Abstract
Mosaic a major disease of sugarcane caused either by Sugarcane streak mosaic virus (SCSMV) or Sugarcane mosaic virus (SCMV) either alone or in combination. Though many serological techniques are available for the detection of viruses, molecular methods are found to be more sensitive in case of low virus titre. For the conventional molecular assays in reverse transcriptase (RT)-PCR, RNA extraction from sugarcane leaves is a cumbersome process. Hence, we optimized immunocapture (IC) using recombinant antiserum (r-antiserum) developed against SCSMV-coat protein (CP) to trap the virus before reverse transcription and optimized a duplex immunocapture reverse transcription (IC-RT) PCR assay. Subsequently we found that the r-antiserum is found to be sensitive to detect both SCMV and SCSMV. Our study established that r-antiserum developed against SCSMV-CP, trapped both SCSMV and SCMV in IC-RT-PCR and it was sensitive enough to detect the two viruses causing mosaic. In ELISA, 12 of 18 sugarcane samples exhibiting varying mosaic symptoms were found to be negative to the virus(es), however they were positive in IC-RT-PCR. We conclude that although DAC-ELISA is simple and cost effective to diagnose these viruses, in samples with very low virus titre, IC-RT-PCR would be more sensitive. This is the first report on the detection of SCSMV and SCMV together in an IC-RT-PCR assay.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mosaic disease is one of the most widespread viral diseases of sugarcane in India and in many other countries (Grisham 2000). Expression of the characterized mosaic symptoms is seen in almost of all the cultivars in the field, however disease expression is influenced by several factors including the environmental conditions and other constraints infecting sugarcane. Worldwide, Sugarcane mosaic virus (SCMV) and Sorghum mosaic virus (SrMV) were reported to cause the disease (Koike and Gillaspie 1989). However, Hema et al. (1999) reported that an unclassified Potyviridae virus, Sugarcane streak mosaic virus (SCSMV) is associated with disease in India. Later, studies of Viswanathan et al. (2007) established that mosaic in sugarcane is caused by both SCMV and SCSMV together or alone in the country. Among the two viruses, the latter was found to more frequently associated with the disease as compared to the former (Viswanathan et al. 2010). Persistence of viral strains in the infected cane is responsible for decline in cane yield and also in sucrose content (Koike and Gillaspie 1989). Impact of mosaic on major sugarcane varieties on cane growth, yield and quality parameters was established from subtropical and tropical regions in India (Singh et al. 2003; Viswanathan and Balamuralikrishnan 2005). The mosaic in association with other viruses causes degeneration of many elite varieties in the country. The phenomenon is referred to as “Varietal degeneration” (Viswanathan and Rao 2011) and due to that performance of many elite sugarcane varieties is affected in the field. Hence, we could not sustain that many of the elite varieties in the field for many years. An uncontrolled viral disease of sugarcane can cause substantial losses, especially in susceptible varieties. In addition, the viruses pose a quarantine risk during germplasm exchange. Therefore, it is essential and necessary to maintain an efficient monitoring programme that would aid in disease management.
The management of a viral disease depends on a detection protocol that is rapid, reproducible and scalable to large number of samples and has to be sensitive enough to detect low virus titre present in the leaf samples. During such situation more sensitive techniques are preferred for precise detection. RT-PCR is considered as a sensitive technique to detect these two viruses as reported earlier (Viswanathan et al. 2007). Later, a duplex-RT-PCR was developed to detect SCMV and SCSMV together in sugarcane (Viswanathan et al. 2008). For these assays primer specificity was established and we were able to detect the viruses more precisely. Since RNA extraction is a tedious process during these assays, simple immunocapture (IC) techniques were employed to trap the viruses using specific antiserum. Earlier, Gaur et al. (2003) used IC-RT-PCR to detect SCMV isolates from North Eastern region of India. In this study, we have optimized a duplex IC-RT-PCR to detect SCSMV and SCMV associated with mosaic in India using a set of mosaic infected sugarcane samples.
Materials and Methods
Detection of SCSMV by DAC-ELISA
Leaf samples from 30 varieties exhibiting varying degrees of streak mosaic and mosaic symptoms were collected at National Hybridization Garden of the institute. The samples were ground in pre-chilled mortar and pestle at a dilution of 1:10 W/V in carbonate buffer (15 mM sodium carbonate, 35 mM sodium bicarbonate, 0.02 % sodium azide, polyvinyl pyrrolindone-0.5 g, pH 9.6). The leaf extracts were then centrifuged at 10,000 rpm for 10 min at 4 °C and the supernatant were stored at −80 °C until use. The crude extracts collected by the above method from different varieties of sugarcane served as an antigen in the experiment. The polystyrene plates were coated with 100 μl leaf extracts as triplicates and incubated. After incubation, 100 μl SCSMV-IgG (1:1,500 dilutions in conjugate buffer) was added as primary antiserum from the rabbits immunized with recombinant SCSMVcp by direct antigen coating (DAC)-ELISA (Viswanathan et al. 2011). The tubes were then coated with 100 μl of goat anti-rabbit IgG (Sigma, USA) conjugated with alkaline phosphatase (1:10,000 dilution in conjugate buffer) and incubated the plate at 37 °C for 2 h at both the steps. Between each coating of antigen, antibody and conjugate, the plates were washed thrice with wash buffer (PBS-T for 5 min each) and tapped upside down on the blotting paper to remove the unbound materials. The final reaction was developed by adding 100 μl of freshly prepared enzyme substrate, p-nitro-phenylphosphate (Sigma, 0.5 mg ml−1) and recorded the absorbance at 405 nm after 30 min in an ELISA reader (Spectra MAX 190, Molecular Devices, USA). The mean values greater than three times that of healthy control were considered as positive.
Detection of SCSMV and SCMV by IC-RT-PCR
Leaf samples from 30 varieties exhibiting varying degrees of streak mosaic and mosaic symptoms were collected at National Hybridization Garden of the institute and homogenized with phosphate buffer, pH 7.4 (135 mM NaCl, 1.5 mM KH2PO4, 8 mM NaHPO4, 3 mM KCl, 0.05 % Tween-20 and 3 mM NaN3) and centrifuged briefly. Specific antiserum was used against viral proteins to immunocapture the virus (Viswanathan et al. 2011). Before IC-RT-PCR, we optimized antiserum and antigen concentration for efficient trapping of the virus in tubes. To standardize optimum antiserum concentration for immunocapture, antiserum dilutions of 1:100 and 1:1,000 in carbonate buffer (pH 9.6) were used. Antigen of 50 μl (1:10 W/V) and/or 100 μl was used. The samples were extracted with either one of the three different buffers namely tris buffer, pH 8.3 (500 mM Tris–HCl, 10 mM Na2SO3, 2 % PVP, 3 mM NaNO3, 140 mM NaCl and 0.05 % Tween-20); carbonate buffer (15 mM Na2CO3, 35 mM NaHCo3, 0.02 % NaN3, PVP-0.5 g, pH 9.6) or phosphate buffer at different ratios viz., 1:3, 1:5, 1:6 and 1:10 (W/V). Between each steps, the tubes were incubated at 37 °C for 30, 45, 60 and 120 min. followed by the addition of 20 μl of DEPC water with 0.2, 0.4, 0.6, 0.8 and 1 % of Triton X100 along with 1 μl of oligo dT. The tubes were incubated at different temperatures viz., 80, 85, 90 and 95 °C for 10 min before adding RT mixture followed by RT-PCR.
Based on this study, antiserum of 1:100 dilution was coated on to 200 μl micro centrifuge tubes followed by the addition of blocking solution (100 μl) and antigen (in phosphate buffer of 1:10 W/V of 100 μl). Between each step the tubes were incubated at 37 °C for 2 h and the tubes were washed twice with 1× phosphate buffer saline Tween 20 (PBST) of each 2 min and quickly washed once with DEPC water. Later, 20 μl of DEPC water was added along with 1 μl of oligo dT. The tubes were incubated at 80 °C for 10 min. After incubation, the tubes were immediately kept on ice.
Complementary DNA was prepared by mixing the following components: 7.0 μl of 5× RT buffer, 3.5 μl of 10 mM dNTP’s, 34.8 U of RNase Inhibitor, 86 U of MMuLV reverse transcriptase enzyme and nuclease free water up to 35 μl. The tubes were mixed gently and incubated at 37 °C for 1 h 30 min. PCR amplification (Applied Biosciences, Veriti 96 well thermal cycler, Germany) was performed to a final volume of 25 μl reaction by adding 2.5 μl of 10× PCR buffer, 2.0 μl of 2.5 mM dNTP’s, 0.5 μl each of forward and reverse primer (Sigma, USA) of both SCSMV and SCMV (Table 1), 0.33 μl of Taq DNA polymerase (3 U/μl, Bangalore Genei, Bangalore, India) and sterile water. The cycling parameters for the amplification of cDNA were: initial denaturation at 94 °C for 5 min followed by 40 cycles of 94 °C for 1 min, 65 °C for 1 min, 72 °C for 1 min and terminated with a final extension cycle at 72 °C for 10 min. The amplified products were run on 1.2 % agarose gel to detect the specific amplification of the virus sequences in a reaction. Presence of 690 and 380 bp bands indicated specific amplification of SCSMV and SCMV, respectively.
Results and Discussion
Among the two viruses associated with mosaic, SCSMV is the recently reported one and is found to infect majority of the mosaic affected sugarcane varieties than SCMV in India. Earlier SCSMV-CP was expressed in an expression vector to produce a recombinant antigen to produce polyclonal antiserum (Viswanathan et al. 2011). Subsequently, antiserum was produced in rabbit and was found to have very high levels of serum titre (Viswanathan, unpublished). Further studies were conducted to standardize immunocapture-RT-PCR to improve the sensitivity of the assay. Among the two antigen extraction buffers used, phosphate buffer was found to be the best for trapping of the virus in the tubes compared to carbonate buffer (Fig. 1a). Further, in this assay antigen dilutions of 1:3, 1:5 and 1:6 gave similar results and 1:10 has given slightly less intense bands. Also, varying templates of cDNA viz., 2, 6, 10, 12 and 16 μl showed progressive increase in amplifications of the virus (Fig. 1b). In the subsequent IC-RT-PCR assays, 12 μl cDNA was used as template for 35 μl reaction volume. The above two independent experiments revealed that phosphate buffer is the ideal one to extract the antigen from leaf samples hence it was used to extract the antigen from leaf samples.
Detection of Sugarcane streak mosaic virus by immunocapture reverse transcriptase polymerase chain reaction. a Influence of sample extraction with two different buffers and different volumes of antigen on virus trapping. M marker (100 bp), Lane 1–8 carbonate buffer, Lane 9–16 phosphate buffer, Lane 1, 2, 9, 10 1:3 antigen dilution, Lane 3, 4, 11, 12 1:5 antigen dilution, Lane 5, 6, 13, 14 1:6 antigen dilution, Lane 7, 8, 15, 16 1:10 antigen dilution (Co 94008), antiseutm dilution 1:100. b Variation in cDNA template on SCSMV amplification. M marker (100 bp), Lane 1 2 μl cDNA, Lane 2 6 μl cDNA, Lane 3 10 μl cDNA, Lane 4 12 μl cDNA, Lane 5 16 μl cDNA for 50 μl reaction, antiserum dilution—1:100, antigen (Co 94008) dilution—1:10
Incubation of trapped virions in DEPC water at 80, 85, 90 and 95 °C before reverse transcription reaction revealed that incubation at 80 °C gave good amplification compared to other temperatures (Fig. 2). In another procedure, a total of seven reactions were performed in which DEPC water was added along with Triton X100 of varying volumes viz, 0.2, 0.4, 0.6, 0.8 and 1 % and the tubes were incubated for different time intervals viz., 30, 45 and 60 min between each coating of antigen and antibody (Fig. 3a). More intense band was observed for the reaction in which Triton X100 was not added when compared with the reaction containing of Triton X100 of different volumes where the reactions were incubated for 60 min. In the fourth lane, due to low volume of Triton X100 the bands observed were darker. In the second and third lanes, bands were not observed due to the tubes incubated for a short period of time i.e., for 30 and 45 min. The same study revealed amplification of partial SCMV-CP genome when SCMV F-380 and SCMV R-380 primers were added in place of SCSMV primers. This assay clearly indicated that r-antiserum of SCSMV-CP traps both SCSMV and SCMV in immunocapture.
Detection of Sugarcane streak mosaic virus (SCSMV) and Sugarcane mosaic virus (SCMV) by IC-RT-PCR using SCSMV r-antiserum. The virus specific primers were used separately. M marker (100 bp), Lanes 1–3 incubated at 95, 90 and 85 °C respectively. Lane 4 incubated at 80 °C, Lane 5 incubated at 80 °C with blocking solution, Lane 6 sample extracted with liquid nitrogen and incubated at 80 °C, Lane 7 antigen added twice (Antigen dilution 1: 10, Co 94008), Lane 8–10 SCMV primers alone added, incubated at 80 °C and for lane 9 blocking solution was added, antigen (Co 94007)—1:7, antiserum dilution used—1:100 and cDNA: 12 μl
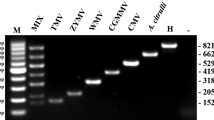
Detection of SCSMV and SCMV in duplex IC-RT-PCR assay using SCSMV r-antiserum. a SCSMV and SCMV primers were added together in RT-PCR. M marker (100 bp), Lanes 1, 2 incubated at 30 and 45 min between each steps of adding blocking solution and antigen, Lane 3 DEPC water without Triton X100 and incubated for 60 min between each steps of adding blocking solution and antigen, Lane 4–7 incubated for 60 min with 0.2, 0.4, 0.6, 0.8 and 1 % Triton X100 respectively between each steps of adding blocking solution and antigen. Antiserum dilution—1:100; antigen dilution (Co 94007)—1:7. b Duplex IC-RT-PCR assay for SCSMV and SCMV with 30 field samples of sugarcane. M marker (100 bp), P SCSMV positive control, Lane positions: 1–30 of the Table 2, antigen dilution—1:7; antiserum dilution—1:100; cDNA: 12 μl
Subsequently, the efficiency of the IC-RT-PCR was tested with a set of 30 samples showing varying degrees of mosaic symptoms which ranged from asymptomatic to severe mosaic symptoms (Fig. 3b). Four varieties Co 86249, CoA 92081, CoH 114 and CoSe 92423 were negative to SCSMV alone in both DAC-ELISA and duplex IC-RT-PCR but showed mild amplification to SCMV. Eight varieties Co 976, Co 98006, CoH 104, CoJ 65, CoJn 86141, CoLk 970221, CoN 91132 and LG 06602 were negative in DAC-ELISA but positive to duplex IC-RT-PCR. Among them, the variety CoJ 65 was negative to SCSMV in DAC-ELISA but positive in duplex IC-RT-PCR but gave negative result to SCMV in duplex IC-RT-PCR whereas the remaining seven varieties were positive to both SCSMV and SCMV. The samples which showed positive in DAC-ELISA gave strong amplification to SCSMV and mild amplification to SCMV in duplex IC-RT-PCR. The variety BO 91 showed negative to SCSMV but positive to SCMV. Remaining 11 samples showed positive to both SCSMV and SCMV. Strong amplification to SCSMV was observed in the varieties Co 740, Co 7204, CoPant 90224, CoC 8201 and Co 86002 in duplex IC-RT-PCR and these samples were considered to contain SCSMV in very high concentration. The varieties Co 740, Co 7204, CoC 8201 and CoC 671 showed mixed infections of SCSMV and SCMV and was confirmed by duplex IC-RT-PCR which amplified both the viruses simultaneously in a single reaction mixture.
We have reported combined infection of these viruses in Co 740 and CoC 671 earlier through RT-PCR (Viswanathan et al. 2007). Mosaic is one of the most widespread and quarantine disease of sugarcane during germplasm exchange it causes decrease in yield significantly. DAC-ELISA is the most commonly preferred method because of its simplicity, reliability, cheap and large number of samples can be tested in a short period of time but has disadvantage in which it is based on the detection of antigenic properties of the viral coat protein. Hence, nucleic acid based methods became the method of choice for more precise detection. RT-PCR is the popular technique for the detection of plant viruses however detection of different viruses infecting the same crop separately by this method is expensive and time consuming. To reduce this, a duplex RT-PCR has been developed by Viswanathan et al. (2008) to detect both SCSMV and SCMV. A further refinement of RT-PCR is IC-RT-PCR in which the virus particles are captured by immobilized antibodies for the detection of specific virus. This method is especially used in concentrating virus particle where virus titre is low or compounds that inhibit PCR are present. We already expressed SCSMV-CP and polyclonal antisera were raised against the expressed protein (Viswanathan et al. 2011).
These immuno-molecular methods unite the immunological with the molecular detection technologies which in turn improved the diagnostic capacity, although the sensitivity of both the methods is highly dependent on the quality of the antibodies produced, measured by its affinity and specificity to the target antigen. From this study, we found that duplex IC-RT-PCR is more sensitive as it detected the viruses in low titre concentration. To test the sensitivity of IC-RT-PCR in routine detection of SCSMV, a comparative analysis was carried out by Hema et al. (2003) with three detection methods viz., DAC-ELISA, dot immunoblotting assay and IC-RT-PCR and reported that IC-RT-PCR technique detected virus in mild concentration. Subsequently, Reddy et al. (2011) found IC-RT-PCR with two antisera was better than DAC-ELISA for the detection of the viruses in sugarcane Table 1.
Trapping of SCMV by r-antiserum of SCSMV-CP is an unexpected finding in this work. Use of single antiserum to detect two viruses is an added advantage in disease detection. This study clearly indicates that duplex IC-RT-PCR procedure would be an effective method for the detection of SCSMV and SCMV in sugarcane and it circumvents the need of total nucleic acids extraction for the standard PCR method which save time and reagent cost for the assays. We conclude that duplex IC-RT-PCR is more sensitive to detect the viruses present in low titre more specifically than DAC-ELISA. This is the first report on the use of duplex IC-RT-PCR to detect SCSMV and SCMV in sugarcane using antiserum developed against SCSMV. Outcome of the studies are expected to have applications in sugarcane quarantine and virus detection in sugarcane in healthy nursery programmes.
References
Gaur, R.K., M. Singh, and G.P. Rao. 2003. Molecular characterization of SCMV isolate from North Eastern Region of India. Sugar Tech 5: 149–153.
Grisham, M.P. 2000. Mosaic. In A guide to sugarcane diseases, ed. P. Rott, R.A. Bailey, J.C. Comstock, B.J. Croft, and A.S. Saumtally, 249–254. Montpellier: CIRAD-ISSCT, CIRAD Publication Services.
Hema, M., J. Joseph, K. Gopinath, P. Sreenivasulu, and H.S. Savithri. 1999. Molecular characterization and interviral relationships of a flexuous filamentous virus causing mosaic disease of sugarcane (Saccharum officinarum L.) in India. Archives of Virology 144: 479–490.
Hema, M., H.S. Savithri, and P. Sreenivasulu. 2003. Comparison of direct binding polymerase chain reaction with recombinant coat protein antibody based dot-blot immunobinding assay and immunocapture–reverse transcription–polymerase chain reaction for the detection of sugarcane streak mosaic virus causing mosaic disease of sugarcane in India. Current Science 85: 1774.
Koike, H., and A.G. Gillaspie. 1989. Mosaic. In Disease of sugarcane—Major diseases, ed. C. Recaud, B.T. Egan, A.G. Gillaspie, and C.G. Hughes, 301–322. Amsterdam: Elsevier.
Reddy, ChV, P. Sreeivasulu, and G. Sekhar. 2011. Duplex-immunocapture-RT-PCR for detection and discrimination of two distinct potyviruses naturally infecting sugarcane (Saccharum spp. hybrid). Indian Journal of Experimental Biology 49: 68–73.
Singh, V., O.K. Sinha, and R. Kumar. 2003. Progressive decline in yield and quality of sugarcane due to Sugarcane mosaic virus. Indian Phytopathology 56: 500–502.
Viswanathan, R., and M. Balamuralikrishnan. 2005. Impact of mosaic infection on growth and yield of sugarcane. Sugar Tech 7: 61–65.
Viswanathan, R., and G.P. Rao. 2011. Disease scenario and management of major sugarcane diseases in India. Sugar Tech 13: 336–353.
Viswanathan, R., M. Balamuralikrishnan, and R. Karuppaiah. 2007. Sugarcane mosaic in India: A cause of combined infection of sugarcane mosaic virus and sugarcane streak mosaic virus. Sugar Cane International 25: 6–14.
Viswanathan, R., M. Balamuralikrishnan, and R. Karuppaiah. 2008. Duplex reverse transcription polymerase chain reaction (D-RT-PCR) a technique for simultaneous detection of viruses causing sugarcane mosaic. Sugar Tech 10: 81–86.
Viswanathan, R., M. Balamuralikrishnan, and R. Karuppaiah. 2010. Detection of three major RNA viruses infecting sugarcane multiplex transcriptase polymerase chain reaction (multiplex RT-PCT). Australasian Plant Pathology 39: 79–84.
Viswanathan, R., R. Karuppaiah, and V. Ganesh Kumar. 2011. Expression of Sugarcane streak mosaic virus (SCSMV) coat protein in expression vector as a fusion protein with maltose binding protein. Journal of Sugarcane Research 1: 63–68.
Acknowledgments
Authors are thankful to Dr. N. Vijayan Nair Director of the Institute for the support and encouragement. Financial support received from DBT, New Delhi under NCS-TCP is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Viswanathan, R., Ganesh Kumar, V., Karuppaiah, R. et al. Development of Duplex-Immunocapture (Duplex-IC) RT-PCR for the Detection of Sugarcane streak mosaic virus and Sugarcane mosaic virus in Sugarcane. Sugar Tech 15, 399–405 (2013). https://doi.org/10.1007/s12355-013-0216-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-013-0216-y