Abstract
The fungal pathogen Fusarium sacchari causing wilt in sugarcane exhibits enormous variation in cultural characters. Detailed studies were conducted on recovery of the pathogen from wilt infected canes and to characterize the pathogen for its cultural characters. From 346 samples collected from 15 states, 263 Fusarium isolates were recovered and no other fungal genera were recovered. Overall, a higher pathogen recovery was recorded in nodal tissues as compared to internodal tissues of wilt infected stalk tissues. Cultural characterization of 117 isolates divided the Fusarium isolates into three groups based on radial growth as slow, moderate and fast. Based on mycelial colour, the isolates were grouped into seven groups viz., white, orange, pinkish orange, pink, dark pink, pinkish violet and reddish brown. The isolates were further divided into 21 groups based on mycelia colour on the top and agar pigmentation at the reverse side of the culture plate and three groups viz., submissive, raised and fluffy based on topology of the mycelium. Based on conidial frequency, the isolates were grouped as those produce microconidia with low, moderate, higher frequencies, micro and macroconidia at lower frequencies and micro and macroconidia production at higher frequencies. The results of this study revealed extensive variation in F. sacchari isolates recovered for their cultural characters from wilt infected stalks and sick soils.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In India, wilt is an important fungal disease which causes significant crop losses in sugarcane. Like red rot, the disease also affects sugarcane stalks which make them unfit for juice extraction and milling. Wilt epidemics in earlier decades of the last century resulted in serious losses to cane production in many commercial varieties (Kirtikar et al. 1972; Agnihotri and Rao 2002). Several commercial varieties like Co 245, Co 321, Co 419, Co 449, Co 453, Co 527, Co 951, Co 1107, Co 1122, Co 1223, Co 89003, etc. were withdrawn from cultivation in North and southern parts of India due to wilt. Although the disease occurs in all the parts of the country, Viswanathan et al. (2006) reported its epidemic in sub-tropical plains, South Gujarat and east coastal regions. Wilt epidemic is very common in delta regions where, conducive environment prevails and susceptible host varieties are available. The disease adversely affects germination. Besides yield reduction, wilt also causes 15–30 % reduction in juice extraction and up to 20 % reduction in sugar recovery. Also partial infection drastically reduces juice extraction and juice quality as in red rot infections. Combined infection of red rot and wilt causes more loss to the crop than their infection alone. Similarly, infestations of sugarcane by borer pests aggravate wilt and cause more damage to the crop (Viswanathan 2012; Viswanathan and Rao 2011).
Butler and Khan (1913) studied sugarcane wilt in detail and described Cephalosporium sacchari as the associated pathogen. Subsequently, several workers reported Fusarium moniliformae var subglutinans as the pathogen. Gams (1971) coined a new species Fusarium sacchari (Butler) W.Gams to which both C. sacchari and F. moniliformae var subglutinans were made synonym. Later Nirenberg (1976) distinguished two varieties of F. sacchari namely, F. sacchari var sacchari and F. sacchari var subglutinans, the former having mostly unseptate conidia in the aerial mycelium, no sporodochia, while the latter with 1–3 septate conidia, macroconidia more commonly formed often in sporodochia. Besides F. sacchari, Acremonium implicatum and Acremonium furcatum were isolated from wilt infected samples in subtropical India by Singh and Singh (1974). Although there are several reports on the incidence of wilt and associated pathogens in India (Agnihotri and Rao 2002) only scattered information was available on disease situation across the country, affected varieties, pathogen variation and associated factors. Hence we made a detailed survey in different sugarcane growing states in the country and assessed occurrence of wilt in major sugarcane growing states, factors influencing disease development and isolation of fungi causing wilt (Viswanathan et al. 2006). Further studies were continued to characterize the isolates collected from different regions using different mycological techniques and on the variability in cultural characters of 117 F. sacchari isolates associated with wilt.
Materials and Methods
Isolation of Wilt Pathogen(s) from Infected Stalks
The infected canes were split open longitudinally and recorded internal symptoms. Later, pathogen isolation was done by tissue segment method, in which about 15–20 nodal and internodal tissues of 8 mm thickness were removed using a cork borer and washed in sterile distilled water followed by 70 % ethanol. The bits were then surface sterilized in 0.1 % HgCl2 for 10 s and then given a few changes of sterile distilled water to remove HgCl2. The bits were transferred to Petri plates containing solidified oat meal agar (OMA) and incubated at room temperature (25–30 °C) for a week and observed for the fungal growth. The fungal isolates were purified subsequently and preserved. Details of 249 isolates recovered from 345 wilt infected sugarcane stalks are given in the Table 1.
Isolation of Wilt Pathogen from Soil
One gram soil adhering rhizosphere of infected canes was taken in a conical flask containing 100 ml of sterile distilled water to give a dilution of 10−2 and mixed well the contents of the flask by placing it on a rotary shaker at 75 rpm for 5 min. Serial dilutions of up to 10−6 and 10−8 were made in sterile distilled water. One ml of the soil suspension from each dilution was transferred with a pipette and spread evenly across the agar surface. Even distribution of the soil suspension was accomplished by carefully manipulating the plate with gentle agitation to move a wetting front across the entire agar surface or by using a glass “hockey stick” applicator to spread the suspension. Among OMA and Coon’s media used initially, the latter was found as the best selective medium for the isolation of Fusarium sp. from rhizosphere soil as it eliminated all other contaminants present in the soil. The inoculated plates were incubated at room temperature for a week. Fourteen isolates obtained from 29 different rhizosphere soil samples collected from 10 different states were subsequently purified by repeated subculturing on plates containing OMA or Coon’s agar. List of isolates recovered from rhizosphere soil samples is given in Table 2.
Cultural Characters
Potato dextrose agar (PDA) was used to assess cultural characters of wilt associated fungal isolates. Mycelial plugs of 0.4 cm from 5 days old mother culture were placed at the center of PDA plates and incubated at 25 °C for 10 days. Three replicates were maintained for each isolate. Later the parameters namely, growth rate, mycelial colour, reverse pigmentation at bottom of the plates and colony topology were observed. Radial growth was recorded by measuring the colony diameter on the reverse side of the culture plate. The isolates that showed growth below 5.5 cm were categorized as slow and that reached more than 7.5 cm in 10 days were categorized as fast growing. The other isolates with growth of 5.5–7.5 cm were rated as medium growth. Mycelial colour of the isolates both at top and reverse pigmentation at bottom of the culture plates were recorded as pink, white or varying shades of pink or white and categorized in one of the following combinations as listed in Table 3. Colony texture of the isolates with cottony hyphal growth above the agar surface (aerial mycelium) was marked as fluffy and the other isolates with powdery mycelia growing along with agar and without any aerial mycelia were noted as submissive.
To record types of conidia produced in the plate, six PDA plugs of uniform diameter of 0.5 cm were taken from the 3 replica plates randomly and spore suspension was prepared by agitating six uniform PDA plugs in 20 ml sterile distilled water for 15 min at 120 rpm. Ten microliters of conidial suspension was pipetted out on to a haemocytometer and was observed under a Leica DMLB2 light microscope. Number of conidia in a small square was counted under 45× objective and average count of 5 such small squares was taken to estimate sporulation. Since one small square has an area of 0.0025 mm2, the number of cells per mm2 was computed with the formula
Types of conidia produced (microconidia, macroconidia or both) were also recorded under 45× magnification. Based on the intensity of sporulation and type of conidia produced the isolates were grouped into 8 classes (Table 5).
Results
Recovery of Wilt Pathogens from Infected Sugarcane Stalks and Rhizosphere Soil
Initially four different media namely OMA, Coon’s medium, PDA and Czapek’s medium were compared for the effective recovery of the pathogen. Isolation by tissue segment method in OMA gave comparatively higher recovery of the pathogen, with few other organisms and PDA gave higher recovery of both Fusarium and other microbes. However, recovery of Fusarium on Czapek’s medium was low compared to Coon’s medium which is specific for Fusarium (results not shown). All our isolates recovered in the study showed typical characters of Fusarium and no other fungi were isolated in the infected samples.
Wilt pathogen was isolated from cane samples collected from 15 different states. Totally 346 wilt infected stalks and 24 rhizosphere soil samples were subjected for isolation. Of them, 249 isolates were recovered from cane samples and 14 isolates from rhizosphere soil samples (Tables 1, 2, 4).
Comparison on the recovery of wilt fungus from nodal and internodal tissues revealed that wilt fungi are recovered both from nodal and internodal tissues in 31 of the 125 cane samples subjected for pathogen isolation. Of the remaining 94 samples, 54 samples yielded wilt fungus only from nodal tissues and 40 samples yielded the fungus only from internodal tissues. Overall, higher recovery of wilt fungus was obtained from samples collected from Uttar Pradesh, Orissa, Bihar, Gujarat, Punjab and Kerala (Table 4). Observation on cultivars indicated that Co 0121 from Uttar Pradesh recorded the highest recovery of 100 and 75 % for nodal and internodal tissues, respectively. However in other cultivars the fungal recovery was between 5 and 100 %. Based on the percentage of recovery, the samples were categorized into three groups viz., high (>50 %), medium (25–50 %) and low (<25 %) recovery. Three samples viz., Co 0121 of Uttar Pradesh, Co 6304 of Tamil Nadu, Black Tanna of Kerala gave higher recoveries of 87.5, 60 and 52.9 % respectively. Nine samples gave medium recovery viz., Co 89003 of Punjab, CoJ 85 of Punjab, CoBln 03176 of Bihar, CoA 89085 of Orissa, Co 85004 of Tamil Nadu, 57NG159 yellow of Kerala, genotype PIR 96-325 of Tamil Nadu and progeny Co 2000-10 × Co 94008 of Tamil Nadu. Overall, higher recovery of the pathogen was made from nodal tissues than internodes (Fig. 1). Coon’s media was proved to be the best medium for isolation of Fusarium from wilt sick soil at dilutions of 10−2 and 10−4. Fourteen wilt fungi were recovered from rhizosphere samples collected from Maharashtra and Tamil Nadu (Table 2).
Cultural Characterization
Of the 263 isolates recovered from wilt infected sugarcane stalks and rhizosphere soil, 117 isolates that differed in their place and source of collection were subjected to cultural characterization. The isolates were grouped into three groups based on the growth rate. Eighteen of 117 isolates studied, were found to be slow in growth (below 5.5 cm), 80 moderate (5.6–7.5 cm) and 19 fast growing (more than 7.5 cm) (Supplementary Table 1). Most of the Kerala isolates (8 of 13) were categorized under slow growth group and on the other hand half the isolates population from Orissa (4 of 8) studied were categorized to be fast growing. However, majority of the isolates studied from other states were of moderate growth. Seven Andhra Pradesh isolates, 17 of Punjab, 12 of Bihar, 3 of Maharashtra, 14 of Gujarat, 15 of Tamil Nadu, one each from Uttar Pradesh and Haryana and two each from Assam and Arunachal Pradesh were of moderate nature (Figs. 2, 3).
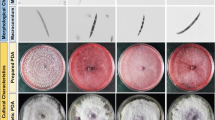
Cultural characterization of Fusarium isolates associated with sugarcane wilt in cv Co 89003 from Punjab. Growth of Fusarium isolates on PDA after 10 days incubation at 28 °C (top and reverse view of the culture plates); 1–5: isolates Fs 003 P1L1, Fs 003 P2L1, Fs 003 P3L1, Fs 003 P4L1, Fs 003 P5L1 from Punjab; arrows point out the variation in growth and pigmentation of the pathogen isolated from the same variety Co 89003
Cultural characterization of Fusarium isolates associated with sugarcane wilt. Growth of Fusarium isolates on PDA after 10 days incubation at 28 °C; Plate a–d represent top and reverse view of the culture plates; a 1–7 isolates Fs 805 O1, FsA 085 O2, FsA 085 O3, FsA 085 O4, FsA 085 O5, FsA 085 O6, FsA 085 O7 from Orissa; b 1–5 isolates FsBln 176 B1, FsSe 231 B, FsBln 175 B4, FsBln 175 B3, FsBln 175 B2 from Bihar; c 1–10 Fs 036 G1, Fs 036 G2, FsSi 071 G, FsV 102 G, Fs 002 G1, Fs 002 G2, Fs 010 G, Fs 006 G1, Fs 006 G2, Fs 006 G3 from Gujarat; d 1–8 FsNG 159 K1, FsNG 159 K2, FsNG 159 K3, FsNG 159 K4, Fs Lajai K1, Fs Lajai K2, FsNG 219 K1, FsNG 219 K2 from Kerala
Characteristic pigmentation of the fungal cultures varied widely among the isolates. Based on the mycelial colour, the isolates were categorized into 7 groups viz., white, orange, pinkish orange, pink, dark pink, pinkish violet and reddish brown. Nineteen of 117 isolates were found to be white, 2 were orange, 3 pinkish orange, 76 pink, 7 dark pink, 8 pinkish violet and 2 reddish brown. More than 75 % of the isolates showed typical pinkish pigmentation and other cultures exhibited varying shades of pinkish pigmentation. However, when reverse pigmentation was compared, the isolates were further categorized into 21 groups (Supplementary Table 2).
The isolates Fs BT K1 and Fs BT K7 were white and 11 other isolates from Kerala had pink mycelia. Similarly 4 Punjab isolates viz., Fs 003 P2L1, Fs 003 P5L1, Fs 003 P10L2 and Fs 120 P4, FsJn 964 MP1 from Madhya Pradesh, FsA 085 O1 and FsA 085 O5 from Orissa, FsC 671 G2, Fs 193 G and Fs 032 G1 from Gujarat, Fs 012 M3 from Maharashtra, Fs 805 AP1L1, FsV 048 AP2 and FsV 048 AP3 from Andhra Pradesh and FsC 063 TN1 and Fs 012 TN from Tamil Nadu had white mycelia. The other isolates from these states exhibited a pinkish mycelium in plates.
When reverse pigmentation was considered, Fs 003 P2L1, Fs 003 P5L1, Fs 003 P10L2, Fs 120 P4 and Fs 120 P5 from Punjab, FsJn 964 MP1 from Madhya Pradesh, FsA 085 O1, FsA 085 O5, FsA 085 O6 and FsA 085 O7 from Orissa, FsC 671 G2 and Fs 193 G from Gujarat, Fs 805 AP1L1 and FsV 048 AP2 from Andhra Pradesh, Fs LC A1 from Assam, Fs 012 M3 from Maharashtra, Fs 121 UP1 from Uttar Pradesh FsC 063 TN1 and Fs 012 TN from Tamil Nadu and Fs BT K1 from Kerala produced no pigments at all. All the other isolates produced varying intensities of different pigments so that bottom of the culture plates was orange, pinkish orange, pink, dark pink, pinkish violet and reddish brown in colour (Figs. 2, 3).
Texture
The 117 isolates were categorized into three groups based on the topology of the culture observed on PDA plates incubated at 25 °C for 10 days. Thirty of 117 isolates studied were found submissive (without aerial mycelium), 68 raised (with aerial mycelium) and 19 fluffy (with abundant cottony mycelia) (Supplementary Table 3). Except the isolate FsV 048 AP3 (fluffy), all the 8 isolates from Andhra Pradesh had raised topology on the agar surface. Six of Punjab isolates were submissive and 17 had raised topology. Four isolates from Bihar, one from Madhya Pradesh, 4 from Gujarat, 3 from Orissa, one each from Uttar Pradesh and Andhra Pradesh, 2 from Maharashtra, 4 each from Tamil Nadu and Kerala were submissive without any aerial mycelium and the remaining isolates from these states produced aerial mycelium. Abundant aerial mycelia resulted in fluffy growth of the isolates FsSe 231 B from Bihar, Fs 036 G2, FsSi 071 G, FsV 102 G, Fs 002 G1, Fs 002 G2, Fs 010 G, Fs 006 G1, Fs 006 G2, Fs 006 G3 (Gujarat), FsV 048 AP3 (Andhra Pradesh), Fs 012 M3 (Maharashtra), Fs LC ArP1 (Arunachal Pradesh), Fs Mclone TN9, FsSi 071 TN1, FsSi 071 TN2, FsV 101 TN3L3, Fs 032 TN8L4 and Fs 032 TN4L2 (Tamil Nadu) (Figs. 2, 3).
Frequency of Sporulation
According to conidial frequency and type of spores produced, the isolates fell into five groups (Table 5; Fig. 4). Two of the Gujarat isolates viz., Fs 036 G1 and FsVi 337 G produced both micro and macroconidia at higher frequencies. Conversely, Fs 036 G2, Fs 032 G1, Fs 032 G2, Fs LC A1, Fs 003 P2L1, FsJ 085 P5, FsBln 175 B3, Fs Lajai K2 and Fs 012 M3 produced micro and macroconidia at lower frequencies. The remaining 106 isolates showed only microconidia and the frequency of sporulation differed among the isolates. In 57 of 117 isolates microconidia concentration was low, moderate concentration of 6–10 × 106/mm2 in 34 and high in 15 isolates. Spore suspension of isolates recovered from wilt infected samples of Andhra Pradesh, Arunachal Pradesh, Uttar Pradesh, Haryana, Madhya Pradesh, Orissa and Tamil Nadu produced only microconidia at different frequencies.
Variation of conidial frequencies in the characterized Fusarium isolates. Conidial frequency of the Fusarium isolates observed on a haemocytometer: a Fs 003 P1L1 with low (<5 × 106/mm2) microconidia production; b Fs 003 P3L1 with medium (6–10 × 106/mm2) production of microconidia; c FsJ 085 P2 showing high (>10 × 106/mm2) concentration of microconidia; d Fs 003 P2L1 with both micro and macro production at low (<5 × 106/mm2) frequency; e Fs 036 G1 with micro and macro production at high (>10 × 106/mm2) frequency
Discussion
Fusarium produces woolly to cottony, flat, spreading colonies, may be white, cream, tan, salmon, cinnamon, yellow, red, violet, pink, or purple. On the reverse, it may be colourless, tan, red, dark purple or brown. Generally a purple reverse is indicative of species in section Liseola (Seifert 1996). The pigmentation of colonies grown on carbohydrate-rich media is variable in some species. Leslie and Summerell (2006) observed that the mycelia of F. oxysporum ranged from white to pale violet in colour and the mycelia floccose, sparse or abundant on PDA. They also reported that F. oxysporum usually produced pale to dark violet or dark magenta pigmentation in the agar but some isolates produced no pigment at all. Mycelia of F. subglutinans and F. sacchari are initially white but becomes violet as the culture ages and agar pigmentation ranged from colourless to dark purple.
The growth of Fusarium isolates was found to vary between 5.5–8.5 cm and most of the isolates, 80 of 117 recorded moderate growth (5.6–7.5 cm). Growth rate of our isolates emphasized that they do not belong to Acremonium as they are faster than Acremonium in their growth rate which is found to be 3 cm in 7 days (http://www.mycology.net/). Campbell et al. (1996) reported growth of 50 mm in diameter in 1 week and the texture was found to be smooth to floccose with orange or pink pigmentation. It is noteworthy that most of the slow growing isolates viz., Fs 003 P2L1, Fs 003 P6L2, Fs 120 P4, FsSe 231 B1, FsJn 964 MP1, Fs 032 M1L1, FsVi 337 G, Fs 032 G1, FsV 048 AP2, Fs Lajai K2, Fs BT K1, Fs BT K2, Fs BT K3, Fs BT K7 were white or orange in colour and the other isolates showed varying shades of pink colour. Gerlach and Nirenberg (1982) and Nelson et al. (1983) also observed pale to violet pigments in different isolates of Fusarium as the culture ages. Most of the isolates were found to have aerial mycelium with raised topography as described by Gams (1971), which clearly concludes that they belong to Fusarium. However, 30 of 117 isolates were submissive in growth, growing along with agar surface. Pink pigmentation and submissive texture of isolates confuse the identity of the pathogen as Acremonium or Fusarium as Butler and Khan (1913) misinterpreted Fusarium as Cephalosporium. However, growth rate of isolates clearly demarcated that our isolates belong to Fusarium but growth rate is not always reliable as it tends to change with environmental conditions. Although several workers characterized sugarcane wilt pathogens from the period of Butler and Khan (1913), currently Fusarium taxonomy is based on the work of Gerlach and Nirenberg (1982) and Nelson et al. (1983) after several reviews in taxonomy. Our results were similar to the observation of Leslie and Summerell (2006) who also observed that the mycelia of F. subglutinans and F. sacchari were white which then turned to violet upon ageing of the culture and pigmentation of the agar ranged from colourless to dark purple observed on the reverse side of the culture plate. On the other hand they also observed mycelia of F. oxysporum which ranged from white to pale violet in colour and the mycelia texture was floccose, sparse or abundant on PDA. They also reported that F. oxysporum usually produced pale to dark violet or dark magenta pigmentation in the agar but some isolates produced no pigment at all.
Growth rate of the fungus is a commonly used secondary character. There can be some variation in this trait (Leslie and Summerell 2006). The linear growth rate of the fungus under controlled conditions was used as a taxonomic characteristic by Booth (1971) and others (Burgess et al. 1988) but must also be used with caution. Isolates within a species may vary considerably with respect to the secondary characters. The degree of variation shown by a particular secondary character may differ between species. Bourne (1953) discussed the identity of the white and purple strains of Fusarium occurring in association with cane stalk rots and pokkah boeng disease in Florida. The fungus was found to be identical with F. verticilloides (F. moniliforme (Sheld.) Snyd. et Hans). Subsequent to publishing these data from Florida, the purple strain of F. verticilloides (F. moniliforme) was maintained in pure culture for 4 years. After this period it still proved highly pathogenic to cane cuttings and growing stalks. However, it was discovered that, after a period of approximately only 2 years in artificial culture stored at 23 °C, this strain completely lost the ability to produce chromogenic substances in nutrient PDA, or to produce septate macroconidia when transferred frequently on this medium. This phenomenon confirms the observations made by Wineland (1924) that certain strains that produced macroconidia in abundance at first, lost this character after a time and afterwards produced nothing but mycelium and microconidia. Loss of colour accompanied this change and in a few instances, cultures never produced pseudo-pionnotes or sporodochia and showed very little colour. This inconsistency in pigment production also agrees well with that recorded by Leonian (1929).
Results of the cultural study imply that there is a good variation among the 117 isolates studied and based on each character they are divided into 3 or more groups. However, most of the isolates fall in a single group. This predicts that amidst cultural variation in some cultures, majority of cultures show uniformity in their characters for cultural observation and these characters may not be adequately distinguish the variation in the fungi. The cultural characters were inconsistent and they tend to change with culture conditions. The same cultures after a month showed a difference in pigmentation and growth rate under different climatic conditions. This change in cultural characters with respect to time and environment was also observed by Butler as mentioned in his personal communication to Bourne in 1939 (Agnihotri and Rao 2002). Although majority of the isolates produced only microconidia in nutrient rich PDA further studies on carnation leaf agar (CLA) clearly established production of macroconidia by the isolates (Viswanathan, Unpublished). This may be the reason that earlier workers have assumed F. verticillioides (F. moniliforme) as the causal organism of the disease.
Singh et al. (1975) reported that frequency of isolation of F. sacchari is more in roots and internodal tissues while of A. implicatum in the nodal tissues. Hence they suggested that the former is a pathogen of parenchymatous tissue and the latter is of vascular tissues. They suggested that higher frequency of A. implicatum in the nodal tissues, but does not prove that this pathogen gets trapped in the nodal tissues because of anastomosis of vascular strands. However, our previous studies indicated that no distinction could be made in isolation of F. sacchari based on nodal and internodal tissues. Cultural characteristics of majority of isolates from different regions revealed that F. sacchari is the most commonly isolated wilt fungi in sugarcane (Viswanathan et al. 2006). Our studies clearly indicated lack of any Acremonium sp. among the 117 isolates which represent the pathogen spread throughout the country. Since cultural characters alone are unreliable for characterizing the isolates further studies were conducted on morphological and molecular characterization. These studies evidenced that F. sacchari is the causative fungus (Poongothai 2010; Viswanathan et al. 2011, 2012). Probably this is the first systematic pathogenic isolation of wilt associated pathogen in sugarcane from different locations and their characterization in India.
References
Agnihotri, V.P., and G.P. Rao. 2002. A century status of sugarcane wilt in India. In Sugarcane crop management, ed. S.B. Singh, G.P. Rao, and S. Eswaramoorthy, 145–160. Houston: SciTech Publishing LLC.
Booth, C. 1971. The genus Fusarium. Kew: Commonwealth Mycological Institute.
Bourne, B.A. 1953. Studies on dissemination of sugarcane diseases. The Sugar Journal 16: 19.
Burgess, L.W., C.M. Liddell, and B.A. Summerell. 1988. Laboratory manual for Fusarium research, 2nd ed. Sydney: University of Sydney.
Butler, E.J., and A.H. Khan. 1913. Some new sugarcane diseases. Part I, wilt, botany series, vol. 6, 180–190. India: Memoirs of Department of Agriculture.
Campbell, C.K., E.M. Johnson, C.M. Philpot, and D.W. Warnock. 1996. Identification of pathogenic fungi. London: PHLS.
Gams, W. 1971. Cephalosporium—artige Schimmelpize (Hyphomycetes), 262. Stuttgart: G Fischer.
Gerlach, W., and H. Nirenberg. 1982. The genus Fusarium—a pictorial atlas. Mitt Biol Bundesanstalt Land-U Forstwirtsch (Berling-Dahlem) 209: 1–406.
Kirtikar, G.P. Singh, and R. Shukla. 1972. Role of seed material in carryover of wilt disease of sugarcane. Indian Sugar 22: 89–90.
Leonian, L.H. 1929. Studies on the variability and dissociation in the genus Fusarium. Phytopathology 19: 753–868.
Leslie, J.F., and B.A. Summerell. 2006. The Fusarium laboratory manual. Oxford: Blackwell Publishing.
Nelson, P.E., T.A. Toussoun, and W.F.O. Marasas. 1983. Fusarium species: An illustrated manual for identification. University Park: Pennsylvania State University Press.
Nirenberg, H. 1976. Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola. Mitt Biol Bundesanstalt Land-U Forstw (Berling-Dahlem) 169: 117.
Poongothai, M. 2010. Cultural, morphological, molecular and pathogenic characterization of pathogen(s) causing sugarcane wilt in India. Ph.D. thesis, Bharathiar University, Coimbatore.
Seifert, K.A. 1996. FusKey-Fusarium interactive key. Agriculture and Agri-Food Canada.
Singh, K., R.P. Singh, and V.P. Agnihotri. 1975. Taxonomy and pathogenicity of fungi causing sugarcane wilt syndrome. Indian Phytopathology 28: 86–91.
Singh, K., and R.P. Singh. 1974. Involvement and pathogenicity of Acremonium in wilt syndrome of sugarcane. Sugarcane Pathologists Newsletter 11(12): 24–25.
Viswanathan, R. 2012. Sugarcane diseases and their management. Coimbatore: Sugarcane Breeding Institute.
Viswanathan, R., P. Malathi, A. Ramesh Sundar, M. Poongothai, and N. Singh. 2006. Current status of sugarcane wilt in India. Sugar Cane International 24(4): 1–7.
Viswanathan, R., M. Poongothai, and P. Malathi. 2011. Pathogenic and molecular confirmation of Fusarium sacchari causing wilt in sugarcane. Sugar Tech 13: 68–76.
Viswanathan, R., M. Poongothai, P. Malathi, and A. Ramesh Sundar. 2012. Sugarcane wilt: New insights into the pathogen identity, variability and pathogenicity. In Functional plant science and biotechnology (special issue 2), vol. 6, ed. R. Viswanathan, and A.R. Sundar, 30–39. Ikenobe: Global Science Books.
Viswanathan, R., and G.P. Rao. 2011. Disease scenario and management of major sugarcane diseases in India. Sugar Tech 13: 336–353.
Wineland, G.O. 1924. An ascigerous stage and synonomy for Fusarium moniliforme. Journal of Agricultural Research 28: 909–922.
Acknowledgments
The authors thank Dr. N. V. Nair, Director of the Institute for providing the facilities and encouragement. The help rendered by different the sugar factory authorities for conducting disease survey and collection of samples is gratefully acknowledged. The work was supported by Network Project from ICAR, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Poongothai, M., Viswanathan, R., Malathi, P. et al. Sugarcane Wilt: Pathogen Recovery from Different Tissues and Variation in Cultural Characters. Sugar Tech 16, 50–66 (2014). https://doi.org/10.1007/s12355-013-0249-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-013-0249-2