Abstract
Background
The use of left ventricular mechanical dyssynchrony (LVMD), which has been reported to be responsible for unfavorable outcomes, might improve conventional risk-stratification by clinical indices including QRS duration (QRSd) and systolic dysfunction in patients with heart failure (HF).
Methods and results
Following measurements of 12-lead QRSd and left ventricular ejection fraction (LVEF), three-dimensional (3-D) LVMD was evaluated as a standard deviation (phase SD) of regional mechanical systolic phase angles by gated myocardial perfusion imaging in 829 HF patients. Patients were followed up for a mean period of 37 months with a primary endpoint of lethal cardiac events (CEs). In an overall multivariate Cox proportional hazards model, phase SDs were identified as significant prognostic determinants independently. The patients were divided into 4 groups by combining with the cut-off values of LVEF (35% and 50%) and QRSd (130 ms and 150 ms). The groups with lower LVEF and prolonged QRSd more frequently had CEs than did the other groups. Patient groups with LVEF < 35% and with 35% ≦ LVEF < 50% were differentiated into low-risk and high-risk categories by using an optimal phase SD cut-off value of both QRSd thresholds.
Conclusions
3-D LVMD can risk-stratify HF patients with mid-range as well as severe abnormalities of QRSd and systolic dysfunction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recent advances in pharmacological and non-pharmacological treatments have contributed to a reduction in the cardiac mortality rate in patients with HF. Besides an increase in the number of HF patients with a preserved LVEF, however, there are HF patients with a reduced LVEF who do not respond to recent combination treatment using several drugs,1,2,3 resulting in a high cardiac mortality rate without benefits. The concept of dys-synchronized cardiac performance being responsible for the development and exacerbation of HF has been accepted and has been supported by the beneficial effects of cardiac resynchronization therapy (CRT).1,2,3 Because of their easy determination and cost-effectiveness and the accumulation of evidence regarding their usefulness, QRS duration (QRSd) and LVEF have been used as classical biomarkers in the major guidelines for HF management and electrical device therapy. Nevertheless, a non-negligible number of HF patients with a reduced LVEF show resistance to treatment with newly developed electrical devices, sometimes together with no benefits but adverse effects, resulting in an unnecessary increase in medical costs and unfavorable outcomes.3,4,5,6,7 Thus, the limitations of current guidelines and conventional biomarkers, including LVEF and electrical dyssynchrony index, indicate the need for a more effective method for risk-stratification and for prediction of outcomes in HF patients in combination with conventional clinical parameters. In this context, 3-D LVMD has been emerging as a important biomarker for predicting unfavorable outcomes and has been shown to have pivotal roles in improving the risk-stratification of chronic HF patients when used in combination with conventional clinical parameters.8,9,10,11,12,13 Recently, 3-D LVMD can be evaluated as a global heterogeneity of the initiation of contraction by mathematical fitting of regional cardiac cycles with higher-ordered Fourier phase analysis in a gated myocardial perfusion imaging (MPI) study.
This study focused on the correlations of 3-DLVMD with QRSd and LVEF and the ability of 3-D LVMD to further risk-stratify HF patients with severe or mid-range abnormalities of QRSd and LVEF.
Methods
Study Patients
A total of 829 consecutive patients with symptomatic HF and with an echocardiographic LVEF < 50% who were admitted to our hospital between April 2011 and March 2017 were enrolled in this study. The entry criteria for this study were as follows: symptomatic HF, echocardiographic LVEF of less than 50% and age of 20 years or older. Patients who refused resuscitation treatment, patients who had overt malignancy or hemorrhagic diseases and patients who were aged less than 20 years were excluded. The patients included 616 males. The mean age of the patients was 67.3 ± 12.1 years and mean LVEF was 36.7 ± 9.8%. The diagnosis of decompensated HF was made by the Framingham criteria including typical symptoms, neck vein distension, peripheral edema, lung rale, S3 or S4 gallop and tachycardia. Chest X-ray and two-dimensional echocardiographic examinations were conducted to support the diagnosis and to exclude other diseases showing similar symptoms or signs. In addition to a definitive history of prior myocardial infarction and/or coronary artery revascularization, HF etiologies such as ischemic or non-ischemic were differentiated by using findings such as stress-induced myocardial ischemia or myocardial infarction (Q-wave infarction or scarred region) on a 12-lead electrocardiogram and/or cardiac imaging together with coronary artery information on computed tomography, magnetic resonance imaging or invasive selective coronary angiography. HF etiologies such as ischemic or non-ischemic were also established using a 12-lead electrocardiogram, exercise stress testing with or without cardiac imaging, and noninvasive or invasive coronary angiographic examination. Patients aged less than 20 years and patients who had overt malignancy or hemorrhagic diseases were excluded from this study. Just before discharge, blood levels of hemoglobin (Hb), creatinine and brain natriuretic peptide (BNP) were measured. Renal function was also evaluated by estimated glomerular filtration rate (eGFR) using the standard formula. Plasma BNP level was measured in the initial 367 patients (43%), but NT-pro BNP level was alternatively measured in the remaining 462 patients (57%). Because of the two different BNP assessments, BNP and NT-pro BNP were classified into 4 stages based on the ESC guidelines for heart failure3) for subsequent statistical analysis: 0 to 40 pg·mL and 0 to 125 pg·mL for stage 1, 41 to 100 pg·mL and 126 to 400 pg·mL for stage 2, 101 to 200 pg·mL and 401 to 900 pg·mL for stage 3, and 201 to pg·mL and 901 to pg·mL for stage 4, respectively.
Measurement of QRS Duration by a 12-Lead Electrocardiogram
A 12-lead electrocardiogram was recorded to measure the widest QRSd in a stable condition of HF.
Echocardiographic Assessment
Two-dimensional echocardiography was performed from parasternal long-axis and apical four-, three- and two-chamber views at a left lateral decubitus position using commercially available ultrasound machines equipped with a 2.5-MHz variable frequency transducer by echocardiographic technicians. The following echocardiographic parameters were measured in a stabilized condition before discharge: left atrium diameter (LAD; mm), left ventricular end-diastolic diameter (LVDd; mm), left ventricular ejection fraction (LVEF; %) calculated using the biplane modified Simpson’s method, left ventricular volume at end-diastole (EDV; mL), left ventricular volume at end-systole (ESV; mL) and septal E/e′.
Assessment of Cardiac Mechanical Dyssynchrony
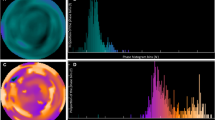
3-D LVMD was quantified using electrocardiogram-gated 99mTc-tetrofosmin MPI with a frame rate of 16 as shown previously.12,13 Briefly, LVMD was evaluated as a standard deviation (phase SD) on a phase histogram of the regional onset-of-mechanical contraction phase angles (unit, degrees) calculated by Fourier phase analysis applied to regional time-activity curves obtained three-dimensionally over the left ventricle (Figure 1). In principle, this mathematical technique measures myocardial count changes that depend on regional wall thickness over a cardiac cycle and can produce regional time-activity curves in each boxel. Thus, a phase histogram was created to calculate phase SD as a 3-D LVMD index. This data analysis was performed by radiological nuclear technicians without knowledge of clinical information using the commercially available gated SPECT software Heart Function View (HFV version 1.1).12,13 The intra- and inter-observer reproducibilities were evaluated by two radiological technicians and confirmed to be sufficient as follows: CV% ranged from 2.93% to 4.56% and the correlation coefficient between operators was R = 0.994 (P < .0001).
Phase histograms for identification of LVMD defined by an increased phase SD in two typical cases. Case 1: a 68-year-old female with ischemic cardiomyopathy who had LVEF of 34%, QRSd of 178 ms and phase SD of 9°. No cardiac event occurred during the follow-up period. Case 2: a 56-year-old male with non-ischemic cardiomyopathy undergoing CRT who had LVEF of 32%, QRSd of 150 ms and phase SD of 68°. He died of progressive pump failure after 1.5 years during the follow-up period
Follow-Up Protocol
Patients with HF were prospectively registered into our HF database and regularly followed up at an out-patient care unit by cardiologists for 1 year or more when patients survived. Primary endpoints used in this study were as follows: lethal cardiac events such as sudden cardiac death, death due to progression of pump failure, lethal ventricular tachyarrhythmias and appropriate ICD therapy against them. Retrospectively, however, clinical outcomes were confirmed by reviewing medical records and then the following outcome analysis was performed. Sudden cardiac death was defined as witnessed cardiac arrest and death within 1 hour after acute onset of symptoms or unexpected death in patients who were well within the previous 24 hours. This study was based on the principles outlined in the Declaration of Helsinki, and informed consent for enrollment in our database and usage for clinical study was obtained according to the guidelines of the ethics committee of our hospital.
Statistical Analysis
A statistical value is shown as mean ± SD. Mean values were compared between two groups using the unpaired t test, and categorical variables were compared using the Chi square test. Kaplan–Meier analysis using key parameters identified in this study was used to create a time-dependent, cumulative event-free curve, and the log-rank test was also used for comparison of the curves if necessary. Following univariate analysis, multivariate analysis with a Cox proportional hazards model was performed using the statistically appropriate number of significant variables identified by univariate analysis, which depended on the number of cardiac events, to calculate hazard ratios and 95% confidence intervals (CIs) of significant variables. Receiver operating characteristic (ROC) analysis was performed to determine an optimal cut-off value of an independent significant parameter. Global Chi square values were calculated to clarify incremental prognostic values of phase SD in combination with other significant variables identified.
A computer software program, SAS for Windows, version 9.4 (SAS Institute, Cary, North Carolina, USA), was used for these analyses. A P value less than .05 was considered significant.
Results
Measurements of phase SD are shown using two typical cases with a wide QRSd and systolic dysfunction (Figure 1). Case 1 with LVEF of 34% and a QRSd of 178 ms had normal phase SD and had no cardiac event documented during the follow-up period. In contrast, Case 2 with LVEF of 32% and a QRSd of 150 ms had a markedly increased phase SD and died of progressive pump failure.
During a mean follow-up period of 37 ± 16 months, cardiac events were documented in 211 (25.5%) of the patients: HF death occurred in 161 patients due to progressive pump failure, lethal ventricular arrhythmias occurred in 22 patients, sudden cardiac death occurred in 16 patients and appropriate ICD shocks against lethal ventricular arrhythmias occurred in 12 patients. Patients with cardiac events were older and leaner, had a greater NYHA functional class and had more reduced eGFR when compared to patients without cardiac events (Table 1). Patients with cardiac events had both greater QRSd and phase SD than did patients without cardiac events: QRSd, 138.6 ± 37.4 vs 121.2 ± 28.0 ms, P < .0001; phase SD, 38.0 ± 11.7 vs 33.3 ± 10.4°, P < .0001 (Table 2). In addition to the results of univariate analysis (Table 3), phase SD as well as NYHA functional class, eGFR and amiodarone use were confirmed to have significant independent prognostic values by multivariate Cox analysis with Chi square values of 5.46 to 78.9 (P < .0001~0.0194). In ROC analysis, phase SD of 36 was determined as an optimal cut-off value that discriminated patients into low-risk and high-risk populations (log-rank 33.1, P < .0001) (Figure 2).
Based on the 2016 ESC guidelines for CRT,14 the 829 patients were divided into 4 groups using both cut-off values of LVEF (35%) and QRSd (130 ms or 150 ms). When QRSd of 130 ms was used, patients were categorized as follows: group I, LVEF > 35% and QRSd < 130 ms; group II, LVEF > 35% and QRSd 130 ms; group III, LVEF 35% and QRSd < 130 ms, and group IV, LVEF 35% and QRSd 130 ms (Figure 3A). Group I had a significantly lower cardiac event rate than those in groups II, III and IV: 14.9% vs 30.2% (P = .0002), 26.5% (P = .0016) and 46.5% (P < .0001), respectively (Figure 3A). Group IV had the highest (P < .0001) event rate among the groups, but there was no significant difference (P = .48) between groups II and III. When QRSd of 150 ms was used instead of 130 ms, similar results were obtained as follows (Figure 3B). Group I (LVEF > 35% and QRSd < 150 ms) had a significantly lower cardiac event rate than those in groups II, III and IV: 16.6% vs 31.3% (P < .001), 30.5% (P < .0001) and 57.3% (P < .0001), respectively. Group IV (LVEF ≦ 35% and QRSd ≧ 150 ms) had the highest (P < .0001) event rate among the groups, but no significant difference (P = .88) was found between groups II and III.
(A) Scatter plots of QRSd and LVEF data for classification of patients into four subgroups in which QRSd of 130 ms and LVEF of 35% are cut-off values (left panel). Group I, LVEF > 35% and QRSd < 130 ms; Group II, LVEF > 35% and QRSd ≧ 130 ms; Group III, LVEF ≦ 35% and QRSd < 130 ms; and Group IV, LVEF ≦ 35% and QRSd ≧ 130 ms. The cardiac event rate (as shown in parenthesis) was significantly lowest in group I and highest in group IV among the four groups (right panel). Open and closed circles indicate patients with and those without lethal cardiac events, respectively. (B) Scatter plots of QRSd and LVEF data for classification
Multivariate Cox analysis was also performed in two ways using QRS durations of ≧ 130 ms (Table 4) and ≧ 150 ms (Table 5). Phase SD was identified as a significant independent predictor of lethal events in groups II, III and IV but not in group I: group I, hazard ratio of 1.020, 95% CI of 0.997 to 1.044, P = .1275; group II, hazard ratio of 1.639, 95% CI of 1.004 to 2.837, P = .0293; group III, hazard ratio of 2.133, 95% CI of 1.003 to 4.142, P = .0409; group IV, hazard ratio of 2.086, 95% CI, 1.191 to 3.733, P = .0099 (Table 4). Phase SD more than 36 further discriminated high-risk patients from low-risk patients in groups II, III and IV but not in group I: group II, log-rank of 3.964, P = .0465; group III, log-rank of 5.004, P = .0253; group IV, log-rank of 15.5, P < .0001 (Figure 4). Likewise, when QRSd of 150 ms was used instead of 130 ms in multivariate Cox analysis (Table 5), phase SD was identified as a significant independent predictor of lethal events in groups II, III and IV but not in group I: group I, hazard ratio of 1.464, 95% CI of 0.894 t 2.370, P = .864; group II, hazard ratio of 4.74, 95% CI of 1.004 to 2.836, P = .0292; group III, hazard ratio of 2.132, 95% CI of 1.004 to 4.764, P = .0390; group IV, hazard ratio of 2.086, 95% CI, 1.191 to 3.733, P = .0099). Phase SD of more than 36 discriminated high-risk patients from low-risk patients in groups II, III and IV but not in group I: group II, log-rank of 3.964, P = .0465; group III, log-rank of 5.004, P = .0253; group IV, log-rank of 15.5, P < .0001 (Figure 5).
When all of the significant variables identified by multivariate Cox analysis (Table 3) were combined, the significant (P < .0001) incremental prognostic value of phase SD was clearly identified with a maximal Chi square value of 225.6 (Figure 6).
Discussion
3-D LVMD was shown in this study to have critical roles for improvement in risk-stratification of HF patients evaluated conventionally by both grades of prolonged QRSd and systolic dysfunction.
Measurements of Mechanical Dyssynchrony
3-D LVMD is more likely than electrical dyssynchrony to impair effective contractile performance, resulting in the development and progression of HF and subsequent cardiac events.6,15 LVMD is produced vertically and extensively, but not planarly as assessed by a body-surface electrocardiogram, and myocyte damage is often heterogeneous even in ischemia-related injury. 3-D LVMD is more closely related to global left ventricular contractile failure leading to cardiac events. QRSd is a classical and easily obtained measurement and is a surrogate marker of dys-synchronized left ventricular wall motion that is responsible for poor prognosis of HF. A prolonged QRSd is not necessarily related to myocardial injury or contractile impairment per se and is sometimes transient even in HF patients.16 QRSd is sometimes prolonged as a sequel to the development of HF or myocardial remodeling.16,17 Besides the discrepancy between electrical disturbance and mechanical dysfunction, no clinical benefit was demonstrated in nearly one-third of patients undergoing CRT under the current guidelines.18 On the other hand, CRT showed adverse as well as favorable prognostic effects when patients with refractory HF had a narrow QRSd.19,20,21 These findings strongly suggest a limitation of QRSd as a dyssynchrony index in prognostic assessment of HF patients and in appropriate selection of CRT responders.
Recent advances in nuclear cardiology techniques have enabled routine assessment of 3-D LV function and LVMD using whole LV slices without dead angles8,11,12 and without any additional exposure or costs. The automated computerized technique can calculate regional alterations of myocardial count data during one cardiac cycle mathematically using a higher-ordered Fourier analysis. Unlike an echocardiographic method,6,7 this method can therefore provide reliable and reproducible measurements of 3-D quantitative data. Among parameters in 3-D LVMD,12,13 phase SD was used in this study because this mechanical dyssynchrony index is simple and is easily understandable in histogram analysis. Furthermore, the mathematical method can minimize artefactual data and operator’s biases and it has been shown to be a prognostic determinant in previous studies.8,9,10,11,12
Clinical Implications of Mechanical Dyssynchrony
Currently, CRT is indicated when patients with refractory HF have both prolonged QRSd and LVEF ≦ 35%. It is still controversial whether QRSd should be 130 ms or more or 150 ms or more for achievement of better clinical outcomes by therapeutic intervention. Despite the difference of QRSd in the criteria, device-related problems and expanding costs relative to the limited clinical benefits have emerged as clinical issues to be resolved. In addition, device treatment has not been established for HF patients with a mid-range reduction of LVEF between 35% and 50%. Group II patients with QRSd ≧ 130 ms or 150 ms and LVEF > 35% and group III patients with QRSd < 130 ms or 150 ms and LVEF ≦ 35% had nearly identical event rates, and they were significantly higher than that in group I with QRSd < 130 ms or 150 ms and LVEF > 35%. LVMD defined as a phase SD of 36 or more further discriminated groups II to IV patients with either prolonged QRSd, reduced LVEF or both into a lower or higher risk category in each group. Together with eGFR and NYHA functional class, 3-D LVMD had definitive prognostic values in HF patients with a mid-range abnormality of LVEF (35%~50%) as well as in those with LVEF ≦ 35% regardless of the QRSd in this study. These findings suggest that LVMD quantified as an increased phase SD due to heterogeneous initiation of contraction can overcome the limitations of LVEF and QRSd by reducing under- and over-estimations of patient risks. 3-D LVDM assessment is expected to precisely identify both patients who are at a relatively low risk and are unlikely to benefit from CRT and those who can benefit from CRT but may have been missed by the current guidelines. Thus, 3-D LVMD quantification can not only further risk-stratify patients assessed by conventional parameters but also possibly increase the cost-effectiveness of device treatment via better risk-assessment by combined use of QRSd and LVEF.
Study Limitations and Future Perspectives
This investigation was designed as a non-interventional, observational cohort study in patients with HF symptoms and established systolic HF. A large-scale, multi-center, interventional study based on the presented results will contribute to the development of a better prophylactic or therapeutic strategy using the appropriate indication for CRT in patients at increased risk for cardiac mortality. It is also necessary to determine the correlation between clinical outcomes and improved 3-D LVMD (phase SD) by CRT in HF patients with mid-range reduction of LVEF as well as in those with LVEF of less than 35%. Radiation exposure, nearly 2 to 4 mSv, by the scintigraphic method must be reduced by innovation of the method, and the additional cost related to the method must verified from a prognostic point of view. It is important to consider how both underuse and overuse of device treatment originating in the conventional guidelines is avoidable by comparing the method presented here with recent echocardiographic techniques such as strain imaging and speckle tracking.22,23 More precise assessment of cardiac mortality risk and actual prognostic benefits of CRT in HF patients would definitively contribute to more cost-effective management of HF by reducing unnecessary costs and radiation exposure related to device implantation.
New Knowledge Gained
LVMD measured three-dimensionally by MPI has been selected as a stronger prognostic factor than QRS complex duration and may be useful for determining the indication and therapeutic effect of CRT in the future.
Conclusion
Three-dimensional LVMD assessed by gated myocardial perfusion imaging can not only identify high-risk HF patients for lethal cardiac events more precisely but can also further discriminate high-risk HF patients who have mid-range as well as severe abnormalities of QRSd and systolic dyfunction into a lower or higher risk category.
Abbreviations
- LVMD:
-
Left ventricular mechanical dyssynchrony
- QRSd:
-
QRS duration
- HF:
-
Heart failure
- NYHA:
-
New York Heart Association
- LVEF:
-
Left ventricular ejection fraction
- CRT:
-
Cardiac resynchronization therapy
- ICD:
-
Implantable cardioverter defibrillator
- Hb:
-
Hemoglobin
- BNP:
-
Brain natriuretic peptide
- eGFR:
-
Estimated glomerular filtration rate
References
Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, Freedman RA, Gettes LS, et al. American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Heart Rhythm Society. 2012 ACCF/AHA/HRS Focused Update Incorporated Into the ACCF/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities. J Am Coll Cardiol 2013;61:e6-75.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37:2129-200.
Tsutsui H, Isobe Mitsuaki, Ito Hiroshi, Okumura Ken, Ono Minoru, Kitakaze Masafumi, et al. Guidelines for diagnosis and treatment of acute and chronic heart failure. Circ J 2019;83:2084-184.
Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002;346:1845-53.
Fox DJ, Fitzpatrick AP, Davidson NC. Optimisation of cardiac resynchronisation therapy: Addressing the problem of ‘non-responders’. Heart 2005;91:1000-2.
Chung ES, Leon AR, Tavazzi L, Sun PG, Nihoyannopoulos P, Merlino J, et al. Results of the predictors of response to crt (prospect) trial. Circulation 2008;117:2608-16.
Hawkins NM, Petrie MC, Burgess MI, McMurray JJ. Selecting patients for cardiac resynchronization therapy: the fallacy of echocardiographic dyssynchrony. J Am Coll Cardiol 2009;53:1944-59.
Chen J, Henneman MM, Trimble MA, Borges-Neto S, Iskandrian AE, et al. Assessment of left ventricular mechanical dyssynchrony by phase analysis of ECG-gated SPECT myocardial perfusion imaging. J Nucl Cardiol 2008;15:127-36.
Wang L, Yang MF, Cai M, Zhao SH, He ZX, Wang YH, et al. Prognostic significance of left ventricular dyssynchrony by phase analysis of gated SPECT in medically treated patients with dilated cardiomyopathy. Clin Nucl Med 2013;38:510-5.
Zafrir N, Nevzorov R, Bental T, Strasberg B, Gutstein A, Mats I, et al. Prognostic value of left ventricular dyssynchrony by myocardial perfusion-gated SPECT in patients with normal and abnormal left ventricular functions. J Nucl Cardiol 2014;21:532-40.
Nakajima K, Okuda K, Matsuo S, Kiso K, Kinuya S, Gracia EV, et al. Comparison of phase dyssynchrony analysis using gated myocardial perfusion imaging with four software programs: Based on the Japanese Society of Nuclear Medicine working group normal database. J Nucl Cardiol 2017;24:611-21.
Doi T, Nakata T, Yuda S, Hashimoto A. Synergistic prognostic implications of left ventricular mechanical dyssynchrony and impaired cardiac sympathetic nerve activity in heart failure patients with reduced left ventricular ejection fraction. Eur Heart J Cardiovasc Imaging 2018;19:74-83.
Doai T, Nakata T, Yuda S, Hashimoto A. Synergistic prognostication of left ventricular hypertrophy and three-dimensional mechanical dyssynchrony in heart failure. ESC Heart Fail 2020;7:361-70.
Bertaglia E, Reggiani A, Palmisano P, D’Onofrio A, Simone AD, Caico SI, et al. The stricter criteria for Class I CRT indication suggested by the 2016 ESC Guidelines reliably exclude patients with a worse prognosis in comparison with the 2013 ESC indication criteria. Int J Cardiol 2018;273:162-7.
D’Andrea A, Mele D, Nistri S, Riegler L, Galderisi M, Agricola E, et al. The prognostic impact of dynamic ventricular dyssynchrony in patients with idiopathic dilated cardiomyopathy and narrow QRS. Eur Heart J Cardiovasc Imaging 2013;14:183-9.
Wang NC, Maggioni AP, Konstam MA, Zannad F, Krasa HB, Burnett JC Jr, et al. Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators: Clinical implications of QRS duration in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction. JAMA 2008;299:2656-66.
Bacharova L, Szathmary V, Potse M, Mateasik A. Computer simulation of ECG manifestations of left ventricular electrical remodeling. J Electrocardiol 2012;45:630-4.
Carità P, Corrado E, Pontone G, Curnis A, Bontempi L, Novo G, et al. Non-responders to cardiac resynchronization therapy: Insights from multimodality imaging and electrocardiography. A brief review. Int J Cardiol 2016;225:402-7.
Bax JJ, Delgado V, Sogaard P, Singh JP, Abraham WT, Borer JS, et al. Prognostic implications of left ventricular global longitudinal strain in heart failure patients with narrow QRS complex treated with cardiac resynchronization therapy: A subanalysis of the randomized EchoCRT trial. Eur Heart J 2017;38:720-6.
Shah RM, Patel D, Molnar J, Ellenbogen KA, Koneru JN, et al. Cardiac-resynchronization therapy in patients with systolic heart failure and QRS interval ≤ 130 ms: Insights from a meta-analysis. Europace 2015;17:267-73.
Ruschitzka F, Abraham WT, Singh JP, Bax JJ, Borer JS, Brugada J, et al. EchoCRT Study Group: Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med 2013;369:1395-405.
Zweerink A, van Everdingen WM, Nijveldt R, Salden OA, Meine M, Maass AH, et al. Strain imaging to predict response to cardiac resynchronization therapy: A systematic comparison of strain parameters using multiple imaging techniques. ESC Heart Fail 2018;5:1130-40.
Fulati Z, Liu Y, Sun N, Kang Y, Su Y, Chen H, et al. Speckle tracking echocardiography analyses of myocardial contraction efficiency predict response for cardiac resynchronization therapy. Cardiovasc Ultrasound 2018;16:30.
Acknowledgements
The authors sincerely thank the staff of Nuclear Medicine Laboratory, Teine-Kijinnkai Hospital (Sapporo), Hokkaido, Japan for their clinical services and their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Conflict of interest to be declared for the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Funding: There was no specific funding.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Doi, T., Nakata, T., Noto, T. et al. Improved risk-stratification in heart failure patients with mid-range to severe abnormalities of QRS duration and systolic function using mechanical dyssynchrony assessed by myocardial perfusion-gated SPECT. J. Nucl. Cardiol. 29, 1611–1625 (2022). https://doi.org/10.1007/s12350-021-02554-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-021-02554-5