature a high removal efficiency, compact machines, easy scaling, fewer processing steps and low energy consumption. Membrane procedures represent advanced and cost-effective purification and fractionation techniques for food-containing solutions based on these properties [9,10,11]. The use of NF membranes in a range of food processing applications has been investigated such as purification of water and sugar, the concentration of juice, whey protein concentration and many more applications making the technology industrially appealing [12].
NF Membranes for Dairy
The nanofiltration method has increased the consistency of dairy products while also increasing process reliability and profitability in the dairy industry. It is commonly used for whey processing [29,30,31], lactose recovery [32], whey demineralization or desalination [33, 34], and lactic acid separation [35, 36]. Whey is a by-product of the casein and cheese industries, with a low solids content (up to 6%) and a high biological oxygen demand (BOD5 = 30–50 g/L), making disposal expensive and difficult [37]. Whey is high in lactose, sugars, fat, mineral salts, vitamins and other nutrients. For several years, whey was regarded as a waste product, and it was mostly used as animal feed (after drying) and as a fertilizer. However, owing to the dietary, biological and practical properties of whey, there has been a growing interest in using it to make value-added products like whey proteins in recent decades [11, 38].
The numerous implementations of membrane-based integrated processes in whey processing in the dairy industry have been shown in Fig. 2. Nanofiltration membrane is effectively applied for concentrating and demineralization of liquid whey to obtain other whey products like whey powder. Acid whey (AW) and sweet whey (SW) are the two common types of whey. AW is obtained as a by-product while making cottage cheese or Greek yoghurt whereas SW is a by-product obtained from hard and semi-hard cheese [39]. The SW is used in the production of whey powder (WP) which is majorly used as a sweetener in bakery, beverages and yoghurt production. Raw whey needs to be treated first as it contains a high amount of minerals and organic acids [40, 41]. To resolve the problem of higher mineral content and higher organic acid contents, preliminary whey desalination comes out to be the best option. The dairy industry utilizes both electrically driven and pressure-driven processes for preliminary whey desalination which includes electrodialysis (ED), ion exchange, NF, dia-nanofiltration (Dia-NF) or integration of both the processes [42]. In the demineralization process of cheese whey, at first, the nanofiltration process results in 90% rejection of protein and lactose with little demineralization then followed by the electrodialysis for high demineralization. In an experiment, the rejection of Na, K, Ca and Mg ions from the NF permeate was done using Ralex membranes and Neosepta membranes [43]. Marx along with his team worked on generating an appropriate technique to produce shelf-stable demineralized whey concentrates. To achieve whey concentrates with different degrees of demineralization, NF and NF incorporated with diafiltration (DF) were employed. A two-step preservation process was also studied which includes microfiltration (MF) combined with thermal treatment for obtaining shelf-stable demineralized whey concentrates. The obtained results demonstrate the suitability of using the NF membrane for demineralizing and concentrating whey along with the retention of worthy components such as lactose or calcium. Also, the integration of NF with DF resulted in increased degrees of demineralization [44]. In one research by Talebi and his team, they combine three processes to study the acid whey desalination process. The combination of three processes used in the study consists of (1) ultrafiltration and electrodialysis; (2) ultrafiltration, nanofiltration and electrodialysis and (3) ultrafiltration, dia-nanofiltration and electrodialysis. The performance of the Dia-NF membrane united with ED comes out to be the best in terms of lactic acid removal with an 88% rejection. The whey powder obtained from a combination process consisting of NF and ED rich in lactose with UF protein retentate was found to have a moisture content of 2.5% and was non-sticky [42]. The research group of Merkel demonstrated adequately the desalination effect on acid whey and sweet whey using an integrated system consisting of nanofiltration and electrodialysis. They observed a reduction in the mineral content up to 27–28% for both AW and SW using nanofiltration followed by electrodialysis. It increases the degree of desalination to 91% for AW and 88% for SW thereby providing a higher removal rate for minerals and organic acids. The integrated system NF + ED shows a rejection of 88% for lactic acid and 93% for citric acid from raw AW resulting in a better quality of whey powders from desalted whey [45]. Lactose recovery from whey is another significant application as demonstrated by Conidi and co-workers in their study. For lactose concentration, an integrated UF and NF technique was used. For milk and whey protein concentration, the UF process was used, accompanied by the NF process for lactose concentration and demineralization followed by spray-drying of the NF retentate. With the help of appropriate operational conditions, more than 90% of lactose can be recovered [13, 29]. Pires and his co-workers reported in their review article that a nanofiltration membrane can be used to recover organic nutrients from cheese whey obtained from the processing of Serpa cheese along with curd production [46]. Nanofiltration processes the cheese whey to produce a concentrate with a high lactose fraction and water treatment with permeate rich in high salt content. Velpula et al. reported a two-stage UF and NF procedure to recycle dairy wastewater. UF protein is concentrated from wastewater during the first stage of the study, and then UF permeate is sent to NF for lactose concentration and reusable water processing. This method is advantageous for using dairy wastewater for bio-energy production by algae cultivation [47]. Concentration polarization and fouling are observed to be significantly reduced during the NF concentration phase in the combined UF and NF process, as proteins are eliminated during the initial UF step.
NF Membranes for Vegetable Oil
The high energy requirements, the need for vast amount of water, nutrient depletion, loss of neutral oil and the other chemical reagents and increased effluent production are some drawbacks of the traditional oil refining method. For solid–liquid and liquid–liquid separation applications, the membrane-based technologies are more efficient and cost-effective due to their low energy consumption, fewer processing steps, high yield as well as being environmentally sustainable process [31, 48]. The majority of progress in the development of membrane technology for oils mainly focused on degumming, deacidification, bleaching, fat/oil hydrolysis, structured lipid synthesis in a membrane reactor, minority compound concentration, solvent extraction from micelles and emulsion separation [49, 50].
In terms of energy consumption, the procedure of solvent recovery from oil extraction has been the most effective; extending 20–25 kWh−1 per tonne of soybean is extracted and approximately 1.7 kg of hexane vapour per tonne of processed oilseeds is exhausted into the atmosphere. On the other side, using the NF technique, this number can be minimized up to a 5% level [12]. Membrane technology can be used to isolate the oil from the solvent in micelles as soon as the oil is extracted, resulting in crude oil that can be refined later. Li et al. utilized AF2400/PTFE composite membrane for the recovery of hexane from the crude soybean oil and hexane mixture by the organic solvent nanofiltration (OSN) method [51]. Several experimental procedures were conducted to study the effect of operational parameters, the concentration of oil, operational temperature, pure solvent permeation along with rejection. It was found that the permeate flux ranged from 0.8 to 1.1 lm−2 h−1 bar−1 with oil rejection of more than 98% before or after physical ageing for 1440 h in pure hexane. An experimental study revealed the effectiveness of the composite membrane in high oil rejection, recovery of hexane and excellent operational stability. Shi and coworkers provided practical guidance for using the OSN method in the vegetable oil processing industry by illustrating a two-stage membrane system for separating and recovering the solvent from oil compounds. This study shows excellent rejection performance and stability by the NF membrane over time [52].
Alternatively, after using the ultrafiltration technique to degum the micelle, solvent recovery using membranes might be used to get hexane-free degummed oil. In a study by Doshi et al., the degumming of crude vegetable oil by membrane separation was studied (Fig. 3). They prepared various polymer membranes and tested their degumming performance of crude peanut oil micelle. They reported 95% rejection of phospholipid gum with hexane and micella permeation 70 and 46 LMH, respectively [49].
Oil/solvent separation after degumming using membrane technology. (Figure taken after some modification from Coutinho et al. [53] with permission, Copyright
In one recent study by Lin and Gazali on palm oil and solvent mixtures using an organic solvent, nanofiltration was done, and the effect of concentration polarization was also studied. They found that the permeation flux of palm oil and acetone mixtures increased with increasing stirring speed but decreased with increasing feed viscosity. Rejection of triglyceride increased with the enhancement of feed concentration; however, no significant change was observed with operating pressure [54]. Novello and his team did the separation study of soybean oil/compressed n-butane and soybean oil/liquefied petroleum gas (LPG) using an integrated system of UF and NF membranes. The consequences of feed pressure, pressure difference, pre-treatment with n-propanol and ethanol on the oil permeate flux as well as its retention were explored in this study [55]. The well-performed membranes Sepa GM and Sepa HL were first conditioned in ethanol. The Sepa GM membrane provided 95–99% oil retention whereas the Sepa HL membrane showed 83–97% oil retention. It was concluded that the pre-treatment with ethanol enhanced the permeate flux. Because of the different gas composition in LPG in comparison to n-butane, changes in the permeate flux and oil retention behaviour were analysed for LPG as solvent. The degummed oil is treated with a sodium hydroxide solution in the refining unit, which reacts and precipitates the free fatty acids (FFAs) as soaps while also removing any leftover phospholipids. To avoid the creation of emulsions, units for refining before the soda treatment, add phosphoric acid to maximize the removal of non-hydratable phospholipids and residues of metals [12]. Membrane technology, in principle, overcomes the bulk of these issues. Hydrophobic membranes would be excellent, with adequate NF membranes exclusively enabling limited separation of FFA [56]. Orm with his co-workers found that the extraction of FFA varied linearly with the quantity of ethanol in the solvent. The drop in the extraction efficiency of FFA with the decrease in ethanol content in the solvent can be recompensed using a higher amount of solvent. This change has a very low impact on the neutral oil losses than FFA extraction. When the quantity of ethanol in solvent decreases, a trade-off relationship between deacidification, oil loss and operating costs is observed. The crude oil extraction resulted in the removal of 95–96% FFA content as well as 4% of neutral oil mass [57]. In an experiment, Li et al. fabricated novel trifluoropropylmethylsiloxane dimethylsiloxane (PDMS-PTFPMS)/polyvinylidene fluoride (PVDF) membranes, having high hexane permeability of PDMS and outstanding hexane stability of PTFPMS, to recover hexane using organic solvent nanofiltration [58]. Under various operating circumstances, the performance of the F50-M membrane was studied, and oil rejection of more than 95% was observed with stable hexane permeability and long-term operational stability. These results are obtained because of the unique microstructure as well as surface qualities due to the presence of the PTFPMS segment [58]. In a similar sense, Hou et al., in their work, used a hydrophobic ceramic membrane to remove phospholipid micelles from crude rapeseed oil. They found that enzyme membrane binding is the most effective degumming method for this work. Through their work, they concluded the enzyme membrane binding can be considered for the production of oil on a small scale [59]. Crude rice bran is a natural product of ɣ-oryzanol, a beneficial antioxidant phytochemical of nutritional value. Rice bran oil (RBO) refining and ɣ -oryzanol enrichment was explored by Sereewatthanawut [60] using NF processing and solvent extraction optimization. Various NF membranes (Evonik, Ltd., UK, StarmemTM, and DuraMemTM membrane series) that are solvent resistant were tested and proven the excellence in a two-step membrane cascade, with fluxes ranging from 39 to 53 Lm−2 h−1. Overall, the integrated treatment increased the antioxidant potential of the oil by more than twofold, increasing an RBO ɣ-oryzanol refinement from 0.95 to 4.1 wt.%. The findings show that organic solvent NF can improve and purify oil-based goods. Deacidification, solvent recovery, and minor chemical concentration procedures in vegetable oils have a lot of promise for further research since the experiments that have been done so far have shown excellent outcomes, even though moderate permeate flow were detected.
NF Membranes for Beverage Application
The most promising application of NF is coming out in the beverage industry. Small monovalent ions and water can flow through the NF membrane to some extent, but bigger molecules are trapped. Fruit juices are generally concentrated by employing a multi-stage vacuum evaporation process, which affects the level of fresh juice fragrance, colour change and cooked flavour due to high-temperature effects. When examining a juice using sensory analysis, heat treatment affects both the colour and odour [61, 62]. This constraint has been proven to be overcome by technological developments in the creation of new membranes and advancements in process engineering. It has a lot of potential for low-temperature clarity, microbial stabilization and concentration of fruit and plant juices and extracts while using very little energy. NF has been used to concentrate a variety of juices and musts [61, 63, 64]. Furthermore, the NF method may be employed in the manufacturing of fermented foods such as beer and wine. Membranes were first used as a clarifying stage following the fermentation process. The use of NF techniques is expected to bring substantial improvements to the beverage sector soon with recent advancements in membrane research and technology. The concentration of juice in present-day methodology includes energy-intensive unit activities such as high-temperature evaporation (90 °C), which has various drawbacks, including loss of scent, nutrients (antioxidants and vitamins), colouring owing to Maillard reactions, cooked odour owing to furfural creation and formation of foam, and enormous energy is required for removing water [64]. On comparing RO from NF membrane, it can be concluded that the NF membrane consumes very less energy because low operational pressure results in an excellent quality of the fruit juices [16]. The employment of NF membrane technology enables the creation of a product that is alike to freshly squeezed juice while also speeding up and simplifying the clarity process. Bhattacharjee reported in their work on the use of integrated RO/NF membranes for the treatment of blackcurrant juice. Integration of nanofiltration membrane with reverse osmosis membrane resulted in overcoming the high osmotic pressure contributed by RO membrane. The integration system resulted in the concentration of the juice up to 70° Brix [61].
For the manufacturing of fermented foods, dead-end filters were originally utilized and then go around with the earliest cross-flow filtering studies for wine and beer clarity [12]. Membrane filtration has made a name for itself in the last decades as a method for clarifying wine and beer, owing to its present dependability in other manufacturing phases. Over the last decade, the order for low-content alcohol and alcohol-free beverages has been increasing steadily [12]. NF can make lower the alcohol content of beer by 8–10 times while keeping the taste. Membrane filtering has long been used in the wine industry. The membrane can be used instead of chaptalization or any other procedures to boost sugar content in wine without adding non-grape components at room temperature; it can also change and maintain the must composition. RO concentrates carbohydrates, as well as numerous other natural substances of must like malic acid, resulting in a lack of wine sensory balance [65]. Furthermore, RO requires a lot of energy and creates a lot of membrane fouling. Using perm-selective NF membranes, these flaws might be overcome. The NF method transports the grape must across the membrane using a pressure gradient, separating the permeate (mainly water) from the concentrate which consists of enriched solutes. Nanofiltration membranes for the grape must concentration might be a new application. To demonstrate the consequences of the separation of sugar content from grape musts which are utilized in the production of wine, Salgado stated in their paper that nanofiltration membranes were successfully able to minimize the sugar concentration from red must. Also, the nanofiltration membrane outperformed in the passage of sugar with less fouling [14, 15].
Wine is one of the world’s most popular alcoholic beverages, and moderate use of these beverages, particularly red wine, is linked to a lower risk of cardiovascular disease. Red wine has antioxidant and cardiovascular effects which are more appreciable than those of other commonly consumed alcoholic drinks [66]. Because of the growing demand for non-alcoholic drinks and the increasing ethanol concentration of wine, the removal of alcohol from wines is becoming increasingly important in the beverage sector. The ethanol in a typical wine may be removed using membrane techniques. A demonstration of using a nanofiltration membrane for preparing low alcohol content red wine was carried out by Banvolgyi [67]. For this study, they used a factorial design to study the influence of temperature and trans-membrane pressure on the rejection of worthy compounds such as anthocyanins and resveratrol. The highest rejection of anthocyanins and resveratrol was obtained at low temperature whereas enhanced permeate flux was observed at high trans-membrane pressure. This study revealed that the nanofiltration membrane is a valid method to obtain low alcohol–content red wine with their sensory properties as that of the original. Salgado and co-workers employed KMS SR3 (manufactured by Koch Membrane Systems) nanofiltration membrane to study the production of low-alcohol wines. Their study demonstrated the feasibility of single- and two-stage nanofiltration processes for reducing sugar from grape must without any notable change in significant compounds such as malic acid, polyphenols and tartaric acids. In this way, they obtained wines with similar sensorial characteristics to the wine obtained from untreated must which can be applied to produce low alcohol content wines [14, 15]. Ivić et al. studied the working of RO and NF membranes to concentrate phenolic and antioxidant compounds under different operational conditions on phenolic retention [68]. Their research work was mainly on the aromatic compound’s permeation in the course of the concentration process, and with this, they discovered that these compounds were rejected in large quantities when the applied pressure was increased and the temperature was decreased. Retention of the phenolic compounds depended on the membrane properties, chemical structure, fouling and the operating conditions. The study revealed no significant change in the colour of the wine [68]. Luo in his experiment refined sugarcane juice by using an integrated membrane system. He concentrated the raw sugarcane juice without treating it with harmful chemicals, which can be further used for sugar production as well. The membrane integrated system consists of a loose UF membrane, a tight UF membrane and an NF membrane as shown in Fig. 4a. The observed results of the experiment can be seen in Fig. 4b where the permeate after every membrane was seen with the final retention from the NF membrane. The results of their experiment concluded that using this system to pilot-scale clarification, decolouration and pre-concentration of raw sugarcane juice can be accomplished. They were able to achieve ~ 96% colour removal and ~ 99.99% turbidity removal from the raw juice. The measurement of the colour value of the final product was below 800 IU which can be converted to white sugar using crystallization/evaporation [69].
a Flow diagram of integrated membrane system consisting of tubular loose UF, tight spiral wound UF and spiral wound NF membranes. b The permeate was obtained from loose UF, tight UF and NF membrane with retentate obtained at last from NF. (Figure taken after some modification from Luo et al. [69] with permission, Copyright
NF Membranes for Plant Extraction
Over the last several decades, phenolic compounds have been extensively investigated for their potent antioxidant, free radical scavenger as well as metal chelator properties and their capacity to inhibit and decrease certain enzymes (lipoxygenase, telomerase, and cycloxygenase) [70, 71]. Because of their potential usage as components in foods, pharmaceutics and cosmetic compositions, recovering bioactive chemicals from natural origin has gotten a lot of interest. Secondary metabolites and polyphenols (PPs), found in all plant species, have lately risen to the top of the majorly searched topic of these chemicals [70]. Those plant metabolites are distinguished by the presence of multiple phenol groups (aromatic rings with hydroxyls generated from tyrosine or L-phenylalanine. They also aid to prevent a variety of human illnesses such as cancer, osteoporosis, diabetes, heart disease, and neurological illnesses. Some phenolic chemicals in particular, e.g. flavan-3-ol, neolignan, tannin and flavonol, have shown unique benefits for well-being, like antimicrobial activities (against bacteria, viruses, and fungus) [70] as well as oral health [72]. Polyphenol’s health-protective properties are largely assigned to their phenolic hydroxyl groups, which act as good hydrogen donating antioxidants and scavenge reactive oxygen species, breaking the chain of radical generation [72, 73]. As a result, the PPs prevent proteins, lipids and DNA from being oxidized. They inhibit the synthesis of enzymes that produce radicals, which have been related to the beginning of diseases [74]. Fruits (berries), leaves, seeds, grains and vegetables have all been proven to contain phenolic chemicals, with the list rising all the time [17]. A key goal in nutraceuticals processing is the development of integrated processing systems, which are capable of minimizing bioactive compound degradation all through the handling of raw materials, manufacturing, packing and storage of the finished product. Membrane techniques, in this opinion, have generated significant attention in recent years as a result of their conventional benefits over classic separation techniques. Membrane techniques have advantages such as lower processing temperatures and pressures, the lack of chemical solvents/reagents, decreased waste treatment quantity and cost [18].
Paun studied the effectiveness of the nanofiltration technique in concentrating polyphenolic components in Geranium robertianum and Salvia officinalis plant extracts [75]. A lab-scale cross-flow system with a flat-sheet structure membrane had been employed in all of the tests (Fig. 5). Two nanofiltration membranes have been employed for this experiment namely an organic–inorganic membrane (polysulfone with SBA-15-NH2) and SelRO MPF-36 (Koch membrane). The rejection ratios for polyphenols and flavonoids were over 70% for both membranes. When compared with the Koch membrane, at the expense of lower flux the organic–inorganic membrane displayed higher rejection ratios approximately 57% for S. officinalis extract and 29% for G. robertianum extract. According to the findings of this study, the maximum antioxidant activity is seen in concentrated extracts produced by nanofiltration membrane via an organic–inorganic membrane, which may be exploited as natural sources for producing antioxidant agents and free radical scavengers.
Diagram of a lab-scale cross-flow system with a flat-sheet structure membrane. (Figure taken after some modification from Paun et al. [75] with little modification)
Pereira [76] studied grape marc extracts rich in anthocyanins and phenolic compounds using an integrated system comprising pressurized liquid extraction (PLE) accompanied by separation through membrane technology.
The permeate fluxes and retention of the bioactive compounds as well as the antioxidant content of the concentrated extracts were estimated (as illustrated in Figs. 6 and 7). The membranes used for the experiment consist of flat sheet MV020 and MP005 MF membranes, UV150 and UP150 UF membranes and NP010, NP030 NF90 and NF270 NF membranes. With a 0.0016-m2 effective permeation area, a bench-scale dead-end filtration was performed whereas MF and NF processes were done using a 0.0077-m2 effective permeation area in a cross-flow filtration bench-scale module. By maintaining the 750 rpm at 35 °C temperature, 4.0 ± 0.5 MPa trans-membrane pressure (TMP) for NF membrane and 1 ± 0.1 MPa TMP were put for MF membrane. For removing larger particles, most potent membrane was found to be the MV020 microfiltration membrane, and its permeate was used for nanofiltration because of the higher permeate flux and lower retention of bioactive compounds. An encouraging result was obtained with the utilization of PLE and MV020-NP010 process for grape marc for concentrating bioactive compounds in cross-flow filtration. It needs to be examined for industrial applications in the nearby future.
Permeate profile of mass flux as a function of time of the A MF at 0.5 MPa, B UF at 0.5 MPa and C NF at 0.8 MPa processes carry out at 35 °C in stirred dead-end filtration mode (Figure taken from Pereira et al. [76] with permission, Copyright
Retention coefficients of the monomeric anthocyanins, total phenolics, and total solids of A MF at 0.5 MPa, B UF at 0.5 MPa and C NF at 0.8 MPa processes represent at 35 °C in stirred dead-end filtration mode. (Figure taken from Pereira et al. [76] with permission, Copyright
Tundis evaluated and compared the chemical description of ethanol (EtOH) and methanol (MeOH) extracts of Sambucus nigra L. Leaves as well as flowers of the Adoxaceae family and their antioxidant and antityrosinase activities, to find suitable representatives for food supplements, pharmaceutical and cosmetics items [18]. Ethylene-chlorotrifluoroethylene (LMP ECTFE) NF membrane with a new lower melting point was utilized to concentrate the bioactive phytochemicals in S. nigra to create concentrated fractions. The LMP ECTFE N2 membrane demonstrated better chemical and mechanical stability, with low swelling in various solvents, particularly methanol and ethanol, which showed 4% and 6% swelling, respectively. At room temperature, the permeabilities of methanol and ethanol were 3.6 and 3.0 Lm−2 h−1 bar−1, respectively. The permeate flux obtained from S. nigra leaves ethanol extract was found to be greater than the methanol extract with a flux of 2 Lm−2 h−1. Similarly, the ethanol and methanol extract of S. nigra flowers showed permeate flux of 0.4 Lm−2 h−1.
Flower extracts contained higher phenolic compounds than leaf extracts and have intriguing bioactivities. The methanol-extracted flower’s retentate portion exhibits the highest inhibitory activity of tyrosinase and antioxidant capacity. Flowers of S. nigra may be an excellent source of tyrosinase inhibitors in the diet and natural antioxidants, according to the research. Cissé studied the possibility of utilizing NF and UF membranes to isolate as well as concentrate anthocyanin components from H. sabdariffa calyces extract. According to the results, to concentrate anthocyanins in roselle extract, any membrane with an apparent MWCO of less than or equal to 20 kDa can be used [77]. At trans-membrane pressure of 3 MPa, anthocyanins were rejected 80% by UF membranes which have molecular weight cut-off (MWCO) between 1 and 20 kDa. At transmembrane pressure of 2 MPa, DK along with DL NF membranes (GE Osmonics production) excel in extracting anthocyanins using roselle extracts. NF membranes had greater permeate flows when trans-membrane pressure was around 2 and 3 MPa, with ≥ 95% rejection of anthocyanins and total soluble solids. These findings suggest that NF and UF are viable alternatives for generating concentrated anthocyanin extract at low temperatures. These membrane techniques might be useful for pre-concentrating extracts without causing heat damage preceding final volume (osmotic evaporation, vacuum evaporation), as well as spray drying. However, more study is required to further assess the method at an industrial scale competitively.
Food is a necessity, and a lot of research is conducted daily for making the food industry better and safer. The target of researchers in the NF membrane sector is to construct an ideal low-cost high-performance membrane which offers high yield with the flexibility of operations. Several experiments regarding membrane food applications have been reported, and an overview of such applications is shown in Table 1.
Fouling Control Strategies
Fouling is a matter of concern for researchers as it interrupts the large-scale application of the membrane. It is a bottleneck issue for the membrane technology for food processing. A fouled membrane has altered pore size leading to a decreased flux rate and selectivity which retards the performance of the membrane. To get an effective antifouling behaviour of the membrane, efforts have been given to modify the physicochemical properties of the selective layer. For antifouling behaviour, the membrane surface must be hydrophilic, have low surface energy or can have self-cleaning behaviour. More hydrophilic surfaces exhibit more permeation and less organic fouling [88]. It is observed from various experiments that the fouling in the nanofiltration membrane used in food industries, especially in refining dairy products, occurs due to the protein molecules [9]. To remove the lincomycin (LIN) from the milk samples, lincomycin molecularly imprinted membranes (LINMIMs) were fabricated, and to test their antifouling characteristic, BSA protein is used. After treatment with poly-ethylenimine (PEI) and (dopamine) DA, the anti-staining ability in the membranes was observed [89]. To amplify the antifouling characteristics of polymeric nanofiltration membrane, Li and his team approached a path. They first dipped the polyethersulfone (PES) polymer membrane into the bioinspired polydopamine (PDA) solution. Then, the surface of the membrane was chemically modified with fluorinated polyamine using the Michale addition reaction. The surface modification (fluorinated polyamine) formed low energy surface to mitigate the accumulation of fouling on the surface. When the membranes were tested for antifouling study against BSA, oil and humic acid the membrane exhibited excellent antifouling behaviour. Flux decrement (~ 8.9%) was observed, and a 98.6% flux recovery ratio states the antifouling characteristic of the membrane is auspiciously good for long-term use [90]. To fabricate a hydrophilic antifouling surface for the TFN nanofiltration membranes, the selection of proper monomers having particular functional groups like compounds containing diamine moieties is considered to be a better option. On the other hand, the incorporation of functional nanoparticles or hydrophilic nanoparticles during the preparation of the NF membrane is also grabbing the attention of researchers [91, 92]. TiO2 nanomaterial incorporation can enhance the antifouling characteristic of the TFN membranes because of its self-cleaning behaviour under UV radiation. The graphene due to its super hydrophilicity does not stick with the organic fouling materials. Nanomaterials like these are a good choice for preparing long-lasting nanocomposite membranes for the food industry’s applications [93]. Also, postmodification of the membrane surface by inserting an additional hydrophilic layer can provide an antifouling characteristic. Ren with coworkers prepared a hydrophilic NF membrane using ring-shaped quaternary ammonium complexes. The ring-shaped complexes mixed in the aqueous phase solution reacted with trimesoyl chloride (TMC) organic phase solution via the interfacial polymerization method. The prepared NF membrane displayed outstanding antibacterial performance with a more than 92% killing rate of bacteria. It also demonstrated good antifouling behaviour as examined using humic acid and bovine serum albumin (BSA) with a flux recovery ratio of > 95%, thus imparting selectivity and antifouling character to the NF membrane [94]. Our group adopted a novel way to synthesize TFN membrane using mesoporous synthetic hectorite (MSH) and the nanoparticle composite of MSH and MOF (UiO-66-NH2) in piperazine and TMC using the interfacial polymerization method. The prepared membrane was tested against several salt solutions, and the antifouling behaviour was investigated. The membrane even demonstrated excellent antibacterial activity with an efficient decrement in the bacterial colony [5]. Our recent work of incorporating carboxylic acid-functionalized TiO2 nanomaterials in the TFN membranes has resulted in a good antifouling tendency (≤ 89% in the first cycle and ≤ 97% in the second cycle) and low irreversible fouling (up to 10% low) after long-term testing against a mixed feed containing BSA protein [95].
Another approach gaining importance in recent times is the development of antifouling membranes by incorporating zwitterionic compounds that combine with the water molecules through electrostatic and H-bonding interaction. It makes a hydration layer on the membrane surface that efficiently removes foulant particles [96]. Li and his team fabricated and designed a new zwitterionic aromatic diamine monomer 3-(4-(2-((4-aminophenyl)amino)ethyl)morpholino-4-ium) propane1-sulfonate (PPD-MEPS) using three-step reaction. The incorporation of this hydrophilic molecule into the selective layer of poly(piperazine-amide) NF membrane effectively enhanced the permeability and antifouling characteristics of the membrane [97]. A research group led by Liu developed a zwitterionic NF membrane consisting of tannic acid (TA) with the help of a multi-layer assembly method (multiple layer-by-layer, mLBL). The prepared membrane includes Fe3+ transition metal ion and illustrated increased roughness, hydrophilicity and antifouling properties as compared with the non-zwitterionic NF membrane. The effects of the TA/Fe3+ bilayer and the concentration of the poly(sulfobetaine methacrylate) (PSBMA) polymer on the membrane operation were examined for salts and dyes. The zwitterionic (TA/Fe3+)/PSBMA membrane showed water flux of 40.36 LMH/bar and > 98% rejection for rose bengal (RB) and ~ 60% rejection for Na2SO4 under appropriate conditions. The incorporation of zwitterionic (TA/Fe3+) and PSBMA polymer resulted in a membrane with excellent antifouling properties along with rapid and economic benefits [98]. One more approach that is widely adopted to increase the antifouling nature of the membrane while simultaneously controlling the perm-selectivity is by preparing an amphiphilic surface with a low-surface-energy and hydrophilic domain. The amphiphilic surface provides an effective way to remove foulant from the surfaces as a hydrophilic domain inhibits unnecessary interactions. Therefore, it prevents the attachment of foulants on the membrane surface. The low-surface-energy domain diminishes the strength of interfacial bonds which provides easy removal of foulants from the surface at low hydrodynamic forces [99]. One more route followed by the researchers is to prepare an antifouling membrane with a low-surface-energy self-cleaning surface. Materials with low surface energy, for instance, fluorine-based and silicone-based materials reduce the energy of the membrane’s surface thereby loosening the attachment of the undesirable substances from the membrane surface which can be easily removed by applying low shear force [22].
Obstacles and Future Prospective
The success of NF membranes so far has been attributed to their unique ability to selectively separate the desired separation species. To give the best separation, a membrane with appropriate selectivity should be chosen based on the application of interest. Membranes made of NF would continuously seek applications in several areas, including isolation of tiny organics in biotechnology, food as well as pharmaceutical applications. It has become one of the most important technologies for addressing global concerns of food processing industries utilizing the NF membranes for beverage, dairy, vegetable oils, plant extracts for concentration, purification, deacidification, demineralization and degumming, etc. Because of its high separation performance, NF membrane preparation and application have made significant development. Researchers have been studying new low-cost NF membranes with enhanced selectivity with good permeation flux, as well as structurally robust and fouling resistance for industrial usage. While several studies have reported on innovative and fascinating uses of NF membranes in a variety of fields, little research has looked at how the mechanisms work on a big scale and over time. For an economically feasible membrane operation, an evaluation of the techno-economic process is required which can be done by experimenting and testing the membranes at the pilot level. This will aid in the resolution of many concerns including the process’s feasibility, profitability, moderation, fouling management and membrane sensitivity to complex feed materials. This sort of research will take a longer time and cost more money, but the results will lead to greater long-term NF membrane operation. Many experiments have been done on making the antifouling membranes, and still, there is a need for further research on this area and their applicability to food items. As fouling is a bottleneck issue for the NF membranes, incorporating nanoparticles in preparing the membranes is one of its solutions. Much research regarding making the membrane antifouling in natured using various nanoparticles has been discussed earlier, and they are needed to test for food applications. Clay nanomaterials and carbon-based nanomaterials including derivatives of graphene oxides, are some potential nanofillers for NF membrane, and further research on these materials can be helpful as they possess biocompatibility and show excellent antifouling behaviour. The technical challenges like membrane fouling, low flux rate, poor durability and instability must be addressed to increase the feasibility of the NF systems. The majority of fouling monitoring evaluations are now focused on permeate fluxes. To allow for a length of time during which the fouling has developed to the point where it can be noticed. Such a study can provide helpful information on detecting early fouling and taking appropriate measures before fouling becomes irreversible. Another approach that can be taken to boost the performance of the NF membranes can be done by integrating different membrane processes with the NF as work has already been started and mentioned in several studies with excellent results.
Conclusions
NF membranes have found success in a range of industries. In NF membranes, steric, dielectric, Donnan and transport effects all play a role in solute transport. Fabrication methodologies for NF membranes are effective in creating membranes with increased selectivity, better flux and antifouling properties. NF membranes prepared by using advanced materials and by employing different methodologies have resulted in a membrane with robust properties such as enhanced hydrophilicity, higher rejection, higher permeance rate and antibacterial activities, and it mainly provides the membrane with an improved antifouling property. The improvement noticed in the NF membranes indicates a good sign for the membrane to be applicable for further usage in the food industry. Different fouling control measures have been tried to cover in this review article. To make thin-film composite or nanocomposite membranes, the principal approach still relies on interfacial polymerization. Nanomaterials added to thin films have had a substantial impact on membrane performance. NF membranes have been proven to be effective in applications in dairy industries, vegetable oil processing, plant extracts and beverage industries due to their variable selectivity. Fouling control and mitigation, however, continue to be an issue. Future research should focus on enhancing the capacity to manage, decrease and mitigate fouling while producing superior membranes and new innovative technologies.
Abbreviations
- NF:
-
Nanofiltration
- DF:
-
Diafiltration
- Dia-NF:
-
Dia-nanofiltration
- UF:
-
Ultrafiltration
- MF:
-
Microfiltration
- RO:
-
Reverse osmosis
- ED:
-
Electrodialysis
- AW:
-
Acid whey
- SW:
-
Sweet whey
- OSN:
-
Organic solvent nanofiltration
- LPG:
-
Liquid petroleum gas
- MWCO:
-
Molecular weight cut-off
- TMP:
-
Trans-membrane pressure
- PL:
-
Phospholipids
- PPs:
-
Polyphenols
- PDMS-PTFPMS:
-
Trifluoropropylmethylsiloxane dimethylsiloxane
- PVDF:
-
Polyvinylidene fluoride
- FFAs:
-
Free fatty acids
- PLE:
-
Pressurized liquid extraction
- RBO:
-
Rice bran oil
- TSS:
-
Total soluble solids
- HFM:
-
Hollow fibre membrane
- FSM:
-
Flat sheet membrane
- PA:
-
Polyamide
- TFC:
-
Thin-film composite
- OA:
-
Organic acids
References
Culler PL, Mcclellan SA (1976) A new approach to partial demineralization. 50th Ann Tech Conf of FS/AWWA/FPCA and FW and PCOA
Conlonw J, Click JD (1984) Surface water treatment with ultrafiltration. 58th Ann Tech Conf of FS/AWWA/FPCA and FW and PCOA
Karki S, Gohain MB, Yadav D, Ingole PG (2021) Nanocomposite and bio-nanocomposite polymeric materials/membranes development in energy and medical sector: a review. Int J Biol Macromol 193:2121–2139
Abdel-Fatah MA (2018) Nanofiltration systems and applications in wastewater treatment: review article. Ain Shams Eng J 9:3077–3092
Gohain MB, Pawar RR, Karki S, Hazarika A, Hazarika S, Ingole PG (2020) Development of thin film nanocomposite membrane incorporated with mesoporous synthetic hectorite and MSH@UiO-66-NH2 nanoparticles for efficient targeted feeds separation, and antibacterial performance. J Membr Sci 609:118212
Ingole PG, Jeon JD, Hazarika S, Lee HK (2021) Polymeric nanocomposite membranes for diverse applications, Handbook of Polymer Nanocomposites for Industrial Applications. Micro and Nano Technologies pp. 169–199
Alves YPC, Antunes FAF, da Silva SS, Forte MBS (2021) From by- to bioproducts: selection of a nanofiltration membrane for biotechnological xylitol purification and process optimization. Food Bioprod Process 125:79–90
Ouyang Z, Huang Z, Tang X, Xiong C, Tang M, Lu Y (2019) A dually charged nanofiltration membrane by pH-responsive polydopamine for pharmaceuticals and personal care products removal. Sep Purif Technol 211:90–97
Kotsanopoulos KV, Arvanitoyannis IS (2015) Membrane processing technology in the food industry: food processing, wastewater treatment, and effects on physical, microbiological, organoleptic, and nutritional properties of foods. Crit Rev Food Sci Nutr 55:1147–1175
Nath K, Dave HK, Patel TM (2018) Revisiting the recent applications of nanofiltration in food processing industries: progress and prognosis. Trends Food Sci Technol 73:12–24
Chen GQ, Leong TSH, Kentish SE, Ashokkumar M, Martin GJO (2019) Membrane separations in the dairy industry, separation of functional molecules in food by membrane technology, pp. 267–304.
Salehi F (2014) Current and future applications for nanofiltration technology in the food processing. Food Bioprod Process 92:161–177
Conidi C, Castro-Muñoz R, Cassano A (2020) Membrane-based operations in the fruit juice processing industry: a review. Beverages 6:18
Salgado CM, Palacio L, Prádanos P, Hernández A, González-Huerta C, Pérez-Magariño S (2015) Comparative study of red grape must nanofiltration: laboratory and pilot plant scales. Food Bioprod Process 94:610–620
Salgado CM, Fernández-Fernández E, Palacio L, Hernández A, Prádanos P (2015) Alcohol reduction in red and white wines by nanofiltration of musts before fermentation. Food Bioprod Process 96:285–295
Yadav D, Hazarika S, Ingole PG (2021) Recent development in nanofiltration (NF) membranes and their diversified applications. Emergent Materials. https://doi.org/10.1007/s42247-021-00302-6
Cassano A, Conidi C, Ruby-Figueroa R, Castro-Muñoz R (2018) Nanofiltration and tight ultrafiltration membranes for the recovery of polyphenols from agro-food by-products. Int J Mol Sci 19:351
Tundis R, Ursino C, Bonesi M, Loizzo MR, Pellicanò VST, Manfredi IL, Figoli A, Cassano A (2019) Flower and leaf extracts of Sambucus nigra L.: application of membrane processes to obtain fractions with antioxidant and antityrosinase properties. Membranes 9, 127
Luo JQ, Wan YH (2013) Effects of Ph and salt on nanofiltration-a critical review. J Membr Sci 438:18–28
Choi O, Ingole PG, Park CH (2022) Precision-aiming tuning of membranes prepared by NIPS and its performance enhancement. J Clean Prod 365:132858
Mohammad AW, Teow YH, Chung WL, Oatley-Radcliffe DL, Hilal N (2015) Nanofiltration membranes review: recent advances and future prospects. Desalination 356:226–254
Zhang H, He Q, Luo J, Wan Y, Darling SB (2020) Sharpening nanofiltration: strategies for enhanced membrane selectivity. ACS Appl Mater Interfaces 12:39948–39966
Park HB, Kamcev J, Robeson LM, Elimelech M, Freeman BD (2017) Maximizing the right stuff: the trade-off between membrane permeability and selectivity. Science 356:1137
Shang WT, Sun FY, Jia W, Guo JX, Yin SM, Wong PW, An AK (2020) High-performance nanofiltration membrane structured with enhanced stripe nano-morphology. J Membr Sci 600:117852
Wu MY, Yuan JQ, Wu H, Su YL, Yang H, You XD, Zhang RN, He XY, Khan NA, Kasher R, Jiang ZY (2019) Ultrathin nanofiltration membrane with polydopamine-covalent organic framework interlayer for enhanced permeability and structural stability. J Membr Sci 576:131–141
Yadav D, Karki S, Ingole PG (2022) Current advances and opportunities in the development of nanofiltration (NF) membranes in the area of wastewater treatment, water desalination, biotechnological and pharmaceutical applications. J Environ Chem Eng 10:108109
Barba FJ, Soto ER, Marszałek K, Kovačević DB, Jambrak AR, Lorenzo JM, Chemat F, Putnik P (2019) Green food processing: concepts, strategies, and tools. Green Food Processing Techniques pp. 1–21
Chemat F, Rombaut N, Meullemiestre A, Turk M, Perino S, Fabiano-Tixier AS, Abert-Vien M (2017) Review of green food processing techniques. Preservation, transformation and extraction. Innov Food Sci Emerg Technol 41:357–377
Conidi C, Muñoz RC, Cassano A (2020) Nanofiltration in beverage industry, Nanotechnology in the Beverage Industry, pp. 525–548
Pant K, Thakur M, Nanda V (2020) Applications of membrane technology in whey processing. application of membrane technology for food processing industries. 1st Edition, CRC Press, eBook ISBN: 9780429276408
Simonic M, Pintaric ZN (2021) Study of acid whey fouling after protein isolation using nanofiltration. Membranes 11:492–502
Esfandian F, Peyravi M, Ghoreyshi AA, Jahanshahi M, Rad AS (2019) Fabrication of TFC nanofiltration membranes via co-solvent assisted interfacial polymerization for lactose recovery. Arab J Chem 12:5325–5338
Kentish SE, Rice G (2015) Demineralization of dairy streams and dairy mineral recovery using nanofiltration. Membrane Processing for Dairy Ingredient separation pp. 112–138
Tavares T, Malcata FX (2016) In: Caballero, B., Finglas, P.M., Toldr´a (Eds.), Whey and whey powders, principles and applications of dialysis. Elsevier, Inc., Oxford, UK, pp. 493–497.
Alexandri M, Schneider R, Venus J (2018) Membrane technologies for lactic acid separation from fermentation broths derived from renewable resources. Membranes 8:94–106
Komesu A, Maciel MRW, Filho RM (2017) Separation and purification technologies for lactic acid – a brief review. BioResources 12:6885–6901
Sluková M, Hinková A, Henke S, Smrž F, Lukačíková M, Pour V, Bubník Z (2016) Cheese whey treated by membrane separation as a valuable ingredient for barley sourdough preparation. J Food Eng 172:38–47
Ganju S, Gogate PR (2017) A review on approaches for efficient recovery of whey proteins from dairy industry effluents. J Food Eng 215:84–96
Bansal N, Bhandari B (2016) Functional milk proteins: production and utilization—whey-based ingredients. In: McSweeney P, O’Mahony J (eds) Advanced Dairy Chemistry. Springer, New York, pp 67–98
Panghal A, Patidar R, Jaglan S, Chhikara N, Khatkar SK, Gat Y, Sindhu N (2018) Whey valorization: current options and future scenario – a critical review. Nutrition and Food Science 48:520–535
Chavan RS, Shraddha RC, Kumar A, Nalawade T (2015) Whey based beverage: its functionality, formulations, health benefits and applications. J Food Process Technol 6:495
Talebi S, Suarez F, Chen GQ, Chen X, Bathurst K, Kentish SE (2020) Pilot study on the removal of lactic acid and minerals from acid whey using membrane technology. ACS Sustain Chem Eng 8:2742–2752
Kaya N, Altıok E, Gökkaya DS, Kabay N, OTLEŞ, S. (2019) Demineralization of cheese whey by sequential nanofilteration (NF) and electrodialysis (ED) processes. Journal of Membrane Science and Research 5:250–255
Marx M, Sixt A, Hofsommer J, Wörthmann M, Kulozik U (2019) Manufacturing of demineralized whey concentrates with extended shelf life: impact of the degree of demineralization on functional and microbial quality criteria. Food Bioprod Process 114:1–11
Merkel A, Voropaeva D, Ondrušek M (2021) The impact of integrated nanofiltration and electrodialytic processes on the chemical composition of sweet and acid whey streams. J Food Eng 298:110500
Pires AF, Marnotes NG, Rubio OD, Garcia AC, Pereira CD (2021) Dairy by-products: a review on the valorization of whey and second cheese whey. Foods 10:1067–1090
Velpula S, Umapathy KS, Thyarla A, Srikanth K, Saraff S (2017) Dairy wastewater treatment by membrane systems - a review. Indian J Pure Appl Biosci 5:389–395
Penttilä PA, Vierros S, Utriainen K, Carl N, Rautkari L, Sammalkorpi M, Österberg M (2019) Phospholipid-based reverse micelle structures in vegetable oil modified by water content, free fatty acid, and temperature. Langmuir 35:8373–8382
Doshi K, Trivedi Y, Ray P, Singh PS (2018) Degumming of crude vegetable oil by membrane separation: probing structure-performance and stability of PVDF membranes. Sep Sci Technol 54:1–10
Subramanian R, Kumar GS, Kuppusamy C (2021) Membrane technology for vegetable oil processing-current status and future prospects. Compr Rev Food Sci Food Saf 20:5015–5042
Li X, Cai W, Wang T, Wu Z, Wang J, He X, Li J (2017) AF2400/PTFE composite membrane for hexane recovery during vegetable oil production. Sep Purif Technol 181:223–229
Shi GM, Farahani MHDA, Liu JY, Chung TS (2019) Separation of vegetable oil compounds and solvent recovery using commercial organic solvent nanofilteration membranes. J Membr Sci 588:117202–117212
Coutinho CDM, Chiu MC, Basso RC, Ribeiro APB, Gonçalves LAG, Viotto LA (2009) State of art of the application of membrane technology to vegetable oils: A review. Food Res Int 42:536–550
Lin KM, Ghazali NF (2021) Nanofiltration of binary palm oil/solvent mixture: experimental and modelling. Materials Today: Proceedings 39:1010–1014
Novello Z, Tres MV, Silva MF, Oliveira JV, Luccio MD (2015) Separation of soyabean oil from liquified n-butane and liquified petroleum gas by membrane separation process. Can J Chem Eng 93:96–101
Ismail DNFA, Ghazali NF (2018) Separation of fatty acid from palm oil using organic solvent nanofiltration. Malaysian Journal of Analytical Sciences 22:561–569
Orm RB, Citeau M, Comitis A, Savoire R, Schiavo CH, Paternault PS, Carré P, Leao JD, Joffre F (2020) Walnut oil deacidification by liquid-liquid extraction with ethanol in a single- and multistage crossflow process. Oilseeds and fats, crops and lipids 27:35–47
Li X, Chen B, Cai W, Wang T, Wu Z, Li J (2017) Highly stable PDMS-PTFPMS/PVDF OSN membranes for hexane recovery during vegetable oil production. RSC Adv 7:11381–11388
Hou ZG, Cao XM, Cao L, Ling GQ, Yu P, M., Yang, P.Z., Jiang, S.T. (2020) The removal of phospholipid from crude rapeseed oil by enzyme-membrane binding. J Food Eng 280:109910–109925
Sereewatthanawut I, Baptista IIR, Boam AT, Hodgson A, Livingston AG (2011) Nanofiltration process for the nutritional enrichment and refining of rice bran oil. J Food Eng 102:16–24
Bhattacharjee C, Saxena VK, Dutta S (2017) Fruit juice processing using membrane technology: a review. Innov Food Sci Emerg Technol 43:136–153
Cai M, Hou W, Lv Y, Sun P (2017) Behavior and rejection mechanisms of fruit juice phenolic compounds in model solution during nanofiltration. J Food Eng 195:97–104
Pruksasri S, Lanner B, Novalin S (2020) Nanofiltration as a potential process for the reduction of sugar in apple juices on an industrial scale. LWT Food Sci Technol 133:110118
Sotoft LF, Christensen KV, Andrésen R, Norddahl B (2012) Full scale plant with membrane based concentration of black currant juice on the basis of laboratory and pilot scale tests. Chem Eng Process 54:12–21
Jordão A, Vilela A, Cosme F (2015) From sugar of grape to alcohol of wine: sensorial impact of alcohol in wine. Beverages 1:292–310
Castaldo L, Narváez A, Izzo L, Graziani G, Gaspari A, Minno GD, Ritieni A (2019) Red wine consumption and cardiovascular health. Molecules 24:3626
Banvolgyi S, Bahçeci KS, Vatai G, Bekassy S, Molnar EB (2016) Partial dealcoholization of red wine by nanofiltration and its effect on anthocyanin and resveratrol levels. Food Sci Technol Int 22:677–687
Ivić I, Kopjar M, Jakobek L, Jukić V, Korbar S, Marić B, Mesić J, Pichler A (2021) Influence of processing parameters on phenolic compounds and color of Cabernet Sauvignon red wine concentrates obtained by reverse osmosis and nanofiltration. Processes 9:89–104
Luo J, Hang X, Zhai W, Qi B, Song W, Chen X, Wan Y (2016) Refining sugarcane juice by an integrated membrane process: filtration behavior of polymeric membrane at high temperature. J Membr Sci 509:105–115
Daglia M (2012) Polyphenols as antimicrobial agents. Curr Opin Biotechnol 23:174–181
Río IGD, Fernández J, Lombó F (2018) Plant nutraceuticals as antimicrobial agents in food preservation: terpenoids, polyphenols and thiols. Int J Antimicrob Agents 52:309–315
Rasouli H, Farazaei MH, Khodarahmi R (2017) Polyphenols and their benefits: a review. Int J Food Prop 20:1700–1741
Tsibranska I, Simeonov E (2020) On the potential of integrating extraction with nanofiltration for separating and concentrating polyphenols from plant materials. Bul Chem Commun 52:509–518
Balyan U, Sarkar B (2016) Integrated membrane process for purification and concentration of aqueous Syzygium cumini (L.) seed extract. Food Bioprod Process 98:29–43
Paun G, Neagu E, Tache A, Radu GL, Parvulescu V (2011) Application of the nanofiltration process for concentration of polyphenolic compounds from Geranium robertianum and Salvia officinalis extracts. Chem Biochem Eng Q 25:453–460
Pereira DTV, Marson GV, Barbero GF, Tarone AG, Cazarin CBB, Hubinger MD, Martínez J (2020) Concentration of bioactive compounds from grape marc using pressurized liquid extraction followed by integrated membrane processes. Sep Purif Technol 250:117206
Cissé M, Vaillant F, Pallet D, Dornier M (2011) Selecting ultrafiltration and nanofiltration membranes to concentrate anthocyanins from roselle extract (Hibiscus sabdariffa L.). Food Res Int 44:2607–2614
Das B, Sarkar S, Sarkar A, Bhattacharjee S, Bhattacharjee C (2016) Recovery of whey proteins and lactose from dairy waste: a step towards green waste management. Process Saf Environ Prot 101:27–33
Babenyshev S, Mamay D, Bratsikhin A, Borisenko A, Mamay A, Amanova S (2020) Concentration of cottage cheese whey permeate by nanofiltration. J Hyg Eng Des 33:243–248
Chandrapala J, Duke MC, Gray SR, Weeks M, Palmer M, Vasiljevic T (2016) Nanofiltration and nanodiafiltration of acid whey as a function of pH and temperature. Sep Purif Technol 160:18–27
Borujeni RT, Akbari A, Gharehbaii A, Lehi AY (2021) Extraction and preparation of dye powders from Reseda luteola L. using membrane processes and its dyeing properties. Environ Technol Innov 21, 101249
Cai M, Xie C, Zhong H, Tian B, Yang K (2021) Identification of anthocyanins and their fouling mechanisms during non-thermal nanofiltration of blueberry aqueous extracts. Membranes 11:200
Muñoz P, Pérez K, Cassano A, Ruby-Figueroa R (2021) Recovery of anthocyanins and monosaccharides from grape marc extract by nanofiltration membranes. Molecules 26:2003
Conidi C, Cassano A, Caiazzo F, Drioli E (2017) Separation and purification of phenolic compounds from pomegranate juice by ultrafiltration and nanofiltration membranes. J Food Eng 195:1–13
Filippou P, Mitrouli ST, Vareltzis P (2022) Sequential membrane filtration to recover polyphenols and organic acids from red wine lees: the antioxidant properties of the spray-dried concentrate. Membranes 12:353
Acosta O, Vaillant F, Pérez AM, Dornier M (2016) Concentration of polyphenolic compounds in blackberry (Rubus Adeno trichos Schltdl.) Juice by Nanofiltration. J Food Process Eng 40, e12343
Tundis R, Loizzo MR, Bonesi M, Sicari V, Ursino C, Manfredi I, Cassano A (2018) Concentration of bioactive compounds from elderberry (Sambucus nigra L.) juice by nanofiltration membranes. Plant Foods Hum Nutr 73:336–343
Maan AMC, Hofman AH, Vos WM, Kamperman M (2020) Recent developments and practical feasibility of polymer-based antifouling coatings. Adv Func Mater 30:2000936
Dong Z, Lu J, Wu Y, Meng M, Yu C, Sun C, Muning C, Da Z, Yan Y (2020) Antifouling molecularly imprinted membranes for pretreatment of milk samples: selective separation and detection of lincomycin. Food Chem 333:127477
Li Y, Su Y, Zhao X, He X, Zhang R, Zhao J, Fan X, Jiang Z (2014) Antifouling, high-flux nanofiltration membranes enabled by dual functional polydopamine. ACS Appl Mater Interfaces 6:5548–5557
Ang MBMY, Pereira JM, Trilles CA, Aquino RR, Huang SH, Lee KR, Lai JY (2019) Performance and antifouling behavior of thin-film nanocomposite nanofiltration membranes with embedded silica spheres. Sep Purif Technol 210:521–529
Koulivand H, Shahbazi A, Vatanpour V, Rahmandoust M (2020) Development of carbon dot-modified polyethersulfone membranes for enhancement of nanofiltration, permeation and antifouling performance. Sep Purif Technol 230:115895
Liu Y, Yu Z, Peng Y, Shao L, Li X, Zeng H (2020) A novel photocatalytic self-cleaning TiO2 nanorods inserted graphene oxide-based nanofiltration membrane. Chem Phys Lett 749:137424
Ren L, Chen J, Lu Q, Wang C, Han J, Huang K, Pan X, Wu H (2020) Construction of high selectivity and antifouling nanofiltration membrane via incorporating macrocyclic molecules into active layer. J Membr Sci 597:117641
Karki S, Ingole PG (2022) Development of polymer-based new high performance thin-film nanocomposite nanofiltration membranes by vapor phase interfacial polymerization for the removal of heavy metal ions. Chem Eng J 446:137303
Ma T, Su Y, Li Y, Zhang R, Liu Y, He M, Li Y, Dong N, Wu H, Jiang Z (2016) Fabrication of electro-neutral nanofiltration membranes at neutral Ph with antifouling surface via interfacial polymerization from a novel zwitterionic amine monomer. J Membr Sci 503:101–109
Li SL, Shan X, Zhao Y, Hu Y (2019) Fabrication of a novel nanofiltration membrane with enhanced performance via interfacial polymerization through the incorporation of a new zwitterionic diamine monomer. ACS Appl Mater Interfaces 11:42846–42855
Liu H, Liu G, Zhang M, Zhao H, Jiang Y, Gao J (2020) Rapid preparation of Tannic acid (TA) based zwitterionic nanofiltration membrane via a multiple layer-by-layer (mLBL) assembly strategy for enhanced antifouling performance. Sep Purif Technol 253:117519
Ruan H, Li B, Ji J, Sotto A, Van der Bruggen B, Shen J, Gao C (2018) Preparation and characterization of an amphiphilic polyamide nanofiltration membrane with improved antifouling properties by two-step surface modification method. RSC Adv 8:13353–13363
Acknowledgements
We are thankful to the Director, CSIR-NEIST, Jorhat for his support and keen interest in this work.
Funding
The Department of Science and Technology (DST), New Delhi, India, provided financial support under the DST Nano mission project DST/NM/NT/2018/143 (GPP-0357) and CSIR, New Delhi, India, under the in-house project OLP-2064. DY received fellowship fund from DST, New Delhi, India, the DST/INSPIRE fellowship award No. IF190678. SK received fellowship fund from DST, New Delhi, India, the DST/INSPIRE fellowship award No. IF190333.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yadav, D., Karki, S. & Ingole, P.G. Nanofiltration (NF) Membrane Processing in the Food Industry. Food Eng Rev 14, 579–595 (2022). https://doi.org/10.1007/s12393-022-09320-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12393-022-09320-4