Abstract
Many plants accumulate betaine in response to water stress. The betaine aldehyde dehydrogenase (BADH) is the key enzyme in betaine biosynthesis in plants and can be regulated by BADH gene. A pot experiment was conducted to compare the relation of betaine content and BADH gene expression in two sugarbeet (Beta vulgaris L.) varieties differing in drought tolerance. We observed an evident correlation between the transcript up-regulation and the betaine accumulation under water stress. Results showed that the betaine content and BADH gene expression in the drought tolerant variety, was significantly increased under drought stress. The betaine accumulation and BADH gene expression might function as one response to eliminate the negative effects of drought and may play a critical role in maintaining photosynthetic activity in sugarbeet plants under water stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing severity of drought worldwide has become the major factor restricting the sustainable development of agricultural production. Under drought stress, betaine often accumulates to maintain and stabilize the complex structure of proteins and the highly organized structures of membrane lipids, which deters injury and enhances the resistance of leaves to stress (Cui et al. 2008). Betaine aldehyde dehydrogenase (BADH) is a key enzyme in betaine biosynthesis (Hanson et al. 1985). Sugarbeet is grown for its root which is used to produce sugar while stems and leaves can be used as feedstocks for bioenergy production. Besides other biotic stress, drought also is a major limiting factor in growing sugarbeet. To understand the role of BADH gene in drought resistance in sugarbeet, we analyzed the BADH gene expression, BADH activity, and glycine betaine accumulation in sugarbeet during water stress condition and recovery from water stress.
Materials and Methods
Plant and Water Stress Treatment
Two sugarbeet varieties were used in this study, a drought resistant variety (HI0466, provided by Syngenta from Swiss) and a drought susceptible variety (NDTY4, provided by Sugarbeet Physiological Institute, Inner Mongolia Agricultural University). Plastic pots (60 × 40 × 20 cm) were filled with 35 kg soil per pot amended with slow-release fertilizer. Field capacity and permanent wilting point of the soil were 21 and 7.5 %, and the pH of the soil was 6.8. The experiment was conducted in a growth chamber at 25 °C using a 14 h photoperiod. The treatments included no water stress (control), i.e., 6.5 L water was added per pot to maintain a 75–80 % water-saturated soil. Soil moisture was assessed by weighing the pots at 6:00 pm daily. Water stress (WS): beginning at the sixth leaf stage, pots were watered with 20 % PEG 6000 for 5 days then watered as usual (rewatered; RW) for 2 days. All leaves were collected on the 1st, 3rd, and 5th day of water stress and on the 1st and 2nd day after rewatering for betaine content and BADH activity measurement and RNA extraction.
RNA Extraction and RT-PCR
The total RNA was extracted using TIANGEN RNA plant plus reagent according to instructions (http://www.tiangen.com/en/). The extracted RNA was reverse transcribed into cDNA using oligo-(dT)18 primers and AMV reverse transcriptase (Takara Biotech Co., Dalian, China).
Gene-specific primers were designed using Primer 5.0 software (Table 1). The amplified products for BADH and Actin were 489 and 350 bp, respectively.
RT-PCR was conducted using RNA PCR Kit Ver.3.0 (Takara) according to the manufacturer’s instructions. PCR was performed with cycling conditions as follows: 1 cycle of 94 °C for 3 min, 30 cycles of 94 °C for 30 s, 53 °C for 30 s, and 72 °C for 90 s, and 1 cycle of 72 °C for 5 min.
Ten microliters PCR product was used for electrophoresis on 1 % agarose gel in Tris-acetate-EDTA (TAE) buffer. After ethidium bromide (EB) staining, a gel imaging system (BIO-RAD GelDoc XR and Quantity One 4.4 software) was utilized for visualizing products and estimating relative product quantities using densitometry.
Analysis of BADH Activity and Betaine Content
BADH activity was assayed using the method described by Bradford (1976) and Weretilnyk and Hanson (1989). Accumulation of betaine in response to water stress was deterined according to protocol of Grieve and Grattan (1983).
Results
Effect of Water Stress on Betaine Content
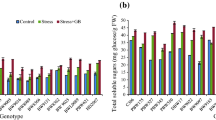
Betaine content in young leaves of sugarbeet seedlings treated with water stress during 1st, 3rd, and 5th day and 1st and 2nd day after rewatering is shown in Fig. 1. In the control treatment, the betaine content in leaves was low, estimating relative product quantities using densitometry, stable, and nearly similar in both the test varieties. However, the betaine content accumulation increased gradually with increasing number of days of water stress and then decreased during rewatering. At 5 days of treatment, the betaine accumulation was significantly stimulated up to the maximum level in both varieties, being approximately 3.4- and 5.5-fold to the control, respectively. At the same time, we also found that the increase in the betaine content was greater in the resistant variety as compared to the susceptible cultivar during water stress (Fig. 1).
BADH Expression in Sugarbeet Leaves Under Water Stress
In order to determine whether induction of betaine accumulation by water stress in young leaves of sugarbeet seedlings correlates with transcriptional regulation, BADH gene transcript levels were assessed by reverse transcriptase-polymerase chain reaction (RT-PCR), using the same samples as those described above for betaine analysis. As shown in Fig. 2, the BADH gene was gradually up-regulated with the greatest increase occurring under the most serious degree of water stress and then down-regulated quickly to normal levels after rewatering. At 5 days of water stress, both of the varieties exhibited the greatest quantity of transcript, although the increase of BADH gene expression was found higher in the resistant variety, HI0466. In addition, compared to the change of the betaine accumulation, we also found that there was a correlation between the transcript up-regulation and the betaine accumulation with the increase in water stress. These results indicated that the BADH gene expression may be induced by water stress and caused the betaine accumulation.
Effect of Water Stress on the BADH Activity
In order to study the betaine aldehyde dehydrogenase, BADH activity was analyzed and is shown in Fig. 3. In control treatments, the BADH activity was low and stable in both the test varieties. However, with water stress, the BADH activity increased quickly and reached its highest level at 3 days of water stress in both varieties. However, a reduction in BADH activity was observed at 5 days of stress. Moreover, BADH activity increased more quickly and decreased more slowly in the resistant variety, HI0466, during water stress. As a consequence, HI0466 maintains higher activity of BADH than the susceptible variety NDTY4.
Discussion
It has been shown that betaine could protect the peripheral polypeptides of PSII, 1,5-bisphosphate ribulose carboxylase/oxygenase, and membrane integrity to maintain relatively greater PSII photochemical activities and increase photosynthetic rates of plants under stress conditions (Sakamoto and Murata 2001; Demirevska-Kepova et al. 2005; Li et al. 2003). This study showed that the betaine content was similar in both varieties under control conditions. However, when subjected to water stress, betaine accumulatted to greater levels in the resistant variety HI0466. The increased betaine content in HI0466 might provide greater protective effects on PSII reaction centers in chloroplasts which may eventually maintain higher PSII photochemical activity (Li et al. 2013). These changes are in turn contribute to greater drought tolerance of the resistant variety HI0466 as compared to the susceptible variety NDTY4.
Changes in betaine response under water stress play an important role in determining the growth, yield, and quality of plants (Wang et al. 2010). The change in betaine content is linked to BADH gene expression (Luo et al. 2001). Changes in BADH expression in response to drought and salt stress were reported for the first time in spinach (Weretilnyk and Hanson 1989). Thereafter, the BADH gene was successively cloned and studied in many plants. Many researchers have reported that plants exposed to water stress show significant enhancement of BADH gene expression and accumulation of betaine in plants (Li et al. 2003; Yamada et al. 2009). Our results are consistent with the above findings. Our study also showed that with application of water stress, the BADH gene expression in the leaves of sugarbeet rapidly increased and reached a maximum level at 5 days of stress. At the same time, the expression in leaves was higher in the resistant variety HI0466 as compared to that in the susceptible variety NDTY4. The study also showed that the trend of betaine content changes are consistent with that of BADH gene expression. The findings indicates that BADH gene expression may be responsible for the increase in betaine accumulation and the improvement of drought resistance of sugarbeet plants.
The BADH gene encodes the protein which catalyzes the second two steps of betaine biosynthesis in plants (Pierre et al. 1986). Previous studies have shown that BADH activity in many plants increased with BADH gene expression in response to water stress (Valenzuela-Soto and Mun˜oz-Clares 1994; Legaria et al. 1998). However, our results showed that BADH gene expression was not parallel with the changes in BADH activity under the serious water stress. So we speculate that the phenomena could be explained by the increasing rate of protein degradation when subjected to serious water stress.
References
Bradford, M.M. 1976. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Analytical Biochemistry 72: 248–254.
Cui, X.Y., Y. Wang, and J.X. Guo. 2008. Osmotic regulation of betaine content in Leymus chinensis under saline-alkali stress and cloning and expression of betaine aldehyde dehydrogenase (BADH) gene. Chemical Research in Chinese Universities 24: 204–209.
Demirevska-Kepova, K., R. Holzer, L. Simova-Stoilova, and U. Feller. 2005. Heat stress effects on ribulose-1,5-bisphosphate carboxylase/oxygenase, rubisco binding protein and rubisco activase in wheat leaves. Biologia Plantarum 49: 521–525.
Grieve, C.M., and S.R. Grattan. 1983. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant and Soil 70: 303–307.
Hanson, A.D., A.M. May, R. Grumet, J. Bode, and G.C. Jamieson. 1985. Betaine synthesis in chenopods: Localization in chloroplasts. Proceedings of the National Academy of Sciences of the United States of America 82: 3678–3682.
Legaria, J., R. Rajsbaum, R.A. Mun˜oz-Clares, N. Villegas-Sepu´lveda, J. Simpson, and G. Iturriaga. 1998. Molecular characterization of two genes encoding betaine aldehyde dehydrogenase from amaranth. Expression in leaves under short-term exposure to osmotic stress or abscisic acid. Gene 218: 69–76.
Li, Y.H., W. Wang, Q.Q. Ma, and Q. Zou. 2003. The osmotic adjustment and photosynthesis of a wheat cultivar Hanfeng 9703 with high yield, drought resistance under drought stress. Acta Agronomica Sinica 29(5): 759–764.
Li, G.L., H.X. Wu, Y.Q. Sun, and S.Y. Zhang. 2013. Response of chlorophyll fluorescence parameters to drought stress in sugarbeet seedlings. Russian Journal of Plant Physiology 60: 337–342.
Luo, A.L., J.Y. Liu, D.Q. Ma, X.C. Wang, and Z. Liang. 2001. Relationship between drought resistance and betaine aldehyde dehydrogenase in the shoots of different genotypic wheat and sorghum. Acta Botanica Sinica 43(1): 108–110.
Pierre, W., E.A. Weretilnyk, and A.D. Hanson. 1986. Betaine aldehyde oxidation by spinach chloroplasts. Plant Physiology 82: 753–759.
Sakamoto, A., and N. Murata. 2001. The use of bacterial choline oxidase, a glycinebetaine synthesizing enzyme, to create stress resistant transgenic plants. Plant Physiology 125: 180–188.
Valenzuela-Soto, E.M., and R.A. Mun˜oz-Clares. 1994. Purification and properties of betaine aldehyde dehydrogenase extracted from detached leaves of Amaranthus hypochondriacus L. subjected to water deficit. Journal of Plant Physiology 143: 145–152.
Wang, G.P., F. Li, J. Zhang, M.R. Zhao, Z. Hui, and W. Wang. 2010. Overaccumulation of glycine betaine enhances tolerance of the photosynthetic apparatus to drought and heat stress in wheat. Photosynthetica 48: 30–41.
Weretilnyk, E.A., and A.D. Hanson. 1989. Betaine aldehyde dehydrogenase from spinach leaves-purification, invitro translation of the messenger-RNA, and regulation by salinity. Archives of Biochemistry and Biophysics 271: 56–63.
Yamada, N., W. Promden, and K. Yamane. 2009. Preferential accumulation of betaine uncoupled to choline monooxygenase in young leaves of sugarbeet importance of long-distance translocation of betaine under normal and salt-stressed conditions. Journal of Plant Physiology 166: 2058–2070.
Acknowledgments
This work was supported by National Natural Science Foundation of China (31360355), Inner Mongolia Natural Science Foundation (2012MS0305), and the China Agriculture Research System (CARS-210304).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, G., Wu, H., Sun, Y. et al. Betaine Aldehyde Dehydrogenase (BADH) Expression and Betaine Production in Sugarbeet Cultivars with Different Tolerances to Drought Stress. Sugar Tech 18, 420–423 (2016). https://doi.org/10.1007/s12355-015-0402-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-015-0402-1