Abstract
The objective of this study was to evaluate the effects of nitrogen and carbon source on cellulase activity of Aspergillus niger ITV 02. The results obtained showed that nitrogen had an influence on the increase in cellulase activity by A. niger ITV 02 during submerged fermentation. Optimal concentrations obtained from nitrogen sources, using a Box–Behnken design were 0.9 g/L urea, 2.4 g/L ammonium sulfate and 1.5 g/L yeast extract, obtaining endoglucanase and β-glucosidase specific activities of 24.63 U/mg and 72.66 U/mg, respectively, at 50 h of fermentation. Subsequently, the effect of three carbon sources was evaluated: carboxymethylcellulose, delignified sweet sorghum bagasse (DSSB) and sweet sorghum bagasse cellulose (SSBC). Maximum cellulase specific activity was obtained when using DSSB, increasing endoglucanase activity fivefold and β-glucosidase activity 1.25-fold (126.72 and 85 U/mg) respectively. SSBC hydrolysis using enzymatic extract produced 18 g/L reducing sugars, equivalent to a 25% residue conversion. These results showed that it is possible to obtain an extract with cellulase activity from A. niger ITV 02 to be used in SSBC hydrolysis using low-cost substrates such as DSSB, which would contribute to a reduction in second-generation ethanol process production costs and an increase in the availability of enzymes for this use.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose, a polysaccharide synthesized by plants, composing between 35 and 50% of their dry weight, is made up of d-glucose molecules linked by β-1,4 glycosidic bonds (Behera et al. 2017). However, its structure constitutes a physical and chemical barrier for enzymes to directly access the polymer and turn it into simple sugars, thus limiting its use. For cellulose hydrolysis, the concerted action of 3 types of cellulases is required: endoglucanases (EC 3.2.1.4); exoglucanases, including D-cellodextrinases (EC 3.2.1.74) and cellobiohydrolases (EC 3.2.1.91); and β-glucosidases (EC 3.2.1.21) (Salmon et al. 2014).
In nature, a low percentage of microorganisms can hydrolyze waste containing cellulose through cellulase production. The main cellulase-producing microorganisms, the filamentous fungi, belonging to the genera of Aspergillus, Fusarium, Humicola, Penicillium, Trichoderma and Neurospora stand out for their high production (Kuhad et al. 2011). Trichoderma reesei is the main cellulase producer; however, it has low β-glucosidase activity which leads to cellobiose accumulation, thus causing product inhibition during cellulase production and lignocellulosic biomass hydrolysis (El-Hadi et al. 2014). In contrast, Aspergillus sp. are effective in β-glucosidase production. Cellulase production and expression are affected by several factors, including the type of strain used, culture conditions and mainly substrate type (Lynd et al. 2002; Niranjane et al. 2007) since it has been reported that cellulase production is regulated and induced in the presence of polymers (Amore et al. 2013). When the fungus comes into contact with polymeric and complex substrates, it secretes hydrolytic enzymes to degrade these and uses them for growth and maintenance (Debeire et al. 2014).
In addition to carbon source, another essential nutrient is nitrogen (N2). Microorganisms have developed different mechanisms for the absorption and assimilation of nitrogen sources which allows them to use a wide range of organic and inorganic compounds (Geisseler et al. 2010). Therefore, in this study, the effects of nitrogen and carbon sources on cellulase production by A. niger ITV 02, an autochthonous strain isolated from sugarcane bagasse residues, were evaluated.
Materials and Methods
Microorganism
Aspergillus niger ITV 02 belonging to the Bioengineering Laboratory of the Veracruz Institute of Technology cell culture collection was isolated from sugarcane bagasse.
Culture Media
Conservation, Activation and Pre-inoculum
Aspergillus niger ITV 02 was stored at 4 °C in the culture medium containing 10 g/L yeast extract, 20 g/L casein peptone, 20 g/L glucose and 25 g/L agar. The strain was activated in this medium without agar, and pH was adjusted to 5.5 using 5% (w/v) H3PO4 or 5% (w/v) NaOH. The pre-inoculum (6 × 106 spores/mL) was prepared in an Erlenmeyer flask containing 100 mL activation medium and incubated at 250 rpm, 30 °C for 24 h.
Subsequently, an adaptation medium was used: 10 g/L yeast extract, 20 g/L casein peptone, 10 g/L glucose and 10 g/L carboxymethylcellulose (CMC).
Evaluation of the Effect of Nitrogen Source on Cellulase Production: Experimental Design and Statistical Analysis of Cellulase Production Using a Box–Behnken Design
Three nitrogen sources were selected to determine the quantitative effect on cellulase production through a Box–Behnken experimental design (Mason et al. 1989). The independent variables selected were yeast extract (X3) concentration, ammonium sulfate (X2) and urea (X1), while the response variable was the endoglucanase activity of the previously centrifuged and microfiltered enzymatic extracts. The range and levels of the experimental variables used are shown in Table 1. Fifteen experiments were performed in duplicate, including three central point repetitions (Table 2). Fermentations were carried out in 250 mL Erlenmeyer flasks with 100 mL culture medium consisting of 10 g/L CMC, 0.3 g/L KH2PO4·7H2O, 0.3 g/L CaCl2, 1 g/L peptone, 5 mg/L FeSO4·7H2O, 1.6 mg/L MnSO4.H2O, 1.4 mg/L ZnSO4·7H2O and 2 mg/L CoCl2, sterilized for 15 min at 121 °C. Concentrations of urea, yeast extract and ammonium sulfate were added according to the Box–Behnken design, and fermentation was carried out at 30 °C, 250 rpm, for 50 h. The resulting fermentation samples were centrifuged for 20 min at 4500 ×g, the supernatant collected and subsequently microfiltered with 0.20 μm nylon membranes (Merck Millipore, 0.20 µm GNWP04700). Statistical analysis was performed using NCSS, Version 7.0 software (NCSS, LLC, USA) to determine the optimal conditions of the nitrogen source for cellulase production.
Evaluation of the Effect of Carbon Source on Cellulase Production
Three carbon sources were used: CMC (technical grade; Meyer, 4000–8000 cps, CAS No. 9004-32-4), delignified sweet sorghum bagasse (DSSB) and sweet sorghum bagasse cellulose (SSBC), all at 10% (w/v). Sweet sorghum bagasse (SSB) was donated by the National Institute of Livestock Agricultural and Forestry Research (INIFAP) Cotaxtla Experimental Field Station. It was dried by sun exposure and pulverized in a hammer mill and ground. DSSB was obtained from SSB acid pretreated with H2O2 (4.5% w/v) in a 16:1 v/w liquid/solid ratio, for 45 h. SSBC was obtained from SSB pretreated with H2SO4 (1.375% w/v) for 36 min and a 4.9:1 v/w liquid/solid ratio, followed by delignification with H2O2 (4.5% w/v) in a 16:1 v/w liquid/solid ratio, for 45 h (Partida-Sedas et al. 2017). Characterization of humidity, cellulose, hemicellulose and lignin contents of SSB, DSSB and SSBC was performed according to AOCS (American Oil Chemists Society) methods and the acid detergent fiber method using the neutral detergent fiber method (ANKOM Technology Method, Model A200, Ankom Technology, Macedon, NY) (Van Soest et al. 1991).
Determination of Enzymatic Activity
Endoglucanase activity was measured by the liberation of reducing sugars in 1 mL reaction volume containing 1% CMC (w/v) and enzyme extract in a sodium acetate buffer pH 5, 0.05 M for 30 min at 50 °C. Reducing sugars formed were quantified by the DNS method (Miller 1959). One unit of enzyme activity was defined as the amount of enzyme that produces 1 μmol reducing sugars per minute under the evaluated conditions.
β-glucosidase activity was determined using 10 mM 4-nitrophenyl β-D-glucopyranoside (β-pNPG, Sigma CAS No. 2492-87-7) as substrate in 0.05 M sodium acetate buffer pH 5. The reaction mixture involved incubating the substrate with the enzymatic extract at 50 °C for 10 min; the reaction was stopped by adding 2 mL 0.2 M Na2CO3 to one mL of the reaction mixture, and absorbance was measured at 400 nm. One unit of β-glucosidase activity was defined as the μM p-nitrophenol released per milliliter of enzyme per minute under the conditions evaluated in the assay (Singhania et al. 2011). Extracellular protein concentration was determined by Bradford reagent (Sigma B6916) using bovine serum albumin as a standard.
Enzymatic Hydrolysis
Crude enzymatic extract, produced using DSSB as a carbon source, was concentrated with a 10 kDa polyethersulfone (PES) membrane, obtaining an endoglucanase activity of 6.11 U/mL and a β-glucosidase activity of 14.49 U/mL. The enzymatic extract concentrate obtained from A. niger ITV 02 was used to hydrolyze SSBC in a 1:1 extract/buffer (50 mM sodium acetate, pH 5) ratio, with a 1:10 solid/liquid ratio and a 100 g/L substrate (SSBC) concentration.
Results and Discussion
Optimization of Nitrogen Sources on Cellulase Activity by A. niger ITV 02
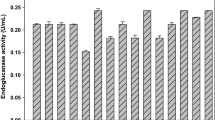
Optimization was carried out using a Box–Behnken design, evaluating different nitrogen sources and concentrations of nitrogen urea, ammonium sulfate and yeast extract, using endoglucanase activity as a variable response. The results obtained from the endoglucanase activity (U/mL) of each of the treatments are shown in Fig. 1. The endoglucanase activity varies in a range of 0.044 to 0.067 U/mL. The maximum value was obtained with the treatment 9 (0.9 g/L urea, 1.8 g/L ammonium sulfate and 1.0 g/L yeast extract). The optimization was carried out, and the results obtained showed that yeast extract and urea do not favor cellulase activity, that is, an increase in yeast extract and urea concentrations result in a lower cellulase activity, as observed in Fig. 2. However, a significant effect (P value < 0.05) was exhibited in the ammonium sulfate/yeast extract interaction (X2/X3), indicating a synergistic effect between these nitrogen sources (Table 3). The presence of additional sources of nitrogen improves growth and in turn enzyme production (Aggarwal et al. 2017) since nitrogen is one of the main protein elements. Stimulation of cellulase activity by ammonium salts may be due to its direct input into protein synthesis (Aggarwal et al. 2017). In a study conducted by Vyas et al. (2005), it was found that ammonium sulfate favored maximum endoglucanase (CMCase) production by A. terreus using pretreated peanut shells. Elsewhere, Sasi et al. (2012) found that A. flavus showed a higher cellulase production using ammonium sulfate as a nitrogen source compared to yeast extract.
On the contrary, Shahriarinour et al. (2011) evaluated the effect of nitrogen source (yeast extract, urea and ammonium sulfate) on A. terreus growth and cellulase production, reporting that the best source was yeast extract at a concentration of 6 g/L, attaining maximum filter paper activity (FPase), endoglucanase activity (CMCase) and β-glucosidase activities of 0.76 U/mL, 8.64 U/mL and 6.81 U/mL, respectively. These authors found that concentrations above 3 g/L urea and ammonium sulfate inhibit cellulase production. The evidence indicates that nitrogen sources induce differential expression of cellulolytic genes in different species of microorganisms (Vyas et al. 2005).
In a study conducted by Lemos et al. (2001) on A. awamori where the effect of ammonium sulfate and yeast extract on xylanase production was evaluated, yeast extract containing approximately 50% free amino acids (glutamate, glutamine, aspartate, asparagine, serine, threonine, arginine and lysine) was found to be preferentially used, as they are assimilated and directly incorporated into proteins. Growth and enzyme production could be favored. In this study, when concentrations less than 6 g/L yeast extract were used, cellulase production was favored; higher concentrations resulted in increased biomass (data not shown).
An analysis of variance (ANOVA) of endoglucanase activity showed a determination coefficient, R2 of 96% for Eq. (1), suggesting a good correlation between experimental and predicted values and indicating that only 4% of the total variation cannot be explained by the model. Figure 3 shows the residual plot for the model obtained. As can be observed, most standardized residuals were within normal limits and evenly distributed.
The model prediction that describes response surface graphs of endoglucanase activity (Y) as a function of yeast extract concentration (X3), ammonium sulfate (X2) and urea (X1) is shown in Eq. (1).
The statistical significance of each of the coefficients was evaluated by a Student´s t test, and the P value is shown in Table 3 at a confidence level of 95%.
Statistical analysis revealed that optimal concentrations of urea, ammonium sulfate and yeast extract were 0.9 g/L, 2.4 g/L and 1.5 g/L, respectively. Under these conditions, the predicted response for endoglucanase activity was 0.06 U/mL; when validating conditions, cellulase activity of 0.07 was obtained, corresponding to specific activities of 24.63 U/mg endoglucanase and 72.66 U/mg β-glucosidase.
Effect of Carbon Source on A. niger ITV 02 Cellulase Activity
After obtaining optimal concentrations of nitrogen sources, two complex carbon sources pretreated (DSSB and SSBC) and CMC were evaluated for cellulase production in submerged culture. Before pretreatment, SSB contained 29.8, 39.42 and 6.33% cellulose, hemicellulose and lignin, respectively. Pretreatment partially removed hemicellulose and lignin (Table 4), producing DSSB with 3.77% and SSBC with 5.47% lignin, thus increasing cellulose content from 29.84% (SSB) to 63.8% in DSSB and to 73.87% in SSBC. In the case of complex substrates, lignin represents an obstacle to biotechnological application, so the purpose of pretreatments is to remove part of the lignin and hemicellulose, reduce crystallinity and improve porosity (Maeda et al. 2011). Lignin elimination leads to a more porous and accessible material for microbial attack, which helps cellulase production (Adsul et al. 2004).
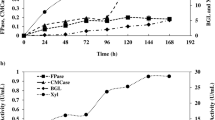
During submerged fermentation, cellulase activity was quantified (Fig. 4); this remained constant after 10 h with all three substrates (DSSB, SSBC y CMC). However, β-glucosidase activity exhibited an increase from 20 h, indicating endoglucanase activity 10 h prior to β-glucosidase activity. Sohail et al. (Sohail et al. 2009) evaluated A. niger MS82 cellulase activity reporting that endoglucanase activity started during lag phase and increased at the end of the experiment, a behavior similar to that experienced in this study. At 50 h of submerged fermentation, maximum endoglucanase activity of 0.085, 0.46 and 0.61 U/mL and β-glucosidase of 0.25, 0.35 and 0.41 U/mL were observed using CMC, DSSB and SSBC, respectively.
Several reports have determined cellulase activity in the Aspergillus species using CMC as a substrate, where activities of 0.061–0.096 U/mL were found after 96 h, similar to those obtained in this study (El-Hadi et al. 2014; Acharya et al. 2008), although here production time was reduced almost by half (50 h).
In addition, it was observed that, the most complex polymer, DSSB exhibited the greatest cellulase production, as this decreased as substrate complexity decreased (Table 5, Fig. 4). When DSSB was used as a carbon source, endoglucanase and β-glucosidase specific activities of 126.72 U/mg and 85 U/mg, respectively, were obtained at 50-h submerged fermentation, representing a 5.15-fold increase for endoglucanase activity with respect to CMC, while β-glucosidase activity was favored when using SSBC, followed by DSSB and CMC (Table 5).
Similar behavior was observed during A. niger Z5 submerged fermentation, where the use of rice husk complex substrate increased cellulase (CMCase and β-glucosidase) expression 1.6 and 2.4 times, compared to the use of simple substrates such as avicel and glucose, respectively (Liu et al. 2013). Penicillium sp LMI01, when using CMC as carbon source, yielded the highest endoglucanase (24.08 U/mg) and β-glucosidase (1345 U/mg) activities at 72-h submerged fermentation (Santa Rosa et al. 2018). It has been reported that cellulase biosynthesis is significant in complex substrates, repressed in the presence of carbon sources that are easily metabolized such as glucose, and induced by low molecular weight compounds, such as cellobiose. Lignocellulosic substrates such as agro-industrial residues have been described as suitable substrates for cellulase and xylanase production by filamentous fungi such as Aspergillus sp. (Amore et al. 2013). Elsewhere, it has been reported that in the case of A. niger, cellulase production is induced in the presence of xylan and leads to cellulose co-utilization; in the absence of xylan, this system could not be activated or only poorly so (Gutierrez-Rojas et al. 2015). Therefore, the best cellulase and β-glucosidase activities are observed in residue complexes with low lignin contents.
pH Behavior During Submerged Fermentation
During submerged fermentation, it was observed that endoglucanase and β-glucosidase production only started when pH was reduced to values close to 4, and at the end of the experiment the pH decreased to 3 using DSSB as the carbon source (Fig. 5). Xiong et al. (2004) reported a correlation between initial pH of the medium and cellulase production by T. reesei Rut C-30; high cellulase yields were produced when lactose was used as a carbon source and the pH of the medium was adjusted to 4–4.5. In the present, it was observed that when the pH decreases (from pH 4 to 3), cellulases were produced, as mentioned by Xiong et al. (2004). It has been reported that all β-glucosidase enzymes of Aspergillus strains have an optimum pH range of 4.5 to 5 (Jäger et al. 2001). McCleary and Harrington (1988) report that this enzyme is stable over a wide pH range of 2.5 to 9. The pH of A. glaucus endoglucanases (pH 4) is low with respect to pH 4.6 of T. reesei (Naika et al. 2007) and pH 6 of A. niger (Okada et al. 1985).
SSBC Hydrolysis Using A. niger ITV 02 Enzyme Extract
The enzymatic cocktail produced with DSSB as a carbon source, with 6.11 U/mL endoglucanase and 14.49 U/mL β-glucosidase activities, was evaluated adding, in a 1: 1 ratio, concentrated extract and 50 mM sodium acetate buffer (pH 5), with 100 g/L SSBC substrate, hydrolysis for 36 h at 50 °C, 250 rpm.
Figure 6 shows reducing sugar production with the enzymatic cocktail. The conversion percentage was 25.64%. Sukumaran et al. (2009) evaluated an enzymatic cocktail of Trichoderma reesei RUT C30 and A. niger MTCC 7956 in solid state using wheat bran as a carbon source, achieving 50 U FPase and 10 U β-glucosidase activities per gram of pretreated biomass. These authors report up to 17.79 g/L reducing sugars in enzymatic hydrolysis of DSSB, while in this work, using A. niger ITV 02 enzymatic cocktail, up to 18.94 g/L reducing sugars were obtained at 36-h enzymatic hydrolysis.
Conclusions
A moderate concentration of nitrogen sources (urea, ammonium sulfate and yeast extract) has a positive effect on enzymatic activity although an increase in yeast extract concentration decreases cellulase activity, favoring fungal growth. The carbon source has a determining effect on cellulase production, and the results showed that using complex sources as DSSB and optimal conditions of nitrogen sources increases endoglucanase activity fivefold with respect to CMC use. A. niger ITV 02 strain has the potential to be used in cellulase production since it expresses a set of enzymes with cellulase activity that can be used in lignocellulosic residue hydrolysis (25.64% conversion) to obtain fermentable sugars which can be used for second-generation bioethanol production.
References
Acharya, P.B., D.K. Acharya, and H.A. Modi. 2008. Optimization for cellulase production by Aspergillus niger using saw dust as substrate. African Journal of Biotechnology 7: 4147. https://doi.org/10.5897/AJB08.689.
Adsul, M.G., J.E. Ghule, R. Singh, H. Shaikh, K.B. Bastawde, D.V. Gokhale, and A.J. Varme. 2004. Polysaccharides from bagasse: Applications in cellulase and xylanase production. Carbohydrate Polymers 57 (1): 67–72.
Aggarwal, N.K., V. Goyal, A. Saini, A. Yadav, and R. Gupta. 2017. Enzymatic saccharification of pretreated rice straw by cellulases from Aspergillus niger BK01. 3 Biotech 7 (3): 158.
Amore, A., S. Giacobber, and V. Faraco. 2013. Regulation of cellulose and hemicellulose gene expression in fungi. Current Genomics 14 (4): 230–249.
Behera, B.C., B.K. Sethi, R.R. Mishra, S.K. Dutta, and H.N. Thatoi. 2017. Microbial cellulases–Diversity and biotechnology with reference to mangrove environment: A review. Journal of Genetic Engineering Biotechnology 15 (1): 197–210.
Debeire, P., F. Delalande, O. Habrylo, J.M. Jeltsch, A. Van Dorsselaer, and V. Phalip. 2014. Enzymatic cocktails produced by Fusarium graminearum under submerged fermentation using different lignocellulosic biomasses. FEMS Microbiology Letters 355 (2): 116–123.
El-Hadi, A.A., S.A. El-Nour, A. Hammad, Z. Kamel, and M. Anwar. 2014. Optimization of cultural and nutritional conditions for carboxymethylcellulase production by Aspergillus hortai. Journal of Radiation Research and Applied Sciences 1: 23–28.
Geisseler, D., W.R. Horwath, R.G. Joergensen, and B. Ludwing. 2010. Pathways of nitrogen utilization by soil microorganism—A review. Soil Biology Biochemistry 42 (12): 2058–2067.
Gutiérrez-Rojas, I., N. Moreno-Sarmiento, and D. Montoya. 2015. Mecanismos y regulación de la hidrólisis enzimática de celulosa en hongos filamentosos: casos clásicos y nuevos modelos. Revista Iberoamericana de Micología 32 (1): 1–12.
Jäger, S., A. Brumbauer, E. Fehér, K. Réczey, and L. Kiss. 2001. Production and characterization of β-glucosidases from different Aspergillus strains. World Journal of Microbiology & Biotechnology 17 (5): 455–461.
Kuhad, R.C., R. Gupta, and A. Singh. 2011. Microbial cellulases and their industrial applications. Enzyme Research . Article ID 280696. https://doi.org/10.4061/2011/280696.
Lemos, J.L., C.D.A Maria, and N. Pereira. 2001. Xylanase production by Aspergillus awamori in solid-state fermentation and influence of different nitrogen sources. In Twenty-second symposium on biotechnology for fuels and chemicals, 681–689. Totowa, NJ: Humana Press. https://doi.org/10.1007/978-1-4612-0217-2_57.
Liu, D., J. Li, S. Zhao, R. Zhang, M. Wang, Y. Miao, and Q. Shen. 2013. Secretome diversity and quantitative analysis of cellulolytic Aspergillus fumigatus Z5 in the presence of different carbon sources. Biotechnology for Biofuels 6. Article No. 49.
Lynd, L.R., P.J. Weimer, W.H. Van Zyl, and I.S. Pretorius. 2002. Microbial cellulose utilization: Fundamentals and biotechnology. Microbiology and Molecular Biololgy Reviews 66 (3): 506–577.
Maeda, R.N., V.I. Serpa, V.A. Rocha, R.A. Mesquita, L.M. Santa Anna, A.M. De Castro, and I. Polikarpov. 2011. Enzymatic hydrolysis of pretreated sugar cane bagasse using Penicillium funiculosum and Trichoderma harzianum cellulases. Process Biochemistry 46 (5): 1196–1201.
Mason, R., R. Gunst, and J. Hess. 1989. Statistical design and analysis of experiments: with applications to engineering and science, 220–221. Hoboken: Wiley.
McCleary, B.V., and J. Harrington. 1988. Purification of β-d-glucosidase from Aspergillus niger. Methods in Enzymology 160: 575–583.
Miller, G.L. 1959. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry 31 (3): 426–428.
Naika, G.S., P. Kaul, and V. Prakash. 2007. Purification and characterization of a new endoglucanase from Aspergillus aculeatus. Journal Agricultural and Food Chemistry 55 (18): 7566–7572.
Niranjane, A.P., P. Madhou, and T.W. Stevenson. 2007. The effect of carbohydrate carbon sources on the production of cellulose by Phlebia gigantean. Enzyme and Microbial Technology 40 (6): 1464–1468.
Okada, G. 1985. Purification and properties of a cellulase from Aspergillus niger. Agricultural and Biological Chemistry 49 (5): 1257–1265.
Partida-Sedas, G., N. Montes-García, O. Carvajal-Zarrabal, L. López-Zamora, J. Gómez-Rodríguez, and M.G. Aguilar-Uscanga. 2017. Optimization of hydrolysis process to obtain fermentable sugars from sweet sorghum bagasse using a Box–Behnken design. Sugar Tech 19 (3): 317–325.
Salmon, D.N.X., M.R. Spier, C.R. Soccol, L.P. de Souza Vandenberghe, V.W. Montibeller, M.C.J. Bier, and V. Faraco. 2014. Analysis of inducers of xylanase and cellulase activities production by Ganoderma applanatum LPB MR-56. Fungal Biology 118 (8): 655–662.
Santa-Rosa, P.S., A.L. Souza, R.A. Roque, E.V. Andrade, S. Astolfi-Filho, A.J. Mota, and C.G. Nunes-Silva. 2018. Production of thermostable β-glucosidase and CMCase by Penicillium sp. LMI01 isolated from the Amazon region. Electronic Journal of Biotechnology 31: 84–92.
Sasi, A., M. Ravikumar, and S. ManthiriKani. 2012. Optimization, production and purification of cellulase enzyme from marine Aspergillus flavus. African Journal of Microbiology Research 6 (20): 4214–4218.
Shahriarinour, M., M.N. Wahab, R. Mohamad, S. Mustafa, and A. Ariff. 2011. Effect of medium composition and cultural condition on cellulose production by Aspergillus terreus. African Journal of Biotechnology 10 (38): 7459–7467.
Singhania, R.R., R.K. Sukumaran, K.P. Rajasree, A. Mathew, L. Gottumekkala, and A. Pandey. 2011. Properties of a major β-glucosidase-BGL1 from Aspergillus niger NII-08121 expressed differentially in response to carbon sources. Process Biochemistry 46 (7): 1521–1524.
Sohail, M., R. Siddiqi, A. Ahmad, and S.A. Khan. 2009. Cellulase production from Aspergillus niger MS82: effect of temperature and pH. New Biotechnology 25 (6): 437–441.
Sukumaran, R.K., R.R. Singhania, G.M. Mathew, and A. Pandey. 2009. Cellulase production using biomass feed stock and its application in lignocellulose saccharification for bio-ethanol production. Renewable Energy 34 (2): 421–424.
Van Soest, P.V., J.B. Robertson, and B.A. Lewis. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. Journal of Dairy Science 74 (10): 3583–3597.
Vyas, A., D. Vyas, and K.M. Vyas. 2005. Production and optimization of cellulases on pretreated groundnut shell by Aspergillus terreus AV49. Journal of Scientific & Industrial Research 64: 281–286.
Xiong, H., N. von Weymarn, M. Leisola, and O. Turunen. 2004. Influence of pH on the production of xylanases by Trichoderma reesei Rut C-30. Process Biochemistry 39 (6): 731–736.
Acknowledgements
The authors acknowledge the economic support from the Sectoral Fund for Research in Agricultural, Livestock, Aquaculture, Agrobiotechnology and Plant Genetic Resources and the National Council of Science and Technology, Mexico (SAGARPA-CONACyT, Project 291143) and the TecNM project 6853.18P, also the critical reading of Patricia Margaret Hayward-Jones, M Sc and Dulce María Barradas-Dermitz, M Sc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Infanzón-Rodríguez, M.I., Ragazzo-Sánchez, J.A., del Moral, S. et al. Optimization of Cellulase Production by Aspergillus niger ITV 02 from Sweet Sorghum Bagasse in Submerged Culture Using a Box–Behnken Design. Sugar Tech 22, 266–273 (2020). https://doi.org/10.1007/s12355-019-00765-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-019-00765-2