Abstract
Background
Appropriate use criteria (AUC) enhance application of cardiovascular imaging techniques but have been applied in limited settings, primarily in common cardiovascular disease processes. There are several complex systemic diseases with cardiovascular implications and special populations with unique cardiovascular considerations that could benefit from appropriateness analysis. Moreover, the high medical complexity of these topics indicate they would benefit from high-yield expert consensus recommendations of the available imaging options. The ASNC Imaging Indications (ASNC-I2) Series will provide a concise overview of relevant disease processes and their multimodality evaluation and will provide consensus clinical indications, diagnostic criteria, and clinical algorithms with representative clinical cases.
Methods
For each ASNC-I2 document, a diverse writing group and rating panel will be composed of experts from societies pertinent to the topic, including relevant imaging societies and clinical societies that manage the disease under evaluation. The rating panel will follow robust modified Delphi methodology and commonly-accepted appropriateness methods to create consensus diagnostic criteria, clinical algorithms, and clinical indications that they will then rate with level of agreement recorded. The clinical and imaging experts will provide concise, high-yield clinical summaries of the disease process, the non-imaging evaluation, and multimodality imaging. Relevant cases will be provided highlighting application of the diagnostic criteria and clinical algorithms.
Conclusion
The ASNC Imaging Indications (ASNC-I2) Series will complement the diverse portfolio of documents from ASNC. It will use a multisocietal approach with robust appropriateness methodology to guide use of radionuclide imaging in the multimodality imaging context for the cardiovascular care of patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The American Society of Nuclear Cardiology (ASNC) is initiating a new series of documents that will be complementary to its robust portfolio of Guidelines and Information Statements. This series will be entitled “ASNC Imaging Indications (ASNC-I2)”. Authors of these documents will use multisocietal consensus and robust modified Delphi methodology to create consensus diagnostic criteria and indications for radionuclide imaging in the context of multimodality imaging to address high-yield and clinically-challenging topics within nuclear cardiology. The rationale, proposed content, and methodology for this new series are provided in this document.
Series Rationale
The ASNC-I2 Series will fill an important gap and serve as a unique educational resource and practice tool in the context of existing guidelines, practice points, and appropriate use (AUC) documents that will be of high clinical value to ASNC members and other interested parties. The field has greatly benefited from AUC documents issued by the American College of Cardiology (ACC), American College of Radiology (ACR), and others.1,2 However, ASNC believes there are several areas related to cardiac radionuclide imaging that have not yet been adequately covered that would benefit from appropriateness analysis. Existing AUC documents focus on major cardiovascular conditions. However, as medical knowledge expands, it is increasingly clear that there are a number of systemic diseases, including inflammatory, infiltrative, and infectious conditions, in which the cardiovascular system is significantly involved but is not necessarily the sole affected organ system; imaging considerations in these disorders have not yet been adequately assessed by existing AUC documents. In addition, there are special populations such as geriatric, pediatric, and bariatric patients with unique imaging considerations that would benefit from focused appropriateness evaluation. Moreover, the high medical complexity of these systemic diseases and special populations indicate these topics would benefit from added clinical focus in the “ASNC-I2 Series”. Use of a multidisciplinary, multisocietal approach involving relevant clinical societies will capture the range of expertise needed to adequately address the topic and focus on clinically relevant indications. These partnerships will be useful to all stakeholders, including practitioners, patients, and payors.
Cardiovascular imaging is complex and multimodal in these systemic conditions due to involvement of multiple aspects of the heart and vascular system. The clinical questions addressed with imaging are often multi-faceted and creative. Collaboration between experts in multiple imaging modalities and the clinicians treating and diagnosing these conditions is essential. The ASNC-I2 Series will foster this collaboration through involvement of the appropriate clinical societies and those specializing in multiple types of cardiovascular imaging. Education through high-yield imaging summaries, consensus diagnostic criteria, and appropriateness rating of radionuclide imaging indications will facilitate application of radionuclide imaging in an appropriate clinical and multimodality context by ASNC members and other interested parties.
Existing AUC documents are robust in methodology and validated. However, current topics that have been addressed are those that are common in cardiovascular clinical practice. Emerging diseases, less common disorders with systemic manifestations, and the unique diagnostic and treatment considerations of special populations in cardiovascular conditions have not been addressed or receive less attention. The ASNC-I2 Series will address these understudied topics incorporating expertise beyond cardiology and cardiovascular imaging.
The clinical and imaging complexity of studied topics mandates a standardized imaging approach that is applied by cardiovascular specialists and other clinical experts addressing these systemic diseases. The ASNC-I2 Series will streamline a uniform approach and common criteria across imaging and clinical societies and facilitate shared diagnostic criteria and an algorithmic approach that appropriately incorporates radionuclide imaging into multimodality imaging paradigms.
Topics will be identified by the ASNC Committee on Guidelines and Scientific Statements or proposed by members or other organizations. They will cover areas of clinical need, particularly in emerging and systemic diseases requiring multimodality imaging evaluation and where there are gaps in the literature and an absence of guidelines. Topics that are selected will be developed using the methodology described below.
Proposed Document Content
Documents in the ASNC-I2 Series will follow a standard structure as shown in Table 1. These sections will be authored by the appropriate content experts. A disease overview will be provided first; this summary is particularly important as many diseases that may be covered in the ASNC-I2 Series are multifaceted systemic disorders. Brief sections on clinical and biomarker criteria for diagnosing cardiovascular involvement will constitute a section on the non-imaging evaluation of cardiac involvement; the cardiovascular focus is particularly important given the complex nature of these systemic diseases.
A section on the multimodality imaging of the disease under assessment will be written by the imaging experts. Each author or multiple authors from the relevant societies will create a concise one-page summary of the use of their imaging modality in the studied condition, giving a high-level summary in the multimodality context, and including a few takeaway key points.
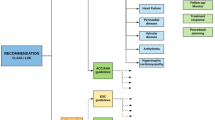
Following the imaging summary section will be a segment incorporating the consensus diagnostic criteria and rated indications for radionuclide imaging in the multimodality context for the assessment of the disease in question. This section will include tables with the criteria and ratings similar to the examples in Tables 2 and 3. A figure with a consensus algorithmic approach to assessment of cardiac involvement of the assessed disease will be provided, similar to the example in Fig. 1. The approach to construction of this section will be based off the methodology in this document and will be provided in an appendix to maintain the concise nature of the documents in the ASNC-I2 Series.
Consensus diagnostic algorithm example. This example from the AHA Expert Consensus Recommendations for the Suspicion and Diagnosis of Transthyretin Cardiac Amyloidosis shows a potential consensus algorithmic approach to the assessment of cardiac involvement of the disease being assessed.18AL, light-chain amyloidosis; ATTR, transthyretin amyloidosis; ATTRm, mutant transthyretin amyloidosis; ATTRwt, wild-type transthyretin amyloidosis; ECG, electrocardiogram; Echo, echocardiogram; MRI, magnetic resonance imaging; MGUS, monoclonal gammopathy of uncertain significance
The clinical expert portion will be followed by a series of case examples illustrating application of the consensus diagnostic criteria and imaging indications to guide the use of radionuclide imaging in the multimodality context to evaluate the disease process under evaluation. The document will end with a concise summary of key imaging and diagnostic concepts and future directions highlighting need to address identified gaps in the literature, imaging indications with insufficient evidence to guide clinical decision-making, and areas of disagreement for future research.
Methodology
The clinical expert section of the ASNC-I2 Series will adhere to a rigorous methodology that incorporates elements of the original RAND/UCLA Appropriateness Method and refinements made in prior radionuclide imaging and other cardiovascular appropriateness documents.3,4,5,6,7,8 An overview of the process flow is provided in Figure 2. The writing group and clinical expert rating panel will be carefully assembled from recommended members of participating imaging and clinical societies. A comprehensive literature review will be performed and considered during the rating process. The clinical expert review panel will create expert consensus diagnostic criteria based on clinical, laboratory, histologic, and imaging features. They will derive consensus clinical indications and then rate them using a commonly-accepted appropriateness scale over multiple rounds through a modified Delphi technique with level of agreement assessment. An open comment period will be included to incorporate feedback from ASNC members and key stakeholders.
Methods process flow. The progression from topic generation to the final document is depicted. The group performing each task and key points are provided for each step. *The full writing group reviews the developed clinical indications, diagnostic criteria, and clinical algorithm. ASNC, American Society of Nuclear Cardiology; EC, Executive Committee; CoGSS, Committee on Guidelines and Scientific Statements
Multisocietal Writing Group Composition
The chair and co-chair of each document will be selected by the ASNC Executive Council and Committee on Guidelines and Scientific Statements and together will have expertise in both the relevant disease and the rating process. They will develop the topic, manage the writing process, and will oversee the imaging indication rating and diagnostic critera development. They will assemble a multisocietal writing group incorporating both imaging experts and a clinical expert rating panel. Nominations will be sought from a broad group of partnering organizations that concentrate on the pertinent imaging modalities or specialize in the clinical care of patients with the relevant disease process. Final members will be selected by the chair and co-chair in consultation with the ASNC Executive Council. A diverse writing group with broad representation and no relevant high-risk conflicts of interest will be sought. Disclosures will be provided for all writing group members.
The entire writing group will review and approve clinical indications, review literature summaries, and review and approve of the final document. The imaging experts will construct the high-yield concise summaries of each pertinent imaging modality.
As outlined in Figure 3, the clinical expert rating panel will be assembled incorporating recommendations from the RAND-UCLA Appropriateness Manual.3 The group will include a substantial representation of clinicians who care for the patient population under study but who are not imaging specialists to foster balanced, equitable ratings and avoid bias toward a single imaging modality. Imagers with expertise in radionuclide imaging will be included to provide imaging and technical expertise. Up to one additional member proposed by each relevant imaging society may be included to inform the panel with balanced, high-level multimodality imaging expertise. The panel will be multi-disciplinary, incorporating experts in diverse fields based on the disease process under consideration, including surgical specialists and other disciplines outside of cardiology (such as infectious disease, rheumatology, and pulmonology) who order the pertinent imaging studies. They will be nominated by the included clinical and imaging societies and will be recruited internationally from diverse geographical locations. Members will ideally work in varied practice settings, but some topics may have sufficient clinical complexity that all will practice in academic settings. The ratings panel will generally include 9-15 clinical experts as recommended by the RAND/UCLA manual.3 This size permits sufficient diversity of expertise but ensures that all have a chance to participate.
Literature Review
A comprehensive literature review will be performed by members of the writing group to synthesize the latest available scientific evidence pertinent to the topic being addressed. This process ensures that all writing group members have access to the same body of evidence and assists the expert rating panel in their process. The review serves as a resource to resolve disagreements that arise during the panel discussion. The review will be undertaken using systematic review methodology but may be less strict in inclusion criteria to incorporate all available evidence.3,9 The specific search methodology and criteria for inclusion/exclusion will be published. When there is enough evidence available, evidence tables will be created to allow key parameters from multiple studies to be compared easily. Example headings are provided in Table 4. Particular note will be made of areas in which studies yield contradictory or uncertain results. Guidelines will be incorporated wherever possible. Some of these reviews will be considered for publication as a separate systematic review document in order to preserve the concise nature of documents in this ASNC-I2 series.
Development of Diagnostic Criteria for Cardiac Involvement
For topics in which there are insufficient existing diagnostic criteria to establish cardiac involvement, expert consensus diagnostic criteria will be developed that incorporate clinical, histologic, biomarker, and imaging features. Efforts will be made to publish a clear and concise algorithmic approach that integrates multimodality imaging wherever possible. Examples of such an approach in cardiac amyloidosis are shown in Figure 1 and Table 210. These criteria will represent expert consensus recommendations, synthesizing available evidence and expert opinion where insufficient evidence exists. They will incorporate systemic diagnosis but will focus on cardiac involvement. Once the rating panel has created draft diagnostic criteria and clinical algorithms, these will be reviewed by the entire writing group including the representatives from participating imaging societies to ensure completeness and provide final feedback.
Clinical Indication Derivation
The clinical expert rating panel will undertake a structured approach to derive clinical radionuclide imaging indications for rating. They will create overarching “clinical scenarios”, broad categories representing key areas of clinical care in which radionuclide imaging might be considered, such as asymptomatic screening, patients with certain presenting symptoms, initial evaluation or follow-up testing for a change in clinical status or after a defined period of time. Within these categories, clinical indications will be created that address specific personal characteristics, comorbidities, key test results, or other nuances not covered in the overlying category. These indications will encompass all situations in which radionuclide imaging may be considered in the context of multimodality imaging, both appropriate and rarely appropriate, including diagnosis, risk stratification, and patient management. The writing group will endeavor to create a list that is comprehensive but manageable with individual indications mutually exclusive and homogeneous per RAND/UCLA recommendations.3 Draft clinical imaging indications will be reviewed by the entire writing group to ensure completeness and provide final feedback.
After clinical indications are finalized, the rating panel will review them using the modified Delphi process. For each indication, the rating panel will be asked to rate the appropriateness of each imaging method assessed in the document.
Modified Delphi Technique
The rating process will be performed using a modified Delphi technique as used in multiple appropriateness documents.4,5,8,10,11 The chair or co-chair of the document will serve as the moderator and liaison to the writing group as detailed in the RAND/UCLA methods.3 He or she will preferentially have familiarity with the ASNC-I2 Series methodology or other appropriateness efforts and will be free of significant relationships with industry and otherwise unbiased relative to the topics under consideration. He or she will establish the goals and procedural rules and facilitate the rating process and meeting as shown in Figure 2 but will not rate the indications. This process will start with review of the assumptions and definitions as detailed below and confirm that the literature was reviewed. Diagnostic criteria and imaging indications will be developed as described above. There will be a strong directive to achieve consensus for the majority of elements.
The imaging indications rating will occur in multiple rounds. The first round will be an individual rating performed independently using the scoring system described in the next section. Scores will be tabulated and a second round will take place in person or over video conferencing. A quorum of at least 70% will be necessary; extensive care will be taken to make sure all can participate. The rating panel members will be given score distributions, the median, and their own score with all ratings de-identified. The participants will then share their perspectives on the use of imaging for different clinical indications. The moderator will guide discussion to the areas with the most variance in initial rating. A second round of ratings will then take place immediately after the meeting to incorporate knowledge of how other panel members initially rated along with the meeting dialogue. Throughout the rating process, clinical indications will be modified as needed or if variables do not appear to differentiate ratings. A third round will be considered if significant dispersion of scores remains.
The level of agreement will be measured using the BIOMED Concerted Action on Appropriateness methods as used in prior appropriateness documents.4,5,8,10,11 For 9-10, 11-13, and 14-15 member panels, disagreement will require ≥3, ≥4, and ≥5 members, respectively, to rate in a different appropriateness category, respectively. Disagreement in the rating of an imaging modality for a particular clinical indication will be categorized as “May Be Appropriate”. Extensive discussion will be undertaken not to force consensus but to make sure that discrepant ratings are due to real clinical disagreement rather than rating fatigue or a misunderstanding.3
Clinical Indication Rating
The following ratings of appropriateness will be used, as used in Part 2 of the ASNC Multisocietal Consensus Statement on Cardiac Amyloidosis and adapted from other documents (such as from the American College of Cardiology) that address appropriate utilization4,11,12,13
An appropriate imaging study is one in which the expected incremental information, combined with clinical judgement, exceeds the expected negative consequences by a sufficiently wide margin for a specific indication that the procedure is generally considered acceptable care and a reasonable approach for the indication.
Rating panels will use a linear scale of increasing appropriateness from 1 to 9. These scores will be divided into three categories: Appropriate (A), May Be Appropriate (M), or Rarely Appropriate (R) in accordance with published appropriate use methodology and as used in prior appropriate use documents.5,8,14
Appropriate (Score 7-9)
An indication scored in the appropriate range (score 7-9) signifies that the imaging procedure is judged to be an appropriate option for management of patients in the population addressed in the document for this particular clinical imaging indication because the benefits generally outweight the risks. The imaging procedure should be considered an effective option for individual care plans but may not always be necessary, preferred, or chosen based on physician judgement and patient-specific preferences. The procedure is judged to be generally acceptable and is generally reasonable for the assessed clinical indication.
May Be Appropriate (Score 4-6)
An indication scored in the 4-6 range signifies that the imaging procedure assessed is at times an appropriate option for management of patients in the population addressed in the document for this particular indication due to variable evidence or agreement regarding the risk-benefit ratio, potential benefit based on practice experience in the absence of evidence, and/or variability in the population. The effectiveness of this imaging procedure for a patient’s individual care plan must be determined by the patient’s physician in consultation with the patient based on additional clinical variables and judgement and patient preferences. The procedure may be acceptable and may be reasonable for the assessed clinical indication. Of note, a May Be Appropriate categorization may also indicate that further research and/or patient information is needed to classify the indication definitively.
Rarely Appropriate (Score 1-3)
An indication scored in the 1-3 range signifies that the imaging procedure is judged to rarely be an appropriate option for management of patients in the population addressed in the document for this particular clinical indication due to a lack of a clear benefit/risk advantage. Physician judgement and patient-specific preferences should be considered, but the imaging procedure should rarely be exercised as an effective option in individual care plans for this indication. Moreover, exceptions should have documentation of the clinical reasons for proceeding with this care option. The procedure is not generally acceptable and is not generally reasonable for the assessed clinical indication.
The division of scores into these three categories is somewhat arbitrary and raters will be instructed to consider the numeric range as a continuum. Acknowledging variability in patient factors, local practice patterns, and a lack of data on use of imaging across all clinical situations, panel members will be asked to independently rate the appropriateness of using each imaging modality for the broad clinical scenario and individual clinical indication based on the best available evidence, using guidelines and key references wherever possible.8 The results of this rating will be provided in a table similar to the example in Table 3.
Assumptions and Definitions
The following list of assumptions are adapted from prior appropriate use documents and methodology recommendations.4,5,8,14,15 They will be generally followed with adjustment as needed for the specific topic assessed and will be communicated clearly to the expert rating panel members prior to their first deliberations. These assumptions minimize issues such as variability in competence, test quality, or other concerns rather than purely the clinical indication in the rating process.
-
1.
All imaging studies will be assumed to be available locally and to be performed in accredited imaging laboratories in accordance with published criteria for quality cardiac diagnostic testing using state-of-the-art, certified imaging equipment.
-
2.
All imaging will be assumed to be performed according to the standard of care as defined by the peer-reviewed medical literature.
-
3.
All interpreting physicians will be assumed to be qualified and certified to supervise the imaging procedure and appropriately report the findings.
-
4.
In clinical scenarios, the clinical status listed will be assumed to be valid as stated (asymptomatic patients are truly asymptomatic) and no extenuating circumstances will be taken into consideration (patient willingness to receive treatment, clinical stability) unless specifically noted.
-
5.
Appropriateness will be rated independently of the appropriateness of any prior diagnostic imaging that may have been performed in the clinical indication/scenario.
-
6.
All patients will be assumed to be receiving optimal contemporary guideline-directed medical therapy and cardiovascular risk-factor modification conforming to current standards of care unless specifically noted.
-
7.
Imaging indicated for surveillance to assess disease progression or response to therapy is assumed to be performed solely because the indicated time period elapsed rather than due to any change in clinical circumstances.
-
8.
Cost of the imaging procedures will not be considered in accordance with recommended appropriateness scoring methods.5,8 These analyses focus purely on whether benefits outweigh risks and do not imply that the imaging procedure must be done for all patients. Cost is recognized to be an important issue from a coverage policy and payment perspective but is beyond the scope of these analyses. Moreover, expert physician appropriateness ratings have been shown to agree well with cost-effectiveness models.16,17
A separate Definitions section will include clear and concise definitions for commonly-used terms and scenarios. These are provided for clarity and to minimize geographical differences in definition that may exist. For instance, clinical observations such as “typical angina” or “high-risk for infection” could be considered differently between parts of Europe and the USA. Explicit definitions also assist with harmonization and application across documents. Definitions will rely on prior published guidelines and key papers wherever possible.
Summary
Appropriate Use documents have been beneficial in major cardiovascular conditions but have not been undertaken in less common and complex diseases with systemic and cardiovascular manifestations. Morover, there are additional specific understudied populations that could benefit from appropriateness analysis. The ASNC Imaging Indications (ASNC-I2) Series seeks to facilitate the application of radionuclide imaging in an appropriate clinical and multimodality context using a multidisciplinary, multisocietal approach. The ASNC-I2 series will assemble a diverse, expert consensus panel, and writing committee from stakeholder imaging and clinical societies and use robust methodology and standard structure to develop consensus diagnostic criteria, rate the appropriateness of radionuclide imaging, and address the high medical complexity in these disorders with high-yield clinical summaries and cases. This algorithmic approach will facilitate incorporation of radionuclide imaging into multimodality imaging paradigms.
New Knowledge Gained
This manuscript provides the rationale and detailed methodology for a novel assessment of indications for radionuclide imaging in the multimodality context in complex systemic diseases and special populations. Readers will understand the background, design, implementation, and optimal application of this valuable series addition to the ASNC diverse portfolio of Guidelines and Information Statements.
References
Ronan G, Wolk MJ, Bailey SR, Doherty JU, Douglas PS, Hendel RC, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Nucl Cardiol 2014;21(1):192-220.
Expert Panel on Cardiac Imaging, Batlle JC, Kirsch J, Bolen MA, Bandettini WP, Brown RKJ, et al. ACR Appropriateness Criteria® Chest pain-possible acute coronary syndrome. J Am Coll Radiol 2020;17(5S):S55-69.
Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR, Lazaro P. The RAND/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: RAND; 2001.
Wolk MJ, Bailey SR, Doherty JU, Douglas PS, Hendel RC, Kramer CM, etal. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol 2014;63(4):380-406.
Hendel RC, Patel MR, Allen JM, Min JK, Shaw LJ, Wolk MJ, et al. Appropriate use of cardiovascular technology: 2013 ACCF appropriate use criteria methodology update: A report of the American College of Cardiology Foundation appropriate use criteria task force. J Am Coll Cardiol 2013;61(12):1305-17.
Hendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 Appropriate Use Criteria for Cardiac Radionuclide Imaging: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. J Am Coll Cardiol 2009;53(23):2201-29.
Patel MR, Bailey SR, Bonow RO, Chambers CE, Chan PS, Dehmer GJ, et al. ACCF/SCAI/AATS/AHA/ASE/ASNC/HFSA/HRS/SCCM/SCCT/SCMR/STS 2012 appropriate use criteria for diagnostic catheterization: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons.J Am Coll Cardiol 2012;59(22):1995-2027.
Patel MR, Spertus JA, Brindis RG, Hendel RC, Douglas PS, Peterson ED, et al. ACCF proposed method for evaluating the appropriateness of cardiovascular imaging. J Am Coll Cardiol 2005;46(8):1606-13.
American Heart Association. Methodology Manual and Policies from the ACCF/AHA Task Force on Practice Guidelines. American Heart Association; 2010.
Dorbala S, Ando Y, Bokhari S, Dispenzieri A, Falk RH, Ferrari VA, et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI Expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 2 of 2-diagnostic criteria and appropriate utilization. J Nucl Cardiol 2019;26(6):2065-123.
Aortic Stenosis Writing Group, Bonow RO, Brown AS, Gillam LD, Kapadia SR, Kavinsky CJ, et al. ACC/AATS/AHA/ASE/EACTS/HVS/SCA/SCAI/SCCT/SCMR/STS 2017 Appropriate Use Criteria for the Treatment of Patients With Severe Aortic Stenosis: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, European Association for Cardio-Thoracic Surgery, Heart Valve Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Soc Echocardiogr 2018;31(2):117-47.
Dorbala S, Ando Y, Bokhari S, Dispenzieri A, Falk RH, Ferrari VA, et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 1 of 2-evidence base and standardized methods of imaging. J Nucl Cardiol 2019;26(6):2065-123.
Brook RH, Chassin MR, Fink A, Solomon DH, Kosecoff J, Park RE. A method for the detailed assessment of the appropriateness of medical technologies. Int J Technol Assess Health Care 1986;2(1):53-63.
Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 Appropriate Use Criteria for Multimodality Imaging in Valvular Heart Disease: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol 2017;70(13):1647-72.
Patel MR, White RD, Abbara S, Bluemke DA, Herfkens RJ, Picard M, et al. 2013 ACCF/ACR/ASE/ASNC/SCCT/SCMR appropriate utilization of cardiovascular imaging in heart failure: a joint report of the American College of Radiology Appropriateness Criteria Committee and the American College of Cardiology Foundation Appropriate Use Criteria Task Force. J Am Coll Cardiol 2013;61(21):2207-31.
Bernstein SJ, Hofer TP, Meijler AP, Rigter H. Setting standards for effectiveness: A comparison of expert panels and decision analysis. Int J Qual Health Care 1997;9(4):255-63.
Kuntz KM, Tsevat J, Weinstein MC, Goldman L. Expert panel vs decision-analysis recommendations for postdischarge coronary angiography after myocardial infarction. JAMA 1999;282(23):2246-51.
Maurer MS, Bokhari S, Damy T, Dorbala S, Drachman BM, Fontana M, et al. Expert Consensus Recommendations for the Suspicion and Diagnosis of Transthyretin Cardiac Amyloidosis. Circ Heart Fail 2019;12(9):e006075.
Kawel N, Turkbey EB, Carr JJ, Eng J, Gomes AS, Hundley WG, et al. Normal left ventricular myocardial thickness for middle-aged and older subjects with steady-state free precession cardiac magnetic resonance: the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging 2012;5(4):500-8.
Disclosures
Jamieson Bourque has received consulting honoraria from Pfizer and GE Healthcare and has an investment interest in Locus Health. Andrew Einsten has received research grants from GE Healthcare and Roche Medical Systems. He has been a speaker for Ionetix.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Funding
Sharmila Dorbala reports research grant support from GE Healthcare, Pfizer, and Attralus. She has received consulting honoraria from GE Healthcare, Pfizer, Attralus, and Ionetix. There are no funding sources to disclose.
Rights and permissions
About this article
Cite this article
Bourque, J.M., Einstein, A.J. & Dorbala, S. ASNC Imaging Indications (ASNC-I2): Multisocietal indications for radionuclide imaging in the multimodality context—Series rationale and methodology. J. Nucl. Cardiol. 29, 2667–2678 (2022). https://doi.org/10.1007/s12350-021-02800-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-021-02800-w