Abstract
Residues of green-harvested sugarcane contribute to nutrient recycling in production systems. Therefore, better understanding of trash decomposition dynamics can help crop fertilisation management. This study was conducted during the 2006–2008 seasons in Jaboticabal, north-eastern Sao Paulo State, Brazil and aimed to evaluate the nitrogen recovery rates from the previous crop residues or from urea applied on sugarcane planting in a minimum tillage system, thus without trash and rhizome incorporation in crop renewal. Previous crop residues consisted of 9 and 3 t/ha of sugarcane trash (dry tops + leaves) and root system (roots + rhizomes) enriched with 1.07 and 0.81% 15N isotope, respectively. These contributed 51 and 33 kg/ha of N. 15N labelled trash laid on the soil surface and buried 15N-root system attempted to simulate the original field residues disposal. The SP81-3250 variety was planted with 80 kg N/ha of a 5.17% 15N-labelled urea. Recovery of sugarcane residues-N (trash-N and root system-N) or urea-N incorporated to the soil at planting were evaluated in distinct plant parts (stem, tops and dry leaves) during three consecutive harvest seasons. Recovery of urea-N was higher in the first harvest season (31% of initial N rate) and its uptake decreased in the second and third to 5 and 4%, respectively. In later harvested seasons, urea-N had probably been turned-over as soil organic matter and/or microbial biomass but remained in the soil N pool and available for plant recovery. Trash-N uptake closely resembled urea-N uptake, and only 13% of its N content was recovered in the first year, followed by 7 and 3% in the second and third seasons. Root system-N recovery was different since the second cut uptake was higher than the first followed by the third, 9, 6 and 2% respectively. Three year cumulative recovery of urea-N, trash-N and root system-N was 39, 23 and 17%, respectively. Most recovered N was found in stems followed by tops and dry leaves.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Brazil is the world’s largest sugarcane producer, totalling 7 million hectares of which more than 50% of harvest is performed without burning in Sao Paulo State (Yank 2009). Sugarcane under green cane trash blanket (GCTB) has been adopted by many producers worldwide as a conservative crop system, reducing soil erosion and greenhouse gas (GHG) emissions. Likewise, minimum or no-tillage systems also present benefits in cost savings for highly competitive growers (Wood 1991).
Residues of GCTB contribute to nutrient recycling in production systems; therefore, better understanding of trash decomposition dynamics can help crop fertilisation management. Those crop residues provide annually about 40–120 kg/ha of N thereby increasing the amount of organic matter and nutrients in the soil (Trivelin et al. 1996; Wood 1991; Oliveira et al. 2002).
Nitrogen tracer (15N) trials indicate that fertiliser-N recovery by the sugarcane crop varies from 5 to 40% (Basanta et al. 2003; Franco 2008; Gava et al. 2005; Meier et al. 2006; Trivelin et al. 1996). These low recovery rates might be due to N losses from soil by denitrification and/or ammonia volatilisation, and gaseous losses from leaf canopy of plants (Trivelin et al. 1996) and microbial immobilisation of N in the soil (Gava et al. 2005; Oliveira et al. 2002).
Crop residues mineralisation is dependent on environmental factors such as temperature, soil moisture and aeration. Nevertheless, chemical composition of residues such as C:N ratio, lignin, cellulose, hemicellulose, and polyphenols content also play an important role in trash decomposition and nutrient dynamics (Janzen and Kucey 1988; Meier et al. 2006; Oliveira et al. 2002).
Sugarcane trash presents on average 390–450 g/kg of carbon and 4.6–6.5 g/kg of N, thus a C:N ratio about 100. Under these conditions, a strong immobilisation of soil N by microbial biomass and a reduced net N mineralisation for the subsequent growing season after harvested unburned sugarcane is expected, as pointed out by several trials using 15N tracer (Franco 2008; Gava et al. 2005; Ng Kee Kwong et al. 1987; Oliveira et al. 2002; Meier et al. 2006).
Nitrogen recovery from crop residues incorporated into the soil varies from 2.4 to 15%. The recovery rate depends basically on residues quality regarding N content, higher than 20 g/kg and a C:N ratio lower than 25. This allows faster mineralisation compared to N immobilisation and enables higher N uptake by plants from those organic sources (Ng Kee Kwong et al. 1987; Janzen and Kucey 1988; Chapman et al. 1992; Gava et al. 2005).
Most studies have considered only one or two harvest seasons to evaluate the effect of N uptake from fertilisers and crop residues. This study was conducted in north-eastern Sao Paulo State, Brazil and aimed to evaluate nitrogen recovery rates from the previous-cycle crop residues and from urea applied to sugarcane under a minimum tillage system during three consecutive harvest seasons, 2006–2008.
Materials and Methods
Location, Trial Design and 15N Labelled Fertiliser/Crop Residues
The trial was planted on March 2nd, 2005 in Jaboticabal county, north-eastern Sao Paulo State (Lat 21°17′ S, Long 48°12′ W) on a clayey Typic Hapludox under minimum tillage system. The previous sugarcane crop, 5th ratoon of RB855536 variety, had been desiccated with herbicide and succeeded by a single subsoiling operation, attempting to preserve previous crop residues on the soil surface.
Soil 0–25 cm layer analysis indicated: 135, 227 and 628 g/kg of total sand, silt and clay, respectively, \( {\text{pH}}_{{ ( {\text{CaCl}}_{2} )}} \) 5.2; OM (g/dm3) 31; P (mg/dm3) 42 and K, Ca, Mg, and CEC, 3.1, 31, 9, 77.4 (mmolc/dm3), respectively.
A randomised block design was adopted with four replicates of 12 rows with 15 m length and spaced 1.5 m apart. Plant-cane fertilisation (N:P205:K2O) was 80:120:120 with a planting density of 12 viable buds per metre of SP81-3250 variety. On ratoons, fertilisation was only KCl (150 kg/ha K2O) applied as a row top dressing after each harvest.
In each plot replicate for plant-cane, micro plots comprising 2.0 m length furrows were assembled and a 5.17% 15N enriched urea was buried as done with ordinary urea in the remaining plot area (Trivelin et al. 1994).
Attempting to simulate past crop residues left on the soil surface, hereafter known as previous crop trash (PCT) and rhizomes (PCR), a small plot of the previous variety (RB855536) was cropped outside this trial, and the plants were labelled by foliar spraying with a 28% 15N urea solution, according to Faroni et al. (2007). The achieved concentrations of C, N (g/kg) and 15N (%) on PCT and PCR were 402, 5.7, 1.07% and 334, 6.6, 0.81%, respectively. Thus, C:N ratios on those materials were around 71:1 for PCT and 51:1 for PCR.
Furthermore, dry leaves and rhizomes were harvested out of this plot in order to assemble other 15N labelled residues micro plots (2.0 × 1.5 m), settled separately in each replication. To simulate the original disposal of crop residues, 9 t/ha of PCT was laid on soil surface and 3 t/ha of PCR was buried into soil as in Franco (2008). Thus, the total N (kg/ha) added by urea, PCT and PCR were 80, 51 and 33, respectively.
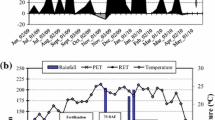
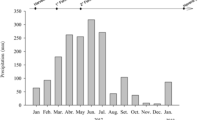
Rainfall during the studied seasons was: 1767, 1715 and 1367 mm for plant-cane, first and second ratoons, respectively. Average maximum and minimum temperatures (°C) in these seasons were 30.4 and 18.0, respectively.
Harvesting, Sampling and Statistical Analysis
The trial has been conducted over three consecutive harvest seasons: 18 month plant-cane was harvested on August 10th, 2006 and first and second 12 month ratoons on August 21st, 2007 and 25th July, 2008, respectively. Agro industrial yield data were evaluated but will not be discussed in this paper.
Harvest of urea, PCT and PCR micro plots comprised the collection of cane in 1.0 m of the central and two adjacent rows, in the same contiguous position as described in Trivelin et al. (1994) and performed in several trials (Trivelin et al. 1996; Basanta et al. 2003; Franco 2008; Gava et al. 2005). All plant parts were harvested separately (stems, dry leaves and tops). Plant parts fresh mass were evaluated directly in the field with a weighing scale and crushed in mechanical forage chopper as in Franco (2008).
Homogenised sub-samples were prepared according to Trivelin et al. (1996) (65°C drying for 72 h and finely ground on Willey type mill) for determination of total N (%) and 15N abundance analysis in a mass spectrometer model Hydra 20-20 SerCon Co., UK, coupled to an automatic N analyser ANCA-SGL (Barrie and Prosser 1996).
Nitrogen recovery from fertiliser and crop residues was calculated using formulae described below (Trivelin et al. 1994, 1996):
where Ndfs, N in plant part derived from 15N source (urea, PCT or PCR); A and B, 15N abundances of harvested samples (plant part) and from 15N source, respectively (0.367% is the natural 15N abundance); NC, nitrogen content in each plant part (stem, dry leaves or tops); R, N recovery from a N source in each sample (plant part); NR, applied N rate (via fertiliser or crop residues).
ANOVA and F test statistical analyses were performed. Means detected as significant by the F test were compared by the Tukey test at 1 and 5% of probability.
Results and Discussion
Nitrogen Recovery from Planting Fertiliser (Urea)
Plant-cane urea-N recovery was higher in the first harvest season, 24.7 kg/ha (31% of applied N) (Table 1). This result is consistent with 28% (22 kg/ha of urea-N) by plant-cane obtained by Franco (2008) who evaluated two other field trials using the same sugarcane variety and planting fertiliser rate (80 kg N-urea/ha). Bologna-Campbell (2007) also used the same N rate in a study with SP80-3280 variety planted in pots and obtained 35% of urea-N utilisation for the whole plant (above ground parts plus roots and rhizomes).
Chapman et al. (1992) also found that 32% of buried urea on planting was present in the first crop, despite the applied rate was twofold compared with this case (160 kg/ha). Basanta et al. (2003) concluded that 63% (40 kg/ha) of N applied on planting was recovered by plant cane. Note that those authors utilised a lower N dose (63 kg N/ha) as ammonium sulfate applied 50 days after planting. Korndörfer et al. (1997) found 53% of apparent urea-N recovery applied on plant cane measured using a non-isotopic method (difference method). Those authors emphasised this method usually overestimates real fertiliser-N uptake.
Slight differences of urea-N accumulation on plant-cane parts were found, compared to literature results. Data shown in this study presented higher uptake by the stems (77%) followed by tops (12%) and dry leaves (11%) (Table 1). Franco (2008) obtained 54% of total urea-N applied on stems, 22% on dry leaves and 24% on tops of plant cane, similar to Basanta et al. (2003) who concluded that 56% of planting fertiliser-N uptake was on stems, 23% dry leaves and 22% on tops.
Korndörfer et al. (1997) found that 56% of urea-N uptake remained on stems; 16% on dry leaves and 28% on tops, respectively. These differences could be explained by the higher tonnage obtained in the present work (average 156 t/ha, data not shown) which could lead to higher urea-N accumulation in millable stalks. This could also reflect on N uptake by cane tops in further years since there were no significantly differences in three seasons for this specific plant part (Table 1).
The cumulative urea-N uptake in three seasons (2006–2008) reached 31.5 kg/ha (21 in stems, 4.5 dry leaves and 6 on tops). The overall recovery of urea-N applied at planting in three consecutive seasons was 39% of which 66% was found in stems, 14% in dry leaves and 20% in tops (Table 1). After the first year, urea-N had probably been turned-over as soil organic matter and/or microbial biomass but remained in the soil as an available N pool for plant recovery.
These results concord with those of Chapman et al. (1992) who obtained 32% of N recovery from buried urea application in the first year and an average of 5% was recovered by the crop above ground part on the second cut. In the present results, 5 and 3.5% of urea-N was recovered in the second and third harvest seasons, respectively (Table 1). Basanta et al. (2003) concluded that 77% (49 kg/ha) of planting fertiliser-N (ammonium sulfate) was recovered after three cropping seasons. From that amount, 53% was exported by stems, 24% dry leaves and 23% by cane tops, respectively.
The urea-N uptake by the whole above ground part over time (1st to 3rd cut) was 31, 5 and 4%, the same pattern obtained by Basanta et al. (2003), 63, 11, 4%, for first second and third cuts, respectively.
Nitrogen Recovery from Previous Crop Residues (PCT and PCR)
Nitrogen recovery from PCT and PCR by plant cane was 13 and 6%, respectively (Table 1). These results are consistent with those of Ng Kee Kwong et al. (1987) who observed up to 14% of trash-N recovery, when this residue remained for 18 months in field conditions. Bologna-Campbell (2007) found 14% of crop residues-N utilisation by sugarcane after 12 mouths planting by using a mixture of sugarcane trash (tops + dry leaves) and rhizomes + roots labelled with 15N incorporated to the soil; 74% of crop residues-N remained in the soil at the end of the 1st season harvested.
PCT-N uptake decreased from the first to third cropping season, 13, 7 and 3%; respectively. PCT-N amount found in stems was 74, 11% in dry leaves and 15% in cane tops (Table 1). This distribution agreed with Gava et al. (2005) who found trash-N uptake as 52% in stems, 7% in dry leaves and 29% in cane tops. PCT-N uptake was closely related to urea-N recovery and was remarkably higher in the first cut, decreasing in the second and third harvest seasons (Table 1). This also indicates that the urea-N added to soil under GCTB and minimum tillage system could have enhanced crop residues mineralisation.
In contrast to PCT-N, a larger amount of PCR-N was recovered after the plant-cane cycle, 9% during the second harvest season, followed by 6% on the first and 2% on the third season (Table 1). This could have happened because this residue was previously buried into soil in a minimum tillage system environment, thus with limited aeration capacity leading to lower mineralisation rates in the first year (Janzen and Kucey 1988). This result differs from those obtained by Chapman et al. (1992) who stated that 4% of the N from a 15N-labelled crop residue (roots and stubbles) was assimilated by sugarcane after two cropping seasons. They mentioned that sometimes temporary flooding may lead to lower decomposition rates of crop residues and can reduce its N availability to plants.
Unburned cane and in this case, minimum tillage system, can lead sugarcane root system to be more superficial and take advantage of surface nutrients (Meier et al. 2006; Oliveira et al. 2002). PCR-N recovered by crop was 57% in stems, 19% in dry leaves and 23% in tops.
After three cropping seasons, N recovery from PCT and PCR by sugarcane aerial part was 12 and 6 kg/ha, respectively. As those residues contributed initially with 51 and 33 kg N/ha, N uptake was approximately 23 and 17% after three harvests (Table 1). Basanta et al. (2003) obtained 7 kg/ha (5%) of trash-N recovered by sugarcane in two consecutive cropping seasons, of which 51% was in stems, 24% in dry leaves and 29% in tops.
Overall N uptake from PCT and PCR found herein (23 and 17%—Table 1) are larger than ones found by Ng Kee Kwong et al. (1987) and Gava et al. (2005), 14 and 9%, respectively. This could be explained by the lower C:N ratio on residues applied during this work (PCT: 71:1 and PCR: 51:1) compared with those used by authors where C:N ratio was higher than 125:1. Furthermore, higher C:N ratios can decrease mineralisation rates as stressed by Janzen and Kucey (1988). Another point to consider is the total time of each trial (three seasons in this work against one in the others) and the local environment temperature, an important factor to be considered in this kind of evaluation.
It is also clear that trash composition is dependent on variety characteristics. Meier et al. (2006) used a 15N-labelled trash from Q166 variety (C:N 105:1) in their research in Queensland, Australia but stated that this ratio usually varies from 70:1 to 120:1. Oliveira et al. (2002) found C:N = 95:1 on recently harvested trashes of two distinct varieties (SP79-1011 and SP80-1842) and two locations in Sao Paulo State, Brazil. Bologna-Campbell (2007) obtained similar values of C:N ratio compared to the present work, 89:1 for trash and 57:1 for root system grown in pots.
Wood (1991) stated that unburned cane management provided 50% of overall urea-N recovery compared to less than 40% in burned cane but emphasised that variety suitability for GCTB crop management is mandatory for successful sugarcane growing.
In general, the amount of N recovered decreased in the order stems > tops > dry leaves. These results are consistent with those of Basanta et al. (2003), Gava et al. (2005) and Ng Kee Kwong et al. (1987) indicating that, despite crop residues-N having slow release and is mainly immobilised in the soil, it can become a complementary source of N for succeeding crops.
Conclusion
Nitrogen recovery from urea, PCT and PCR after three harvest seasons was 39, 23 and 17%, respectively, indicating that N from crop residues is an important long-term source of nitrogen to sugarcane.
The larger portions of recovered nitrogen from urea and crop residues were found on stems followed by tops and dry leaves.
References
Barrie, A., and S.J. Prosser. 1996. Automated analysis of light-element stable isotopes by isotope ratio mass spectrometry. In Mass spectrometry of soil, ed. T.W. Boutton, and S. Yamsahi, 1–46. New York: Marcel Dekker.
Basanta, M.V., D. Dourado Neto, K. Reichardt, O.O.S. Bacchi, J.C.M. Oliviera, P.C.O. Trivelin, L.C. Timm, T.T. Tominaga, V. Correchel, F.A.M. Cássaro, L.F. Pires, and J.R. Macedo. 2003. Management effects on nitrogen recovery in a sugarcane crop grown in Brazil. Geoderma 116(8): 235–248.
Bologna-Campbell, I. 2007. Balanço de nitrogênio e enxofre no sistema solo-cana-de-açúcar no ciclo de cana-planta. PhD Thesis, soils and plant nutrition—Escola Superior de Agricultura ‘Luiz de Queiroz’, University of Sao Paulo, Piracicaba.
Chapman, L.S., M.B.C. Haysom, and P.G. Saffigna. 1992. N cycling in cane fields from 15N labelled trash and residual fertiliser. Proceedings of the Australian Society of Sugarcane Technologists 14: 84–89.
Faroni, C.E., P.C.O. Trivelin, P.H. Silva, I.R. Bologna, A.C. Vitti, and H.C.J. Franco. 2007. Marcação da fitomassa de cana-de-açúcar com aplicação de solução de uréia marcada com 15N. Pesquisa Agropecuária Brasileira 42: 01–07.
Franco, H.C.J. 2008. Eficiência agronômica da adubação nitrogenada de cana-planta. PhD Thesis, soils and plant nutrition—Escola Superior de Agricultura ‘Luiz de Queiroz’, University of Sao Paulo, Piracicaba.
Gava, G.J.C., P.C.O. Trivelin, A.C. Vitti, and M.W. Oliveira. 2005. Urea and sugarcane straw nitrogen balance in a soil-sugarcane crop system. Pesquisa Agropecuária Brasileira 40(7): 689–695.
Janzen, H.H., and R.M.N. Kucey. 1988. C, N, and S mineralisation of crop residues as influenced by crop species and nutrient regime. Plant and Soil 106: 35–41.
Korndörfer, G.H., M.R. Valle, M. Martins, and P.C.O. Trivelin. 1997. Aproveitamento do nitrogênio da uréia pela cana planta. Revista Brasileira de Ciência do Solo 21: 23–26.
Meier, E.A., P.J. Thorburn, M.K. Wegener, and K.E. Basford. 2006. The availability of nitrogen from sugarcane trash on contrasting soils in the wet tropics of north Queensland. Nutrient Cycling in Agroecosystems 75: 101–114.
Ng Kee Kwong, K.F., J. Deville, P.C. Cavalot, and V. Riviere. 1987. Value of cane trash in nitrogen nutrition of sugarcane. Plant and Soil 102: 79–83.
Oliveira, M.W., P.C.O. Trivelin, G. Kingston, M.H.P. Barbosa, and A.C. Vitti. 2002. Decomposition and release of nutrients from sugarcane trash in two agricultural environments in Brazil. Proceedings of the Australian Society of Sugarcane Technologists 24: 40.
Trivelin, P.C.O., W.A.R. Lara Cabezas, R.L. Victoria, and K. Reichardt. 1994. Evaluation of a 15N plot design for estimating plant recovery of fertiliser nitrogen applied to sugar cane. Scientia Agricola, Piracicaba 51(2): 226–234.
Trivelin, P.C.O., J.C.S. Rodrigues, and R.L. Victoria. 1996. Utilização por soqueira de cana-de-açúcar de início de safra do nitrogênio da aquamônia-15N e uréia-15N aplicado ao solo em complemento à vinhaça. Pesquisa Agropecuária Brasileira 31(2): 89–99.
Yank, M.S. 2009. Global sustainable biofuels: The role of sugarcane ethanol. União da indústria Canavieira, Available at http://english.unica.com.br/multimedia/apresentacao.
Wood, A.W. 1991. Management of crop residues following green harvesting of sugarcane in north Queensland. Soil and Tillage Research 20: 69–85.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fortes, C., Trivelin, P.C.O., Vitti, A.C. et al. Recovery of Nitrogen (15N) by Sugarcane from Previous Crop Residues and Urea Fertilisation Under a Minimum Tillage System. Sugar Tech 13, 42–46 (2011). https://doi.org/10.1007/s12355-011-0074-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-011-0074-4