Abstract
A comparative study on the effect of nonthermal processing using cold atmospheric plasma (CAP), ozonation (OZ), pulsed electromagnetic fields (PEMF) and high pressure (HP) on the quality and shelf life of fresh sea bream fillets was conducted. The aim was the selection of the optimum nonthermal processing technology for minimum effect on the fish quality and maximum shelf-life extension, based on pre-selected processing conditions for each technology. Air plasma was generated using a surface dielectric barrier discharge (SDBD) source (3 kV, 45 kHz) applied for 15 min at 25 °C. PEMF (80 J/pulsed energy, 12.5 mT, 3 Hz) and OZ (1.3 mM of aqueous ozone, flow rate 640 ppm) were also applied for 15 min at 25 °C. HP treatment at 300 MPa/5 min at 25 °C was studied. Processed and unprocessed fillets were stored at 4 °C for 22 days. The fish fillets were analysed for their microbial load, pH, texture, colour, lipid oxidation, and sensorial characteristics, during storage. The shelf life of the product was extended by 4 and 2.5 days after HP and CAP processing, respectively. HP was more effective on the microbial load reduction compared to the other processes; nevertheless, colour and texture alteration of the fish fillets was observed. CAP processing decreased the initial total microbial load by 1.03 logCFU/g, while also resulted to reduced intension of the fish odour. PEMF and OZ affected slightly the quality indices nevertheless found to be less effective on the microbial load reduction with only 1 day shelf-life extension of the fillets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fresh fish fillets are considered among the most nutritive food products consumed worldwide; however, they are highly perishable thus requiring proper handling and preservation to ensure long shelf life. Concerning the latter, in order to decrease the rate of fish quality deterioration, various techniques are applied such as the use of modified atmosphere packaging (MAP), vacuum packaging, hot or cold smoking, and marination. However, the use of such techniques leads either to a slight extension of shelf life or to significant effect on the sensorial characteristics of that kind of products, making them not comparable to the fresh ones.
Nonthermal processing technologies could potentially lead to microorganisms’ inactivation and shelf-life extension while maintaining the organoleptic characteristics of fish products. In the literature, high pressure (HP) is considered to be a process leading to microbiologically stable and quality-improved food products [15]. Processing conditions include pressures ranging from 100 to 1000 MPa, combined with mild temperatures (5–60 °C). The positive effect of HP on the microflora of several fish is cited in the literature. Rode and Hovda [29] presented a reduction of 3 and 5 logCFU/g in the total viable count (TVC) for cod and mackerel, respectively, after HP processing at 500 MPa, 2 min, 8–9 °C. Teixeira et al. [33] observed a reduction of 2 logCFU/g in the total viable count (TVC) of sea bass (Dicentrarchus labrax), after HP processing at 400 MPa, 30 min, 6 °C. Mengden et al. [22] also presented > 6 logCFU/g reduction in Listeria monocytogenes and Escherichia Coli of European catfish (Silurus glanis) after HP processing at 600 MPa, 5 min at room temperature. Tsironi et al. [35] reported a significant shelf-life extension for sea bream fillets; nevertheless, the intense treatment conditions used (600 MPa, 25oC for 5 min) led to significant colour and texture alteration (fillets were evaluated as being cooked).
Cold atmospheric plasma (CAP), frequently called the “fourth state of matter”, is a weakly ionised gas consisting of a mixture of neutral and charged particles, free radicals, excited species and photons. CAP is an emerging nonthermal processing technology that could also be used for fish decontamination without affecting the quality characteristics of the final product [2]. Air plasma effectively inactivates microorganisms mainly via chemical interaction with reactive oxygen-nitrogen species (RONS) produced in the gas phase [25]. The effect of the application of CAP technology on the quality characteristics and microorganism inactivation of food products has so far been investigated in meat [6], fruits [32], fruit juices [37], vegetables [10, 23] and grains [30]. The results mainly indicate microbial inactivation and significant or not quality changes depending on the product characteristics, the processing intensity and the CAP source used.
Ozonation is a commercially accepted technology that is applied for many aspects in the food industry. Ozone is widely used in industry for surface disinfection of meat, fruit and vegetable products [11, 14]. Numerous studies have been conducted to investigate the efficacy of ozone in microbial inactivation mainly in fruits and vegetables with promising results [1, 19].
Pulsed electromagnetic fields (PEMF) technology is mainly studied for its use for human therapeutic purposes. Although this technology does not appear to have been studied for its effect on food products, PEMF is considered to be a promising technology for microbial inactivation as well [31, 34].
The aim of the research presented herein was to investigate and compare the effect of novel processing technologies such as high pressure, cold atmospheric plasma, pulsed electromagnetic fields and ozonation on fresh fish fillets in order to extend their shelf life without affecting their quality characteristics. This is a comparison between all these technologies, based on the available equipment for each technology. CAP and PEMF are not commercially available, so there is significant space for their equipment improvement. However, a methodology to establish a holistic approach for comparing such diverse technologies is established, which can be repeated in the future after extensive optimisation of each technology for the particular or similar food matrixes.
Materials and Methods
Sample Description
A number of 70 sea bream fillets (Sparus aurata) were supplied by SELONDA Aquaculture SA (leader in Mediterranean sea bream production). After 24-h storage at a 0 °C chamber, a number of 56 samples were shared and processed using four different nonthermal technologies. A number of 14 fish fillets remained unprocessed (control) for comparative evaluation.
Nonthermal Processing
Sea bream fillets were processed under a wide range of conditions in order to select the reference processing conditions for each nonthermal technology (preliminary experiments—data not shown). The selection of the reference conditions was based on microbiological and sensory analyses taking into account the minimum effect on the fish quality and maximum effect on their initial spoilage microbial load.
High Pressure
A number of 14 sea bream fillets were vacuum packed in sterile HDPE pouches for HP processing at 300 MPa for 5 min at 25 °C. The process conditions were selected by preliminary experiments among the range of pressure 100–600 MPa, at 25 °C for 1–10-min processing time. HP processing was carried out in a laboratory-scale HP system with a maximum operating pressure of 1000 MPa (Food Pressure Unit FPU 1.01, Resato International BV, Roden, Holland). A number of 3 sea bream fillets were placed each time into the HP chamber for processing. The system pressure transfer medium was polyglycol (ISO viscosity class VG 15, Resato International BV, Roden, Holland). During processing the rate of compression was about 100 MPa/7 s, while the rate of decompression was less than 3 s. During high-pressure processing of the fish fillets, the adiabatic heating phenomenon was taken into consideration. The temperature of the samples when processed was 18 °C and was increased to 24–26 °C during processing, while temperature immediately returned to the initial value after pressure released.
Cold Plasma in Atmospheric Pressure
CAP was performed in a closed rectangular glass reactor chamber with a surface dielectric barrier discharge (SDBD) source adjusted on the plastic lid at the upper side. Plasma was excited in air and in batch conditions using the atmospheric air trapped inside the enclosure. The plasma device, able for in-storage food decontamination, is described in detail by Dimitrakellis et al. [9]. A number of 14 unpacked fish fillets were placed individually in the chamber at a distance of 4 cm from the surface of the SDBD source. The SDBD device operated using sinusoidal high voltage at around 3-kV amplitude and 45-kHz frequency for processing time 15 min (process durations from 1 to 30 min were studied to select the most efficient and less destructive time for the fillets) at 25 °C.
Pulsed Electromagnetic Fields
A number of 14 sea bream fillets were packed in sterile HDPE pouches for PEMF processing at 80 J/pulsed energy, amplitude 12.5 mT and repetitive frequency of 3 Hz for 15 min (process durations from 1 to 30 min were studied to select the most efficient and less destructive time for the fillets) at 25 °C. The pulsed magnetic field generator Papimi was used. Papimi is a class IIα device type BF with maximum operating conditions of 240 V input voltage, 2500 VA power, energy per pulse 96 J and 300 MHz frequency. The device has a mechanical arm holder (optional) for the application coil (probe) and two types of application coils (probes).
Ozonation
Ozonation was performed using an appropriate ozone generator creating a level of 1.3 mM of aqueous ozone at a flow rate 640 ppm. A number of 6 sea bream fillets were placed each time into the ozone chamber. The processing time and temperature conditions were set to 15 min (process durations from 1 to 30 min were studied to select the most efficient and less destructive time for the fillets) and 25 °C, respectively. A number of 14 fish fillets were used for the experiments.
Shelf-Life Study
For each one of the nonthermal technologies studied, including control samples, a number of 14 sea bream fillets were stored in a dark cooling incubator at 4 °C for up to 22 days. Fish fillets were packed in open-air HDPE pouches. At storage times 0, 1, 4, 7, 10 and 15 days, the fillets were analysed for their microbial load, pH, lipid oxidation, texture, colour and sensory characteristics (odour, texture, colour, overall impression), while at day 22 only microbial analysis was conducted. The shelf life of the untreated (control) and nonthermally treated fish fillets was estimated based on Eq. 2.1.
where SL is the estimated shelf life, N(max) is the maximum value of the main deterioration factor for non-acceptability limit, N(0) is the initial value of the main deterioration factor and k the response constant rate.
Microbiological Analysis
The fish fillets were analysed for the existence of lactic acid bacteria (LAB), Pseudomonas spp., Enterobacteriaceae and total viable bacteria (TVC). The surface plating technique (ISO 4833-2:[13]) was used for the growth of TVC and Pseudomonas spp. while pour plate method (ISO 4833-1:[12]) was used for the growth of LAB and Enterobacteriaceae. TVC and Pseudomonas spp. were grown on plate count agar (Biokar Diagnostics, Beauvais, France) and Pseudomonas agar base (Lab M), respectively. The columns of the TVC and Pseudomonas spp. were enumerated after incubation at 25 °C for 72 h. De Man-Rogosa-Sharpe Agar (MRS Agar (ISO), Lab M) and incubation at 30 °C for 96 h were used for LAB growth. Enterobacteriaceae were enumerated after incubation at 37 °C for 24 h on violet red bile glucose agar (VRBG) (Biokar Diagnostics, Beauvais, France). Three replicates of at least three appropriate dilutions were enumerated.
Analysis of Physicochemical Characteristics
The pH value of the fish fillets was measured by means of a combined glass electrode in a dispersion of minced flesh in distilled water (1:1 ratio) using an ORION ion analyser model EA 940 (ORION-scientific, Limena (PD), Italy).
The colour of the samples was measured using colorimeter Minolta CR-300 (Minolta Company, Chuo-Ku, Osaka, Japan) with illuminant D65 and 10o standard observer, and expressed through CIEL*a*b* colour scale where L*-value is the lightness and represents the change from black (0) to white (100), a*-value the change from green (− 60) to red (+ 60) and b*-value the change from blue (− 60) to yellow (+ 60). The calibration of the colorimeter was performed using a standard white plate (L* = 21, a* = (− 0.05), b* = 3.21). The colour change of the fish fillets during storage was described through Eq. 2.2.
where Lo*, ao* and bo* are the L*, a* and b* values at time zero, respectively.
The lipid oxidation of the fish fillets was evaluated spectrophotometrically according to Mendes et al. [21] method, through the determination of the formed thiobarbituric acid reactive substances (TBARs). TEP (1,1,3,3-tetraethoxypropane) was used as the malonaldehyde (MDA) standard. A solution of TEP in 7.5% TCA at concentrations 2.0 to 10.0 μM was used in order to prepare a standard curve for the determination of TBAR concentration, which was expressed as mg malonaldehyde (MDA) per kg. The absorbance was measured using a digital UV-Vis spectrophotometer (Varian Cary®50 UV-Vis Spectrophotometer, Agilent) at 530 nm.
Texture Analysis
Texture analysis was performed using a ΤΑ.HD Plus texture analyser (Stable Micro Systems Ltd., UK). A flat ended cylinder with diameter 20 mm (type P/20) was used in order to simulate the human finger. The speed of the probe was defined at 1 mm/s during the test and 2 mm/s for pre- and post-test throughout the analysis, and the penetration depth was set at 7.00 mm.
All measurements were carried out at temperature 25 ± 1 °C, and the hardness of the fish fillets was determined. For statistical analysis, 10 replicates in each fillet took place.
Sensory Analysis
A panel of at least ten members of laboratory personnel familiar to fish products were semi-trained prior the experiments to distinguish deterioration of the fillets, evaluated the nonthermal-processed fish fillets at all sampling points. All the samples were compared with untreated ones coded as control. Only raw fish fillets were scored for their colour, appearance, texture (hardness), odour and overall impression by means of a 1 to 9 hedonic scale. The colour, hardness (cutting using knife) and odour of the fish fillets were scored in terms of intensity (score 1—low intensity; score 9—high intensity) while the appearance and overall impression of the fillets were scored in terms of likeliness (score 1—low likeliness; score 9—high likeliness). In the case of the latter, the score of 5 corresponded to the characterisation “neither like nor dislike” and was considered the limit for product acceptability.
Statistical Analysis
At each sampling point, all quality parameters were measured in two different untreated or treated with each studied technology samples. At least two replicates were performed for each sample and sampling measurement according to the parameter to be measured. The deviation among the measurements was expressed through standard deviation (average ± stdev). In the case of kinetic modelling of quality parameters, the deviation among the calculated values of the constant rates (k) was expressed through standard error (s. error). The 95% confidence upper and lower values were also estimated via a regression statistical routine. To assess the significance of the impact of the different nonthermal technologies on fish fillets at time zero and also during storage at 4 °C, all the received data were analysed statistically by one-way ANOVA using least significant difference (LSD) at p < 0.05 to evaluate differences between samples. Significant differences among data were assessed by Duncan’s multiple range test.
Results and Discussion
The Effect of Nonthermal Processing on Sea Bream Fillet Quality
Microbiological Analysis
Microbial load of control samples at time zero was estimated for TVC, Pseudomonas spp., LAB and Enterobacteriaceae as 5.57 ± 0.07, 5.11 ± 0.06, 1.65 ± 0.07 and 4.23 ± 0.07 logCFU/g, respectively. Based on the results, nonthermal processing of fish fillets led to microbial inactivation depending on the applied processing technology (Table 1).
Based on the results and statistical analysis, Enterobacteriaceae seemed to be the most sensitive microorganism after PEMF treatment, while for HP and OZ treatments the highest and similar (with no statistical differences, p > 0.05) sensitivity was observed for Enterobacteriaceae and Pseudomonas spp. CAP technology seemed to be more effective on Pseudomonas sp. inactivation (Table 1). CAP technology seemed to be more effective on Pseudomonas sp. inactivation (Table 1). The highest reduction of TVC was observed for HP-treated samples by 3.57 ± 0.10 logCFU/g, followed by CΑP-treated ones by 1.03 ± 0.04 logCFU/g, compared with control samples. TVC was decreased by 0.49 ± 0.06 logCFU/g and 0.42 ± 0.05 logCFU/g for OZ- and PEMF-treated fish fillets, respectively (no statistically significant differences were observed between the two processes, p > 0.05) (Table 1).
Ortega Blázquez et al. [28] estimated a reduction of 2.36 log cycles for TVC in HP-treated at 300 MPa/5 min sea bream fillets. Albertos et al. [2], after CAP processing of fresh mackerel fillets (DBD source, 70-80 kV, 1–5 min), reported no significant changes in TVC load in contrast to the Pseudomonas spp. and LAB loads which were decreased significantly. On the other hand, Chen et al. [8] reported significant TVC reduction from 5.02 ± 0.48 to 2.64 ± 0.16 logCFU/g for the fish flesh, after DBD processing (60 kV, 45 s) of chub mackerel. Based on the literature, during DBD processing, reactive oxygen species (ROS) are generated. Especially for ozone formation generated in large quantities and for which a long relative half-life has been reported [36], its reaction in combination with other species can cause bacteria inactivation [18]. Zhao et al. [40] also reported a decrease of TVC from 4.67 ± 0.07 to 4.35 ± 0.04 log CFU/g after soaking of Nile tilapia fillets in flow-ozonated water (4.0 mg/L) for 30 min.
Physicochemical Characteristics and Texture Analysis
Based on the data obtained from colour analysis, the nonthermal technologies applied seemed to have ambiguous effect on brightness (L*-value), redness (a*-value) and yellowness (b*-value) of the flesh of the sea bream fillets (Table 2). CAP, OZ and PEMF processing led to a slightly increased brightness (L*-value) of the fish flesh as instrumentally measured.
Based on one-way ANOVA statistical analysis no significant differences were observed on the brightness (L*-value) of the OZ- and CAP-processed sea bream fillets, as well as on the colour parameters a* and b* of the control and CAP-processed fillets.
PEMF-processed fillets L*-values were found to be slightly lower compared with control, and CAP- and OZ-processed samples. Although HP processing conditions applied (300 MPa/5 min) considered to be of low impact, L*- and b*-values were increased and a*-value was decreased (appearance as of a slightly cooked product) compared with control samples at day zero. Ortega Blázquez et al. [28], also observed a negative effect of increased pressures on the colour of the sea bream fillets, selecting conditions of 300 MPa/5 min as the optimum for microbial reduction while having lowest impact on the natural colour of the fillets.
The pH value of the HP samples increased slightly from 6.33 ± 0.02 to 6.44 ± 0.01 after processing in contrast to the other technologies which led to decreased pH values as 6.35 ± 0.02, 6.28 ± 0.03 and 6.22 ± 0.02 for the PEMF-, CAP- and OZ-processed fish fillets (Table 2), respectively. In the case of HP, the increase of pH value of the fish flesh after HP processing could be attributed to the denaturation of some protein fractions which cause a decrease in available acidic groups [3]. In contrast, several researchers have reported a decrease in pH value after DBD treatment [2, 18, 36] of different food products which could be attributed to H+ dissociation during treatment and the formation of nitric acid (HNO3) and nitrous acid (HNO2) [27].
Based on the results on the lipid oxidation through the determination of the formed thiobarbituric acid reactive substances (TBARs), CAP fillets showed the highest values of TBARs, indicating increased formation of secondary lipid oxidation products. OZ-treated samples followed CAP samples, while the lowest values without statistically significant differences (p > 0.05) appeared for HP and PEMF samples and control sea bream fillets (Table 2). Albertos et al. [2] reported no significant differences between control and in-pack CAP-processed (70, 80 KV/1, 3, 5 min) fresh mackerel. In contrary, Kim et al. [18] observed higher TBAR values after DBD CAP processing of pork loin.
Based on one-way ANOVA statistical analysis, no significant differences were observed in the hardness between the control and CAP-processed sea bream fillets (p > 0.05). PEMF-treated were slightly softer (reduction in hardness) compared with all the other samples (p < 0.05), potentially attributed either to destruction of fish muscle microstructure by disintegration of muscle fibres and the rupture of perimysial and epimysial connective tissue networks or by increased electroporation due to the magnetic fields resulting in bond rupture. In contrary, OZ-treated fillets found to be slightly harder compared with control, and CAP- and PEMF-treated ones (p < 0.05), while HP-processed fillets showed the highest values in hardness compared with all other samples (p < 0.05) (Table 2). Several researchers have observed an increase in hardness of fish flesh after HP processing, i.e. for 100 MPa/30 min processed bluefish [4], 300 MPa/15 min processed mahi mahi [38] and 400 MPa/20 min processed cod [3]. Zare [39] has attributed this phenomenon to increased protein-protein interactions and bond formation during HP processing of the fish muscles.
Sensory Analysis
In Fig. 1, the scores of the sensory panel for the hardness, odour and colour in terms of intensity and the overall acceptance of the unprocessed and nonthermal-processed raw sea bream fillets at time zero, are presented.
Sensory score for the intensity of colour, odour, hardness and overall acceptance of unprocessed (control) raw sea bream fillets and after processing using high pressure (HP), cold atmospheric plasma (CAP), ozonation (OZ) and pulsed electromagnetic fields (PEMF). Values with different superscripts (a, b, c, d) were significantly different as shown by Duncan's multiple range test. The different superscripts are referred to each one of the studied sensory parameters and evaluated among the different processing technologies
After CAP, ozonation and PEMF processing, all the sea bream fillets were sensory accepted and they were given the highest score on the hedonic scale. HP samples were scored lower due to colour alteration observed compared with the control samples (slight cooked appearance). A slight change in the colour of the OZ-processed fillets was observed by the panel while no differences were observed on the colour between control, and CAP- and PEMF-processed fillets. These findings were in accordance with the results obtained for chromatic parameters, as presented above. Similarly to our results, Kovačević et al. [20] and Xu et al. [37] observed a slight change in the total colour difference after CAP treatment of pomegranate and orange juice, respectively, which was not perceptible by the naked eye.
The hardness of the HP-processed fillets was increased compared with control samples with the ΟZ-processed ones to follow. No significant differences (p > 0.05) were observed on the hardness of the control and the CAP-processed sea bream fillets, while PEMF processing resulted in reduction of the hardness of the fillets, as perceived by the sensory panel, in accordance with the results obtained for hardness parameter evaluated instrumentally. Concerning the intensity of the odour of the fish fillets, CAP processing led to a significant decrease of the intensity of the fishy odour compared with control and all other technologies studied. This phenomenon which was evaluated positively by the sensory panel may be due to partial decomposition of trimethylamine which is mainly responsible for the fish odour. It has been demonstrated that plasmas are able to decompose volatile organic compounds (VOCs) such as aldehydes, fatty acids, alkanes, formic acid, amines, esters or thiols [7, 16, 17, 24, 26]. A slight decrease of the intensity of the fishy odour was also observed for the ΟZ-processed fillets.
The Effect of Nonthermal Processing on the Shelf Life of the Sea Bream Fillets
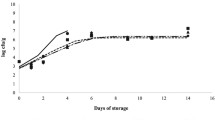
The effect of the applied processing technology and storage time on TVC, Pseudomonas spp., LAB and Enterobacteriaceae of the sea bream fillets during storage at 4 °C, is shown in Fig. 2a, b, c, and d.
The effect of storage time (days) at 4 °C on the (a) total viable count (TVC), (b) Pseudomonas spp., (c) Enterobacteriaceae and (d) lactic acid bacteria (LAB) of unprocessed (control) (  ) and nonthermal-processed sea bream fillets using high pressure (HP) (
) and nonthermal-processed sea bream fillets using high pressure (HP) (  ), cold atmospheric plasma (CAP) (
), cold atmospheric plasma (CAP) (  ), ozonation (
), ozonation (  ) and pulsed electromagnetic fields (PEMF) (
) and pulsed electromagnetic fields (PEMF) (  )
)
For the modelling of the microbial growth, the Baranyi growth model [5] was used. In Table 3, the estimated growth constant rates of TVC, Pseudomonas spp., Enterobacteriaceae and LAB of the unprocessed and nonthermal-processed sea bream fillets, are presented.
Based on the results, during storage of the sea bream fillets at 4 °C, the dominant microorganisms were Pseudomonas spp. For all the studied microorganisms, the HP-processed sea bream fillets had lower initial loads and also lower growth rates, resulting in slower quality degradation. The growth constant rates of TVC of the sea bream fillets seemed to be affected by the applied nonthermal technology and were decreased to 0.406, 0.381, 0.377 and 0.367 day−1 for PEMF-, OZ-, CAP- and HP-processed fillets, respectively, compared with 0.478 day−1 for control samples. In all the studied samples, LAB showed slight increase during storage at 4 °C, reaching in 10 days logCFU/g values equal to 3.86 ± 0.04, 1.92 ± 0.03, 3.31 ± 0.05, 3.63 ± 0.09 and 3.38 ± 0.02 for control, and HP-, CAP-, OZ- and PEMF-processed samples, respectively.
In Table 4, the effect of storage at 4 °C on the colour change (ΔΕ), hardness (N) and TBAR value (mg MDA/kg) of the unprocessed (control) and nonthermal-processed sea bream fillets, is presented. Storage at 4 °C appeared to have a significant effect on the colour (ΔE value) of the sea bream fillets for periods longer than 4 days (p < 0.05). Although HP-treated fillets exhibited the highest colour change after processing compared with the untreated ones, during storage they retained their colour more than the other fish fillets. The highest ΔΕ values were observed for control; nevertheless based on statistical analysis, no significant differences were observed in the colour change of the control, and CAP- and ΟZ-processed fillets throughout storage.
Concerning the effect of the storage on the hardness of the nonthermal treated and untreated fish fillets, a decrease was observed for all samples. No significant differences were observed in the reduction of hardness when storing CAP- and OZ-processed fish fillets. The highest decrease in hardness was observed for control and PEMF-treated fillets with no statistically significant differences.
Storage at 4 °C seemed to significantly affect TBAR value for storage times over 1 day for all the samples (p < 0.05). CAP-treated fillets showed the highest values of TBARs, followed by OZ-treated ones. No significant differences were observed on the increase of TBAR value for control and PEMF-processed fish fillets, while the lowest values appeared for HP-treated fillets. However, this indicator was not the dominant factor for the degradation of food quality.
Sensory scorings (odour, texture, colour, overall impression) were modelled with apparent zero order equations (R2 ranging from 0.9982 to 0.9993), considering as 9 the scoring at time zero. The constant rates of the overall impression scores during storage at 4 °C for the control, and HP-, CAP-, OZ- and PEMF-processed fillets were estimated as 0.793 ± 0.012, 0.432 ± 0.024, 0.531 ± 0.016, 0.648 ± 0.021 and 0.667 ± 0.016, respectively.
A sensory score of 5 was taken as the average score for minimum acceptability of the sea bream fillets. For all the fish fillets, the time of sensory rejection coincided with TVC load of 7.5 logCFU/g. The shelf life of the sea bream fillets at 4 °C was determined by sensory evaluation as 5, 9, 7.5, 6 and 6 days for control, and HP-, CAP-, OZ- and PEMF-processed fish fillets, respectively (Fig. 3), without including the 1 day the fillets were stored at 0 °C, prior their processing.
Conclusions
A comparison of four nonthermal technologies for processing of fish fillets was conducted targeting reduction of their microbial loads without affecting their sensory characteristics. High-pressure (HP) processing appeared to be more effective in microbial inactivation after treatment (64.1%) and during storage at 4 °C. For HP samples, the lipid oxidation (TBAR value) rate was lower during storage, compared with all other samples. The colour and hardness of the HP-processed sea bream fillets were retained compared with all other samples during storage. However, an effect on colour and texture of the fish fillets was observed immediately after HP processing (appearance of slightly cooked product) compared with the other treatments. CAP processing reduced the microbial load to a satisfactory degree of 18.5%. A slight increase in the brightness of the fish fillets was instrumentally observed, while a decreased intense of the fish odour was sensory perceived.
The application of ozonation and PEMF appeared to be less effective in reducing the microbial load, compared with HP and CAP processes. PEMF processing led to a slight effect on the instrumentally measured colour of the fillets which was not perceived during sensory evaluation and to a reduced hardness of the fish flesh after processing and during storage compared with all the other samples.
Sea bream fillets’ shelf life was determined based on a sensory acceptance score of 5 which coincided with a TVC load of 7.5 logCFU/g. Treatment of sea bream fillets with nonthermal technologies resulted in shelf-life extension by 4, 2.5, 1 and 1 day after treatment with HP, CAP, OZ, and PEMF, respectively, for the selected process conditions. A more detailed optimisation will follow for each technology by a second detailed comparison in the future. However, the first trend advantages and disadvantages of each technology have been revealed and presented for further uptake by the community.
References
Achen M, Yousef AE (2001) Efficacy of ozone against Escherichia coli O157:H7 on apples. J Food Sci 66:1380–1384
Albertos I, Martín-Diana AB, Cullen PJ, Tiwari BK, Ojha SK, Bourke P, Álvarez C, Rico D (2017) Effects of dielectric barrier discharge (DBD) generated plasma on microbial reduction and quality parameters of fresh mackerel (Scomber scombrus) fillets. Innov Food Sci Emerg Technol 44:117–122
Angsupanich K, Ledward DA (1998) High pressure treatment effects on cod (Gadus morhua) muscle. Food Chem 63(1):39–50
Ashie INA, Simpson BK, Ramaswamy HS (1997) Changes in texture and microstructure of pressure-treated fish muscle tissue during chilled storage. J Muscle Foods 8(1):13–32
Baranyi J, Roberts TA (1994) A dynamic approach to predicting bacterial growth in food. Int J Food Microbiol 23:277–294
Bauer A, Ni Y, Bauer S, Paulsen P, Modic M, Walsh JL, Smulders FJM (2017) The effects of atmospheric pressure cold plasma treatment on microbiological, physical-chemical and sensory characteristics of vacuum packaged beef loin. Meat Sci 128:77–87
Chang JS (2008) Physics and chemistry of plasma pollution control technology. Plasma Sources Sci Technol 17:450–462
Chen J, Wang SZ, Chen JY, Chen DZ, Denga SG, Xu B (2019) Effect of cold plasma on maintaining the quality of chub mackerel (Scomber japonicus): biochemical and sensory attributes. J Sci Food Agric 99:39–46
Dimitrakellis P, Giannoglou M, Zeniou A, Katsaros G, Gogolides E (2020) Food container employing a cold atmospheric plasma source for prolonged preservation of plant and animal origin food. MethodsX Journal, under review, MEX-S-20-00728
Giannoglou M, Stergiou P, Dimitrakellis P, Gogolides E, Stoforos N, Katsaros G (2020) Effect of cold atmospheric plasma processing on quality and shelf life of ready-to-eat leafy salads. Innovative Food Science and Emerging Technologies, under review, IFSET-D-20-00103
Guzel-Seydim Z, Greene AK, Seydim AC (2004) Use of ozone in the food industry. Lebensm-Wiss Technol 37:453–460
ISO 4833-1:2013. Microbiology of the food chain — horizontal method for the enumeration of microorganisms-Part 1: Colony count at 30 degrees C by the pour plate technique. ICS: 07.100.30, Food Microbiol
ISO 4833-2:2013. Microbiology of the food chain — Horizontal method for the enumeration of microorganisms-Part 2: Colony count at 30 degrees C by the surface plating technique. ICS: 07.100.30, Food Microbiol
Karaca H, Velioglu YS (2007) Ozone applications in fruit and vegetable processing. Food Rev Int 23(1):91–106
Katsaros G, Alexandrakis Z, Taoukis P (2014) High pressure processing of foods: technology and applications. In: Varzakas T, Tzia C (eds) Handbook of Food Processing Vol.1: Food Preservation, Chapter 14. CRC Press, pp 443–468
Kim HH (2004) Nonthermal plasma processing for airpollution control: a historical review, current issues and future prospects. Plasma Process Polym 1:91–110
Kim HH, Ogata A, Futamura S (2007) Complete oxidation of volatile organic compounds (VOCs) using plasma-driven catalysis and oxygen plasma. Int J Plasma Environ Sci Technol 1:46–51
Kim H, Yong HI, Park S, Choe W, Jo C (2013) Effects of dielectric barrier discharge plasma on pathogen inactivation and the physicochemical and sensory characteristics of pork loin. Curr Appl Phys 13(7):1420–1425
Koseki S, Yoshida K, Isobe S, Itoh K (2001) Decontamination of lettuce using acidic electrolyzed water. J Food Prot 64:652–658
Kovačević DB, Putnik P, Dragović-Uzelac V, Pedisić S, Jambrak AR, Herceg Z (2016) Effects of cold atmospheric gas phase plasma on anthocyanins and color in pomegranate juice. Food Chem 190:317–323
Mendes R, Cardoso C, Pestana C (2009) Measurement of malondialdehyde in fish: A comparison study between HPLC methods and the traditional spectrophotometric test. Food Chem 112(4):1038–1045
Mengden R, Röhner A, Sudhaus N, Klein G (2015) High-pressure processing of mild smoked rainbow trout fillets (Oncorhynchus mykiss) and fresh European catfish fillets (Silurus glanis). Innovative Food Sci Emerg Technol 32:9–15
Min SC, Roh SH, Niemira BA, Boyd G, Sites JE, Uknalis J, Fan X (2017) In-package inhibition of E. coli O157:H7 on bulk Romaine lettuce using cold plasma. Food Microbiol 65:1–6
Mizuno A (2007) Industrial applications of atmospheric non-thermal plasma in environmental remediation. Plasma Phys Control Fusion 49:1–15
Moisan M, Barbeau J, Crevier M, Pelletier J, Phillip N, Saoudi B (2002) Plasma sterilization. Methods and mechanisms. Pure Appl Chem 74:349–358
Müller S, Zahn RJ (2007) Air pollution control by non-thermal plasma. Contrib Plasma Phys 47:520–529
Oehmigen K, Hähnel M, Brandenburg R, Wilke C, Weltmann KD, von Woedtke T (2010) The role of acidification for antimicrobial activity of atmospheric pressure plasma in liquids. Plasma Process Polym 7:250–257
Ortega Blázquez I, Grande Burgos MJ, Pérez-Pulido R, Gálvez A, Lucas R (2018) Treatment with high-hydrostatic pressure, activated film packaging with thymol plus enterocin AS48, and its combination modify the bacterial communities of refrigerated sea bream (Sparus aurata) fillets. Front Microbiol 9:314
Rode TM, Hovda MB (2016) High pressure processing extend the shelf life of fresh salmon, cod and mackerel. Food Control 70:242–248
Selcuk M, Oksuz L, Basaran P (2008) Decontamination of grains and legumes infected with Aspergillus spp. and Penicillum spp. by cold plasma treatment. Bioresour TechnolJ 99:5104–5109
Tadevosian A, Kalantarian V, Trchounian A (2006) The effects of electromagnetic radiation of extremely high frequency and low intensity on the growth rate of bacteria Escherichia coli and the role of medium pH. Biofizika 52(5):893–898
Tappi S, Gozzi G, Vannini L, Berardinelli A, Romani S, Ragni L, Rocculi P (2016) Cold plasma treatment for fresh-cut melon stabilization. Innov Food Sci Emerg Technol 33:225–233
Teixeira B, Fidalgo L, Mendes R, Costa G, Cordeiro C, Marques A, Saraiva J, Nunes M (2014) Effect of high pressure processing in the quality of sea bass (Dicentrarchus labrax) fillets: pressurization rate, pressure level and holding time. Innovative Food Sci Emerg Technol 22(0):31–39
Torgomyan H, Kalantaryan V, Trchounian Α (2011) Low intensity electromagnetic irradiation with 70.6 and 73 GHz frequencies affects Escherichia coli growth and changes water properties. Cell Biochem Biophys 60:275–281
Tsironi T, Maltezou I, Tsevdou M, Katsaros G, Taoukis PS (2015) High pressure cold pasteurization of gilthead sea bream fillets: Selection of process conditions and validation of shelf-life extension. Food Bioprocess Technol: Int J 8:681–690
Wang J, Zhuang H, Zhang J (2016) Inactivation of spoilage bacteria in package by dielectric barrier discharge atmospheric cold plasma-treatment time effects. Food Bioprocess Technol:1–5
Xu L, Garner AL, Tao B, Keener KM (2017) Microbial inactivation and quality changes in orange juice treated by high voltage atmospheric cold plasma. Food Bioprocess Technol 10:1778–1791
Yagiz Y, Kristinsson HG, Balaban MO, Marshall MR (2007) Effect of high pressure treatment on the quality of rainbow trout (Oncorhynchus mykiss) and Mahi Mahi (Coryphaena hippurus). J Food Sci 72(9):509–515
Zare Z (2004) High pressure processing of fresh tuna fish and its effects on shelf life. High pressure processing of fish. McGill University, Quebec 45-76.
Zhao Y, Yang S, Yang X, Li L, Hao S, Cen J, Wei Y, Li C, Zhang H (2019) Effects of ozonated water treatment on physico-chemical, microbiological and sensory characteristics changes of Nile tilapia (Oreochromis niloticus) fillets during storage in ice. Ozone Sci Eng. https://doi.org/10.1080/01919512.2019.1688133
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Giannoglou, M., Dimitrakellis, P., Efthimiadou, Α. et al. Comparative Study on the Effect of Cold Atmospheric Plasma, Ozonation, Pulsed Electromagnetic Fields and High-Pressure Technologies on Sea Bream Fillet Quality Indices and Shelf Life. Food Eng Rev 13, 175–184 (2021). https://doi.org/10.1007/s12393-020-09248-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12393-020-09248-7