Abstract
Background
Transcatheter aortic valve replacement (TAVR) can rapidly improve cardiac sympathetic nervous function (CSNF) within 2 weeks in patients with aortic stenosis (AS). However, whether such short-term improvements will be sustained thereafter remains unclear.
Methods
Patients with severe AS who underwent TAVR between October 2017 and June 2019 were enrolled in this single-center, prospective, observational study. 123I-meta-iodobenzylguanidine imaging was performed at baseline, within 2 weeks after TAVR, and at 6 to 12 months post-TAVR to evaluate the heart–mediastinum ratio (H/M) and washout rate.
Results
Of 183 consecutive patients, 75 (19 men; median age: 86 years) were evaluated. The late H/M significantly improved within 2 weeks after TAVR (P = .041) and further improved over 6 to 12 months after TAVR (P = .041). Multivariate analysis revealed that the baseline mean aortic valve pressure gradient (mPG) was an independent predictor of mid-term improvement in the late H/M (> 0.1) (P = .037). Patients with a high baseline mPG (≥ 58 mmHg) exhibited a significantly greater increase in the late H/M than those with a low baseline mPG (< 42 mmHg) (0.24 vs 0.01; P = .029).
Conclusion
CSNF demonstrated sustained improvement from within 2 weeks after TAVR until 6 to 12 months later. Such improvement was related to baseline hemodynamic AS severity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aortic stenosis (AS) is one of the leading causes of cardiac morbidity and mortality among elderly patients.1 While surgical aortic valve replacement is considered as the established treatment for severe symptomatic AS, transcatheter aortic valve replacement (TAVR) is another important treatment option, particularly for elderly adults or patients with prohibitive surgical risk.2
Patients with AS exhibit impairments in cardiac sympathetic nervous function (CSNF), and several studies have indicated improvements in CSNF consequent to TAVR.3,4 Our previous research has demonstrated that CSNF, as assessed by 123I-meta-iodobenzylguanidine (MIBG) imaging, significantly improves within a short duration (i.e., < 2 weeks) after TAVR.4 However, whether such short-term improvement in CSNF will be sustained thereafter remains unclear.
Functional improvement in cardiac sympathetic nerve endings is generally thought to require substantial time (i.e., more than a few months). Previous studies on the effects of beta-blockers or angiotensin-converting enzyme inhibitors (ACEIs) evaluated changes in MIBG parameters, including the heart–mediastinum ratio (H/M) and washout rate (WR), over a period of 6 to 12 months.5,6 To adequately evaluate the effects of TAVR on CSNF, it is necessary to observe not only the short-term as well as long-term changes in CSNF indices; hence, it is important to examine whether TAVR-dependent early improvements in CSNF are transitory or sustainable. To address this, the present study aimed to investigate the mid-term effects of TAVR on CSNF using serial cardiac MIBG imaging.
Methods
Study Population
This single-center, prospective, observational study enrolled consecutive patients who were scheduled to undergo TAVR for severe AS between October 2017 and June 2019. Severe AS was defined according to the current American College of Cardiology/American Heart Association guidelines.2 Cardiac MIBG imaging was scheduled at baseline, within 2 weeks after TAVR, and at 6 to 12 months after TAVR to evaluate the H/M and WR. All baseline and procedural data, including laboratory and echocardiographic findings, were prospectively collected at the same time as MIBG scintigraphy. Echocardiographic measurements during the study period were conducted in accordance with the recommendations of the American Society of Echocardiography.
The exclusion criteria for the initial MIBG evaluation of patients were as follows: (a) active cancer and/or Parkinson’s disease; (b) incapability to perform MIBG imaging due to severe dementia and/or mental disorder; (c) ongoing therapy with medications known to interact with MIBG (e.g., labetalol, reserpine, tricyclic antidepressants, sympathomimetic amines, and serotonin–norepinephrine reuptake inhibitors)7; (d) utilization of trans-apical and trans-aortic TAVR or of valve-in-valve TAVR for structural valve deterioration in bioprosthetic aortic valves; (e) unstable preprocedural conditions (e.g., cardiogenic shock) treated with intravenous catecholamine administration; (f) insufficient MIBG imaging quality; and (g) refusal to provide consent for study participation. Patients with serious procedure-related complications (e.g., left ventricular perforation, annular rupture, coronary obstruction, and cardiac tamponade) were excluded from the subsequent postoperative MIBG evaluation. Furthermore, patients treated with newly added beta-blockers, ACEIs, and/or angiotensin II receptor blockers after TAVR were excluded from the final MIBG evaluation.
All procedures were performed in accordance with the ethical standards of the institutional research committee of Kyoto Prefectural University of Medicine (ERB-C-1081-3) and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all participants. The corresponding author (K.Z.) had full access to all data in the study and takes responsibility for data integrity as well as data analyses.
Cardiac MIBG Imaging
The first MIBG imaging was performed at the time of admission for TAVR, which was a few days prior to TAVR in most cases. The second MIBG imaging was carried out at > 2 days post-TAVR and when patients’ conditions were stable in order to avoid the influence of perioperative transfusion or infection. In our institution, all patients were routinely followed up at 1, 6, and 12 months after TAVR. The third MIBG imaging was performed at the 6-month follow-up visit; depending on patients’ availability, the third MIBG imaging was also permitted to be conducted at the 12-month follow-up visit.
MyoMIBG (FUJIFILM RI Pharma Co. Ltd., Tokyo, Japan) was intravenously administered at a dose of 111 MBq. Anterior planar images were acquired at 15 min (early image) and 240 min (late image) after MIBG injection using a large-field-of-view gamma camera (Discovery NM/CT 670; GE Healthcare, Waukesha, WI, USA) equipped with a medium-energy collimator. To obtain semi-quantitative parameters for tracer distribution, MIBG imaging analysis was performed based on the region of interest using smart MIBG software (FUJIFILM RI Pharma Co. Ltd., Tokyo, Japan), which was developed to semi-automatically determine the H/M and correct the H/M to standard medium-energy collimator conditions.8 The H/M was determined by measuring the average counts in each region, whereas the WR from the heart was calculated as the time decay-corrected difference between early and late images. Experienced radiology technicians who were unaware of patients’ information conducted all analyses.
TAVR Procedure
The TAVR procedure was performed using the transfemoral or trans-subclavian approach under general anesthesia or conscious sedation with local anesthesia. Either the Edwards SAPIEN 3 (Edwards Lifesciences, Irvine, CA, USA) or Medtronic CoreValve Evolut R/Pro (Medtronic, Minneapolis, MN, USA) was used as a prosthesis. The multidisciplinary heart team determined the indication, valve type, and/or vascular access for TAVR.
Statistical Analysis
Normality of distributions was assessed using the Shapiro–Wilk test. Categorical variables are presented as counts and percentages, whereas continuous variables are expressed as median (25th to 75th percentile) given that few variables followed a normal distribution. With respect to normally distributed variables, the median and mean values were nearly identical. Depending on variable distribution, Friedman’s test or repeated-measures analysis of variance was performed for the comparison of laboratory, echocardiographic, and MIBG findings at the three time points. In case of significance, pairwise post hoc tests with Holm correction were performed. Multiple regression analysis was conducted to determine the predictors of improvement in the late H/M at 6 to 12 months after TAVR compared to baseline values. Following our previously established methodology, the cut-off values for the improvement in the H/M and WR were set at 0.1 and 3%, respectively, considering measurement reliability and clinical significance.9,10,11 The following variables were included as possible confounders: age, sex, body mass index, New York Heart Association class ≥ III, Canadian Study of Health and Aging Clinical Frailty Scale ≥ 5, Society of Thoracic Surgeons surgical mortality risk score, estimated glomerular filtration rate, post-TAVR new-onset complete left bundle branch block, post-TAVR pacemaker implantation, baseline brain natriuretic peptide, baseline left ventricular end-diastolic volume, baseline left ventricular ejection fraction (LVEF), baseline aortic valve area (AVA), baseline ratio of early diastolic left transmitral flow velocity to septal mitral annular velocity, baseline peak velocity (Vmax), baseline mean aortic valve pressure gradient (mPG), and baseline late H/M. Among all variables with P < .2 according to the univariate analysis, clinically important variables with a lower P value were selected as confounders, considering the number of endpoints and multicollinearity. For example, we selected baseline mPG as the variable instead of ΔmPG (6 to 12 months—baseline) in the multivariable model, given that baseline mPG had a lower P value of 0.043 in the univariate analysis, and we considered it to be a more clinically important confounder.
To further investigate the association between the baseline mPG and mid-term improvement in the late H/M, the study participants were retrospectively divided into three groups according to the baseline mPG: the low-gradient (LG) group, which consisted of patients with baseline mPG < 42 mmHg (n = 24); the high-gradient (HG) group, which comprised patients with mPG ≥ 42 mmHg and < 58 mmHg (n = 26); and the very-high-gradient (VHG) group, which included patients with mPG ≥ 58 mmHg (n = 25). The baseline mPG thresholds were set to ensure an even distribution of the number of patients. Subgroup analyses of changes in the late H/M were subsequently performed. To compare the late H/M and its changes among these three groups at three time points (at baseline, within 2 weeks after TAVR, and at 6–12 months after TAVR), one-way analysis of variance or Kruskal–Wallis test was conducted for normally distributed or non-normally distributed variables, respectively. In case of significance, pairwise post hoc tests with Holm correction were performed.
Statistical analyses were performed using R software packages version 3.1.1 (R Development Core Team, Auckland, New Zealand), with the significance level for statistical hypothesis testing set at 0.05 and with the alternative hypothesis being two-sided.
Results
Patient Characteristics
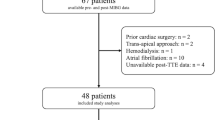
Of 183 consecutive patients who underwent TAVR, 65 were excluded from the initial evaluation, whereas 7 were excluded from post-TAVR evaluation due to periprocedural complications. During the follow-up period, 21 patients who required additional medical treatment, eight patients who were followed up at other hospitals, and seven patients who died before the final MIBG imaging were excluded from the final MIBG evaluation. The final study population comprised 75 patients (Figure 1).
The baseline patient characteristics are summarized in Table 1. The median age of the study population was 86 years; 19 patients (25.3%) were males. A total of 57 patients were classified as having high-gradient severe AS (baseline mPG ≥ 40 mmHg), whereas 18 patients were deemed to have low-gradient severe AS (baseline mPG < 40 mmHg); among these 18 patients, 15 were diagnosed with “paradoxical low-flow low-gradient severe AS” (LVEF ≥ 50%). The median period between TAVR and second MIBG scintigraphy and between TAVR and third MIBG scintigraphy was 6 and 205 days, respectively.
Changes in Laboratory, Echocardiographic, and MIBG Findings After TAVR
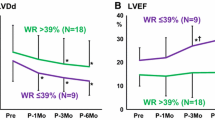
Serial changes in laboratory, echocardiographic, and MIBG findings are summarized in Table 2. Hemodynamic AS parameters, such as Vmax, mPG, and AVA, improved immediately after TAVR and were well maintained until 6 to 12 months post-procedurally. The LVEF increased after TAVR and was sustained after 6 to 12 months.
Changes in MIBG parameters are presented in a boxplot in Figure 2. Late H/M significantly improved within 2 weeks after TAVR (P = .041) and further improved over 6 to 12 months after TAVR (P = .041). WR rapidly improved after TAVR (P = .003) but showed no further improvement over 6 to 12 months (P = .827). Nevertheless, compared to the baseline level, the WR values were still significantly improved at 6 to 12 months after TAVR (P = .003).
The increase in the late H/M at 6 to 12 months post-TAVR [i.e., Δlate H/M (6 to 12 months—baseline)] was significantly greater than that just after TAVR [i.e., Δlate H/M (< 2 weeks—baseline)] (P = .021). In contrast, the decrease in the WR at 6 to 12 months after TAVR [i.e., ΔWR (6 to 12 months—baseline)] was not significantly different from that immediately after TAVR [i.e., ΔWR (< 2 weeks—baseline)] (P = .827). No changes in the early H/M were observed among the three time points.
Baseline mPG and Improvement in Late H/M at 6–12 Months After TAVR
Overall, 43 out of 75 patients showed improvement in the late H/M (> 0.1) at 6–12 months after TAVR. Multivariate analysis revealed that baseline mPG was associated with such mid-term improvement in the late H/M (adjusted odds ratio: 0.035; 95% confidence interval 0.004 to 0.070; P = .037) (Table 3).
The late H/M and associated clinical parameters in the three groups who were categorized according to the baseline mPG (LG group, < 42 mmHg; HG group, ≥ 42 and < 58 mmHg; and VHG group, ≥ 58 mmHg) are presented in Table 4. No significant difference in baseline LVEF was observed among the three groups. In the LG group, 21 (87.5%) out of 24 patients had baseline LVEF ≥ 50%. The increase in late H/M within 2 weeks after TAVR [i.e., Δlate H/M (< 2 weeks—baseline)] was comparable among the three groups, whereas the increase in late H/M at 6 to 12 months after TAVR [i.e., Δlate H/M (6 to 12 months—baseline)] tended to be greater in the HG group than in the LG group and in the VHG group than in the HG group. The VHG group had significantly higher Δlate H/M (6 to 12 months—baseline) than the LG group (P = .029) (Figure 3). The changes in MIBG parameters (early H/M, late H/M, and WR) in the three groups (LG group, HG group, and VHG group) are presented in the Online Resource 1.
Increase in the late H/M at 6–12 months after transcatheter aortic valve replacement [i.e., Δlate H/M (6–12 months—baseline)] in three groups classified according to the baseline mPG. H/M, indicates heart–mediastinum ratio; mPG, mean aortic valve pressure gradient; TAVR, transcatheter aortic valve replacement
Discussion
The present study yielded the following two main findings: (i) CSNF, as denoted by late H/M, demonstrated a sustained improvement from within 2 weeks after TAVR until 6–12 months later, and (ii) higher baseline mPG was an independent predictor of mid-term improvement in late H/M after TAVR. As no studies have evaluated the long-term (> 6 months) effects of TAVR on CSNF, our study could provide physicians with new insights into this field.
Mid-Term Effects of TAVR on CSNF
In our results, late H/M exhibited a sustained improvement from within 2 weeks after TAVR until 6–12 months later, whereas WR displayed a rapid improvement just after TAVR but remained unchanged over the succeeding 6–12 months. Early H/M represents the amount of cardiac sympathetic nervous terminal endings and uptake-1 function, whereas WR represents sympathetic nervous tone12; in comparison, late H/M represents both factors. In general, the recovery of the amount of sympathetic nervous terminal endings and uptake-1 function is expected to require more than a few months, whereas sympathetic nervous tone can rapidly respond to hemodynamic changes and is susceptible to other clinical factors. The present results indicate a gradual trend toward improvement, as demonstrated by the late H/M, and a rapid but non-continuous improvement in the WR, which were thought to be consistent with the theories that have been stated.12
In our study, the absolute value of the change in the late H/M before and after TAVR was not large. However, the change in the late H/M in our study seems comparable with the values reported in previous studies that investigated the effects of medical treatments.5,13 Moreover, even small changes such as these are associated with an improved prognosis.14 Therefore, we believe that the improvement in the late H/M demonstrated in our study is clinically significant.
Predictors of Improvement in Late H/M at 6–12 Months After TAVR
A higher baseline mPG was an independent predictor of mid-term improvement in the late H/M after TAVR; in particular, patients with a very high baseline mPG (≥ 58 mmHg) experienced significantly greater increase in the late H/M than those with a low baseline mPG (< 42 mmHg).
The mPG assessed using Doppler echocardiography is one of the most powerful markers of AS severity in patients with normal cardiac output and preserved LVEF. High mPG is an independent predictor of mortality in patients with severe AS.15 Moreover, patients with “very severe AS,” defined as mPG ≥ 60 mmHg and/or Vmax ≥ 5 m/s, are considered at high risk, and current Japanese guidelines recommend early surgery, even when these patients are asymptomatic.16 In our present study, patients with a very high mPG (≥ 58 mmHg) exhibited a higher degree of improvement in CSNF after TAVR, suggesting that patients with a very high baseline mPG would gain the greatest prognostic benefit from TAVR, considering the association of TAVR-related improvement in the late H/M with post-TAVR prognosis.17
The effects of TAVR on patients with a low mPG should be carefully discussed. In this study, the increase in the late H/M at 6–12 months after TAVR (i.e., Δlate H/M [6 to 12 months—baseline]) was minimal among patients with a low baseline mPG (< 42 mmHg). According to current guidelines,16,18 AS with low mPG, so-called “low-flow low-gradient” (LF-LG) severe AS can be divided into two groups, namely, classical LF-LG AS with LVEF < 50% and paradoxical LF-LG AS with LVEF ≥ 50%. The prognosis of patients with paradoxical LF-LG AS remains controversial, with some studies reporting poor prognosis19,20 and others indicating good prognosis.21,22 In the present study, most patients with a low mPG (< 42 mmHg) were classified as having paradoxical LF-LG AS. Therefore, the present results may represent poor CSNF response after TAVR among patients with paradoxical LF-LG AS, suggesting that patients with paradoxical LF-LG AS may gain benefit from TAVR with respect to symptomatic relief but not in terms of prognostic improvement.
Clinical Implications
The clinical outcomes of TAVR have improved due to device development and technical advancement, and its application has expanded to include younger patients as well as those with lower surgical risk.2 Consequently, simpler, easier, and more accurate prognostic indicators are desired. Furthermore, there is an increased need for the appropriate selection of patients who would most benefit from TAVR. In our previous study, we demonstrated that CSNF assessment using MIBG imaging could provide essential information about patients’ prognosis after TAVR and that patients exhibiting early improvement in late H/M after TAVR had a significantly lower incidence of cardiac events than those without such improvement.17 The present results indicate that late H/M displayed a clearer trend toward improvement at 6 to 12 months than immediately after TAVR. It is assumed that MIBG parameters immediately after TAVR will inevitably be influenced by perioperative procedural factors such as infusion; therefore, a comparison of baseline and 6- to 12-month data may provide a better prognostic indicator for patients who have undergone TAVR. Furthermore, our results suggest that more careful consideration may be given to the indications for TAVR among patients with a low baseline mPG, particularly those with paradoxical LF-LG AS. Serial CSNF assessment using MIBG imaging may be useful in validating TAVR treatment for this AS category.
New Knowledge Gained
The late H/M assessed using MIBG imaging displayed a clearer trend toward improvement at 6 to 12 months than immediately after TAVR. Higher baseline mPG was an independent predictor of mid-term improvement in late H/M after TAVR.
Study Limitations
The present study has certain limitations. First, the relatively small sample size of this study may be insufficient to fully reveal the long-term effects on CSNF by TAVR procedure. In addition, the final study population comprised only 75 of the 183 consecutive patients enrolled, which raises concern about the external validity of this study. The applicability of certain imaging modalities, including MIBG scintigraphy, can be limited by the patient’s background and the presence of intraoperative complications. Therefore, potential limitations to the applicability of MIBG imaging in clinical settings exist. Second, the prognostic benefit from the improvement in CSNF at 6 to 12 months after TAVR remains unclear because the patients’ prognostic information was not included in this study. However, because the association of early TAVR-related improvement in the late H/M with better prognosis has previously been confirmed,17 the increase in the late H/M revealed in this study should also represent good clinical outcomes after TAVR. Third, there may be some selection bias in sampling, as baseline MIBG imaging could not be performed for critically ill patients with unstable general conditions. Fourth, as this study included Japanese patients only, our results cannot be generalized to other ethnicities.
Conclusion
CSNF, as denoted by late H/M, demonstrated a sustained improvement from within 2 weeks after TAVR until 6–12 months later. Such improvement was related to baseline hemodynamic AS severity. Patients with very high baseline mPG would gain the greatest prognostic benefit from TAVR.
Abbreviations
- ACEIs:
-
Angiotensin-converting enzyme inhibitors
- AS:
-
Aortic stenosis
- AVA:
-
Aortic valve area
- CSNF:
-
Cardiac sympathetic nervous function
- H/M:
-
Heart–mediastinum ratio
- LVEF:
-
Left ventricular ejection fraction
- MIBG:
-
123I-meta-iodobenzylguanidine
- mPG:
-
Mean aortic valve pressure gradient
- TAVR:
-
Transcatheter aortic valve replacement
- Vmax:
-
Peak velocity
- WR:
-
Washout rate
References
Otto CM, Prendergast B. Aortic-valve stenosis—from patients at risk to severe valve obstruction. N Engl J Med 2014;371:744-56. https://doi.org/10.1056/nejmra1313875.
Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;143:e35-71. https://doi.org/10.1161/cir.0000000000000932.
Sobajima M, Ueno H, Onoda H, Kuwahara H, Tanaka S, Ushijima R, et al. Transcatheter aortic valve implantation improves cardiac sympathetic nerve activity on 123I-metaiodobenzylguanidine myocardial scintigraphy in severe aortic valve stenosis. Circ J 2018;82:579-85. https://doi.org/10.1253/circj.cj-17-0817.
Kadoya Y, Zen K, Tamaki N, Ito N, Kuwabara K, Yamano M, et al. Early effects of transcatheter aortic valve replacement on cardiac sympathetic nervous function assessed by 123I-metaiodobenzylguanidine scintigraphy in patients with severe aortic valve stenosis. Eur J Nucl Med Mol Imaging 2020;47:1657-67. https://doi.org/10.1007/s00259-019-04523-0.
Cohen-Solal A, Rouzet F, Berdeaux A, Le Guludec D, Abergel E, Syrota A, et al. Effects of carvedilol on myocardial sympathetic innervation in patients with chronic heart failure. J Nucl Med 2005;46:1796-1803.
Takeishi Y, Atsumi H, Fujiwara S, Takahashi K, Tomoike H. ACE inhibition reduces cardiac iodine-123-MIBG release in heart failure. J Nucl Med 1997;38:1085-89.
Jacobson AF, Travin MI. Impact of medications on mIBG uptake, with specific attention to the heart: Comprehensive review of the literature. J Nucl Cardiol 2015;22:980-93. https://doi.org/10.1007/s12350-015-0170-z.
Okuda K, Nakajima K, Hosoya T, Ishikawa T, Konishi T, Matsubara K, et al. Semi-automated algorithm for calculating heart-to-mediastinum ratio in cardiac iodine-123 MIBG imaging. J Nucl Cardiol 2011;18:82-89. https://doi.org/10.1007/s12350-010-9313-4.
Nakata T, Nakajima K, Yamashina S, Yamada T, Momose M, Kasama S, et al. A pooled analysis of multicenter cohort studies of (123)I-mIBG imaging of sympathetic innervation for assessment of long-term prognosis in heart failure. JACC Cardiovasc Imaging 2013;6:772-84. https://doi.org/10.1016/j.jcmg.2013.02.007.
Veltman CE, Boogers MJ, Meinardi JE, Al Younis I, Dibbets-Schneider P, Van der Wall EE, et al. Reproducibility of planar (123)I-meta-iodobenzylguanidine (MIBG) myocardial scintigraphy in patients with heart failure. Eur J Nucl Med Mol Imaging 2012;39:1599-1608. https://doi.org/10.1007/s00259-012-2180-2.
Kasama S, Toyama T, Sumino H, Nakazawa M, Matsumoto N, Sato Y, et al. Prognostic value of serial cardiac 123I-MIBG imaging in patients with stabilized chronic heart failure and reduced left ventricular ejection fraction. J Nucl Med 2008;49:907-14. https://doi.org/10.2967/jnumed.107.047548.
Carrió I, Cowie MR, Yamazaki J, Udelson J, Camici PG. Cardiac sympathetic imaging with mIBG in heart failure. JACC Cardiovasc Imaging 2010;3:92-100. https://doi.org/10.1016/j.jcmg.2009.07.014.
Kasama S, Toyama T, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, et al. Effects of candesartan on cardiac sympathetic nerve activity in patients with congestive heart failure and preserved left ventricular ejection fraction. J Am Coll Cardiol 2005;45:661-7.
Narula J, Gerson M, Thomas GS, Cerqueira MD, Jacobson AF. 123I-MIBG imaging for prediction of mortality and potentially fatal events in heart failure: The ADMIRE-HFX study. J Nucl Med 2015;56:1011-18.
Bohbot Y, Kowalski C, Rusinaru D, Ringle A, Marechaux S, Tribouilloy C. Impact of mean transaortic pressure gradient on long-term outcome in patients with severe aortic stenosis and preserved left ventricular ejection fraction. J Am Heart Assoc 2017;6:e005850. https://doi.org/10.1161/jaha.117.005850.
Izumi C, Eishi K, Ashihara K, Arita T, Otsuji Y, Kunihara T, et al. JCS/JSCS/JATS/JSVS 2020 guidelines on the management of valvular heart disease. Circ J 2020;84:2037-2119. https://doi.org/10.1253/circj.cj-20-0135.
Kadoya Y, Zen K, Tamaki N, Yashige M, Takamatsu K, Ito N, et al. Prognostic value of cardiac 123I-metaiodobenzylguanidine imaging for predicting cardiac events after transcatheter aortic valve replacement. ESC Heart Fail 2021;8:1106-16. https://doi.org/10.1002/ehf2.13123.
Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91. https://doi.org/10.1093/eurheartj/ehx391.
Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 2007;115:2856-64. https://doi.org/10.1161/circulationaha.106.668681.
Jander N, Minners J, Holme I, Gerdts E, Boman K, Brudi P, et al. Outcome of patients with low-gradient “severe” aortic stenosis and preserved ejection fraction. Circulation 2011;123:887-95. https://doi.org/10.1161/circulationaha.110.983510.
Tribouilloy C, Rusinaru D, Maréchaux S, Castel AL, Debry N, Maizel J, et al. Low-gradient, low-flow severe aortic stenosis with preserved left ventricular ejection fraction: Characteristics, outcome, and implications for surgery. J Am Coll Cardiol 2015;65:55-66. https://doi.org/10.1016/j.jacc.2014.09.080.
Yamashita E, Takeuchi M, Seo Y, Izumo M, Ishizu T, Sato K, et al. Prognostic value of paradoxical low-gradient severe aortic stenosis in Japan: Japanese Multicenter Aortic Stenosis Study, Retrospective (JUST-R) Registry. J Cardiol 2015;65:360-8. https://doi.org/10.1016/j.jjcc.2014.12.019.
Disclosures
Yoshito Kadoya, Kan Zen, Nagara Tamaki, Shunsuke Nakamura, Tomotaka Fujimoto, Masaki Yashige, Kazuaki Takamatsu, Nobuyasu Ito, Michiyo Yamano, Tetsuhiro Yamano, Takeshi Nakamura, Hidetake Kawajiri, Satoshi Numata, Hitoshi Yaku, and Satoaki Matoba have no conflicts of interest.
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarizes the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Supplementary Information
Below is the link to the electronic supplementary material.
12350_2021_2799_MOESM1_ESM.pdf
Supplementary file1 Changes in 123I-meta-iodobenzylguanidine parameters at baseline, < 2 weeks after TAVR, and at 6–12 months after TAVR in the three groups classified according to the baseline mPG. Low mPG group: (a) Early H/M, (b) late H/M, and (c) WR. High mPG group: (d) Early H/M, (e) late H/M, and (f) WR. Very high mPG group: (g) Early H/M, (h) late H/M, and (i) WR. TAVR indicates transcatheter aortic valve replacement; H/M, heart–mediastinum ratio; mPG, mean aortic valve pressure gradient; and WR, washout ratio (PDF 406 kb)
Rights and permissions
About this article
Cite this article
Kadoya, Y., Zen, K., Tamaki, N. et al. Serial changes in cardiac sympathetic nervous function after transcatheter aortic valve replacement: A prospective observational study using 123I-meta-iodobenzylguanidine imaging. J. Nucl. Cardiol. 29, 2652–2663 (2022). https://doi.org/10.1007/s12350-021-02799-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-021-02799-0