Abstract
Background
To evaluate the diagnostic value of 18F-FDG PET/CT in distinguishing benign versus malignant cardiac tumors as well as to assess its prognostic value.
Methods
We analyzed 38 patients with cardiac tumors who underwent 18F-FDG PET/CT and followed for median 8.5 ± 12.5 months. SUVmax and TBRmax (maximum tumor-to-background ratio) by receiver-operating characteristic (ROC) curve analysis were used to obtain threshold for the diagnosis of malignancy as defined by histology (n = 38). Survival was assessed and correlated with the dignity of the lesions and PET parameters.
Results
Optimal cut-off values indicating malignancy were as follows: SUVmax = 3.44, with 100% sensitivity and 92.9% specificity, and TBRmax = 1.55, with 95.8% sensitivity and 92.9% specificity. A significant difference of 18F-FDG uptake was observed between primary benign (n = 14, SUVmax = 2.35 ± 1.31, TBRmax = 1.05 ± 0.50) compared to primary malignant cardiac tumors (n = 11, SUVmax = 8.90 ± 4.23, TBRmax = 3.82 ± 1.44) as well as cardiac metastases and lymphoma (n = 13, SUVmax = 14.37 ± 8.05, TBRmax = 6.19 ± 3.38) (all P < .001). Survival rate was significantly lower in patients with malignant as compared to benign cardiac tumors (P < .05). Regression analysis revealed that the lesion dignity determined by the cut-off value of SUVmax was an independent predictor for death in patients with cardiac tumors (P < .05).
Conclusion
18F-FDG uptake in cardiac tumors can differentiate between benign and malignant cardiac tumors and predicts survival.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With an incidence of only 0.0017% to 0.02%, primary cardiac tumors are rare;1 however, cardiac metastases of extracardiac malignancies occur much more frequently.2 For primary cardiac tumors, the likelihood of malignancy is reported between 20% and 25%.3,4
Primary cardiac tumors are mainly treated by surgical resection. Metastatic cardiac tumors in most cases are not referred to surgery; this especially applies to patients with cardiac manifestations of lymphoma,5 which can be treated by chemotherapy and/or radiation therapy. Hence, a thorough initial staging is essential to the management of cardiac tumors in order to choose the best treatment strategy.
Noninvasive imaging plays an important role for the work-up of an undetermined cardiac masses, since percutaneous biopsy typically is not feasible, and surgical biopsy or resection carries a high risk of perioperative complications.5,6 Echocardiography, cardiac magnetic resonance imaging (CMR) or computed tomography (CT), particularly with contrast enhancement, allow assessment of multiple morphologic features. In some instances, these modalities permit to diagnose a specific tumor type, e.g., myxoma.7 Even though morphologic features can help to differentiate malignant from benign tumors,8 individual morphological feature usually does not allow for a precise determination of dignity. Cardiac MR has a very high resolution; however, the duration of image acquisition can be fairly long. Also, its use is limited in patients with pacemakers or other metal implants.
18F-fluorodeoxyglucose (18F-FDG) PET/CT has become an essential tool in managing a variety of malignancies.9,10,11,12 This modality provides noninvasive whole-body information and can also identify the most aggressive malignant lesion.13 In many tumors, accurate diagnosis is nowadays essential for individual decision-making to decide on the right treatment strategy, and further, to improve patient’s outcome. Nonetheless, to date, only a few studies, with a limited number of patients, have been published, which used 18F-FDG PET/CT to differentiate benign from malignant cardiac tumors.14,15 So far, the prognostic value of 18F-FDG PET/CT in patients with cardiac tumors has not been reported.
This retrospective study aimed to evaluate the diagnostic value of 18F-FDG PET/CT in the determination of the dignity of cardiac tumors with histopathology serving as the gold standard. Also, the prognostic value of 18F-FDG PET/CT in patients with cardiac tumors was assessed.
Materials and Methods
Study Population
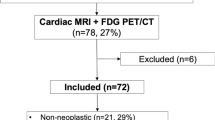
Initially included in the analysis were 66 consecutive patients with cardiac tumors referred to Beijing Anzhen Hospital, China, from January 2015 to May 2019 for staging by whole-body 18F-FDG PET/CT. 38 patients with available histopathology could be enrolled in current study (Figure 1). Electronic medical records were reviewed; this included clinical characteristics, symptoms, imaging findings by echocardiography, CT, cardiac MR (if available), histological results, and treatment strategy.
This analysis was approved by the Beijing Anzhen Hospital ethics committee and conducted according to the Helsinki Declaration. Patients provided written informed consent for the imaging procedures as well as for the participation in anonymized analyses such as this.
Patient Preparation and 18F-FDG PET/CT
All patients underwent 18F-FDG PET/CT imaging after a high-fat diet.16 Additionally, they fasted for ≥ 12 h and had blood glucose < 10 mmol/L before imaging. The average 18F-FDG activity of 254 ± 67 MBq (6.9 ± 1.8 mCi), 5 MBq/kg (0.13 mCi/kg) of body weight was injected intravenously; radiation exposure for the combined PET/CT ranged from 11.6 to 17.8 mSv per patient per procedure.
Imaging was performed 60 min after 18F-FDG injection, using a high-spatial-resolution, full-ring PET scanner (Biograph mCT, Siemens Healthcare, Erlangen, Germany). The imaging data were reconstructed using a point spread function and a time-of-flight algorithm (TrueX+time-of-flight, UltraHD-PET), with 2 iterations and 21 subsets. Subsequently, a Gaussian filter with 2 mm full-width half-maximum was applied to the reconstructed images. For attenuation and scatter correction, a CT without contrast agent was used. Here, slice thickness was 5 mm with a pitch of 0.8 and a tube voltage of 120 kV. The tube current automatically modulated according to the patient’s size and body shape; a 210 mAs maximum reference value was used to achieve good image quality. Representative 18F-FDG PET/CT images from the patient’s collective are shown in Figures 2, 3, and 4.
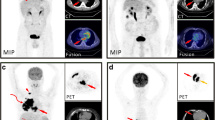
44-year-old female presenting with dyspnea, abdominal distention, and lower extremity edema for 6 months. A, B, C: Transverse views of CT, PET, and PET/CT fusion. D: coronal view of PET. The blue arrow indicates the cardiac tumor. On CT, the right ventricle (RV) mass appears isodense or hypodense relative to the myocardium. 18F-FDG PET shows mildly increased uptake in RV lesion (SUVmax 3.90, TBRmax 1.45): E: Intraoperative image of the tumor and histopathology, which revealed a primary cardiac myxoma (hematoxylin and eosin [HE] staining magnified 200 times)
47-year-old female presenting with palpitations and shortness of breath for 2 months. A, B, C: Transverse views of CT, PET, and PET/CT fusion. D: coronal view of PET. The blue arrow indicates the cardiac tumor. On CT, the right atrium (RA) masses appeared isodense or hypodense relative to the myocardium and a large pericardial effusion can be noted. 18F-FDG PET/CT shows an increased uptake in this tumor (SUVmax 8.92; TBRmax 4.07). E: Gross biopsy specimen of the masses; histopathology revealed the lesion to be a primary angiosarcoma (HE staining magnified 200 times)
73-year-old female presenting with increasing dyspnea for 2 years. A, B, C: Transverse views of CT, PET, and PET/CT fusion. D: Coronal view of PET. The blue arrow indicates the cardiac tumor, the red arrow increased uptake in lymph node manifestations. On CT scan, the RA masses appear isodense or hypodense relative to the myocardium. 18F-FDG PET/CT depicts distinctly increased uptake in the RA tumor (SUVmax 21.00; TBRmax 8.47). E: Histopathology revealed the tumor to be DLBCL (HE staining magnified 200 times)
Image Analysis
PET/CT datasets were reviewed and analyzed by two readers, both board-certified nuclear medicine physicians and PET/CT experts, to determine 18F-FDG uptake of the cardiac tumors and to screen for extracardiac malignant manifestations by visual interpretation. The maximum standardized uptake value (SUVmax) was derived from a 3-dimensional volume of interest (VOI) assigned to the tumor. This VOI was placed within the tumor at the location of the highest tracer uptake. Caution was taken to exclude unspecific myocardial uptake from the analysis. In case of very low glucose metabolism, the coregistered CT data were used to help assign the VOI. In order to calculate the maximum tumor-to-background ratio (TBRmax), the SUVmax of each tumor was normalized by the mean SUV of a spherical reference VOI, 3 cm in diameter, assigned to the right lobe of the liver. If an extracardiac hypermetabolic lesion was verified as tumor by histology or biopsy and the 18F-FDG uptake pattern was similar with the cardiac tumors, the cardiac tumors were deemed to be of the same entity.
Histological Analysis
Pathological materials from all patients studied were available for gross and histological examinations. Specimens were fixed in formalin, routinely processed for light microscopy and stained with hematoxylin-eosin, periodic acid-schiff (PAS), azan and Weigert van Gieson. Also, transmission electron microscopy and immunohistochemistry studies were performed to confirm the histological diagnosis. The results were confirmed by two experienced pathologists who were blinded to the previous clinical findings and location of the tumors. According to histological diagnosis,17 the 38 patients were divided into three groups, Group 1 with primary benign cardiac tumor, Group 2 with primary malignant cardiac tumor, and Group 3 with cardiac metastases and lymphoma.
Follow-Up
Follow-up was performed by review of patients’ clinical records and by phone contact with patients or their relatives. Thirty-eight patients were followed for 8.5 ± 12.6 months [median ± interquartile range (IQR) 25th to 75th percentiles], range 1-52 months. 13 patients (34.2%) died during follow-up.
Statistics
Data were analyzed using SPSS 19.0 (SPSS Inc. IBM, Armonk, NY). Continuous parametric variables were expressed as the mean ± standard deviation (SD). Non-parametric variables were displayed as the median ± IQR. The mean values of continuous variables were compared between groups by 1-way ANOVA followed by the Scheffe post hoc test. Categorical data are reported as percentages. Via receiver-operating characteristic (ROC) curve analysis, we determined the optimal SUVmax and TBRmax cut-off values to differentiate benign versus malignant cardiac tumors based on histology results as the gold standard. Sensitivity, specificity, accuracy, and area-under-the-curve (AUC) were calculated by χ2 testing. Pearson correlation analysis was used to assess a relationship between tumor size and SUVmax. Kaplan–Meier survival curves were generated in subgroups classified using SUVmax and TBRmax cut-off values and were compared by log-rank test. The Cox proportional hazards regression analysis model was used to identify independent predictors of all-cause mortality in patients with cardiac lesions (n = 38). In the model, significant predictors were chosen via a stepwise forward method with P < .05 as the entry criterion and P > .10 as the removal criterion. Statistical significance was defined as P < .05.
Results
Baseline characteristics of the patients with histological results (n = 38) are summarized in Table 1 and Supplemental data Table 1, respectively. Group 1, benign cardiac tumor (n = 14), was proven by surgery and histopathology. In Group 2, the primary malignant cardiac tumors (n = 11), only one case was verified by lung lesion biopsy; all others were confirmed by surgery and histopathology. In Group 3, cardiac metastasis and lymphoma were included (n = 13). Of 7 cases with DLBCL, two cases were diagnosed by extracardiac lesion biopsy (liver and bone). One patient was diagnosed by cardiac surgery and histopathology; the others were proved by lymph node biopsy. Four of the six patients with cardiac metastases were confirmed by extracardiac lesion biopsy (lung, bone, esophagus); two of them were verified by cardiac surgery and histopathology. All of the biopsied lesions were positive on PET.
Tumor Types and 18F-FDG Uptake
As shown in Table 1, 14 (36.8%) of the patients presented with primary benign cardiac lesions were all verified histologically by surgery (Group 1, mean SUVmax = 2.35 ± 1.31, mean TBRmax = 1.05 ± 0.50), eleven patients (28.9%) had primary malignant cardiac tumors (Group 2, mean SUVmax = 8.90 ± 4.23, mean TBRmax = 3.82 ± 1.44), and 13 patients (34.2%) with cardiac metastases or lymphoma (Group 3, mean SUVmax = 14.37 ± 8.05, mean TBRmax = 6.19 ± 3.38).
A significant difference was observed in both SUVmax (P < .001) and TBRmax (P < .001) among three groups. Both mean SUVmax (P < .001) and mean TBRmax (P < .001) were significantly lower in Group 1 than those in Group 3. Moreover, both mean SUVmax (P < .05) and mean TBRmax (P < .05) in Group 1 were significantly lower than those in Group 2. TBRmax were significantly lower in Group 2 than that in Group 3 (P < .05). Pearson correlation analysis showed a significant correlation between tumor size and SUVmax (R2 = 0.44, P = .006) and a significant difference of the size of tumor between the benign and malignant tumors was observed (P = .04).
Among these 38 patients, 5 myxoma patients (all female, range of age 18-52) presented with a considerable variability of 18F-FDG uptake (mean SUVmax = 3.31 ± 1.21, range 1.91-5.23 and mean TBRmax = 1.45 ± 0.37, range 1.00-2.02). In 10 cases of different types of sarcoma, the mean SUVmax is 8.20 ± 3.73 (ranging 3.80-14.37) and the mean TBRmax is 3.57 ± 1.26 (ranging 1.61-5.50), were calculated; 2 patients had bilateral pulmonary metastases.
Remarkably, a very high 18F-FDG uptake (mean SUVmax = 19.66 ± 6.05, range 13.61-31.60; mean TBRmax = 8.46 ± 2.56, range 5.25-12.85) was observed in 7 patients with histologically confirmed as cardiac involvement of diffuse large B-cell lymphoma (DLBCL). The SUVmax in patients with DLBCL was significantly higher than the other malignant tumors (including primary malignant and metastatic cardiac tumors) (n = 17, mean SUVmax = 8.79 ± 4.31, range 3.49-18.12; mean TBRmax = 3.72 ± 1.59, range 1.27-7.05, P < .05).
SUVmax and TBRmax CUT-OFFS
Table 2 shows the diagnostic performance for the differentiation of benign vs malignant using these cut-off values for SUVmax and TBRmax. According to ROC curve analysis, the optimal cut-offs to differentiate benign and malignant (primary and metastatic) cardiac lesions were a SUVmax of 3.44 with AUC = 0.988, P < .001, and a TBRmax of 1.55 with AUC = 0.979, P < .001. Using a best discriminative SUVmax cut-off of 3.44 for the cardiac tumors, the sensitivity, specificity, and accuracy of 18F-FDG PET/CT for identification of malignancy were 100% (24/24), 92.9% (13/14), and 97.4% (37/38), respectively. Additionally, the TBRmax cut-off value of 1.55 proved to be equally suitable in detecting malignancy with a sensitivity of 95.8% (23/24), a specificity of 92.9% (13/14), and the diagnostic accuracy of 94.7% (36/38), respectively.
Survival
As shown in Figure 5, Kaplan–Meier cumulative overall survival curves of three groups differed significantly (P < .05). Malignant tumors as categorized by SUVmax ≥ 3.44 or TBRmax ≥ 1.55 was associated with significantly higher mortality as compared to benign tumors with SUVmax < 3.44 or TBRmax < 1.55 (P = .018 and P = .002, respectively) (Figure 6). All benign patients underwent surgery. Yet, there were different treatment methods for malignant patients. The Kaplan–Meier survival curve showed a significant difference with different treatment methods among patients with malignant cardiac tumors (Log rank 14.997, P = .002) (Figure 7).
Kaplan–Meier cumulative survival curves of patients with cardiac tumors (n = 38) classified by SUVmax and TBRmax using the calculated optimal cut-off values. A: Estimated overall survival was significantly lower in the malignant group (SUVmax ≥ 3.44, n = 25) than in the benign group (SUVmax < 3.44, n = 13) (log rank 5.596, P = .018). B: Similarly, estimated overall survival was significantly lower in the malignant group (TBRmax ≥ 1.55, n = 24) than in the benign group (TBRmax < 1.55, n = 14) log rank 9.710, P = .002)
COX Regression Analysis
As shown in Table 3, univariate Cox regression analysis revealed that the lesion dignity as determined by the cut-off value of SUVmax (HR 95% CI 7.834 [1.016-60.411], P = .048) was an independent predictor for death in patients with cardiac tumors, so was the pathological diagnosis (HR 95% CI 9.275 [1.201-71.645], P = .033). The other variables, including age, gender, tumor location, size, and treatment strategy had no independent predictive value for mortality.
Discussion
To date, literature regarding cardiac tumors evaluated by 18F-FDG PET/CT is scarce.14,15,16,18 In our current study, so far with a largest number of patients, we assessed the ability of 18F-FDG PET/CT imaging to determine cardiac tumor dignity. The diagnostic value of 18F-FDG PET/CT was validated by histopathology. Additionally, the PET imaging characteristics of primary benign, primary malignant, and metastatic cardiac lesions were assessed and survival was also evaluated.
In this study, 18F-FDG PET/CT showed excellent diagnostic performance in differentiating benign from malignant cardiac tumors. Cut-off values obtained for both measures of 18F-FDG uptake, SUVmax and TBRmax, resulted in very good sensitivity, specificity, and accuracy. Our observations aligned well with those of the previous studies in smaller patient populations,14,15 as well as those of the only study yet published using 18F-FDG PET/MR.19 Our SUVmax cut-off of 3.44 is well consistent with the SUVmax cut-off of 3.5 obtained by Rabhar et al 14 and SUVmax cut-off of 3.5-4.0 by Shao et al.15
In contrast to the previous studies,14,15,19 we also investigated a second variable of 18F-FDG uptake, the TBRmax. This variable was calculated using a reference VOI in the liver, because hepatic 18F-FDG uptake has been shown to correlate with circulating 18F-FDG levels,20 and the liver’s size makes it easy to assign VOIs. Additionally, TBRmax appears to be less influenced by, for e.g., body composition, presumably also image reconstruction methods or PET equipment, as compared to SUVmax.21,22,23,24 Remarkably, TBRmax at the calculated optimal cut-off, 1.55, proved to have similar diagnostic accuracy as the calculated optimal SUVmax cut-off of 3.44.
As another main finding, our analysis suggested that in patients with cardiac tumors, 18F-FDG PET/CT was able to detect suspicious hypermetabolic extracardiac lesions, and hence may be a valuable staging tool in this setting. In our study, 2 patients with primary cardiac malignancy were found to have bilateral lung metastases and 13 had lesions of non-cardiac origin, including 7 cases of DLBCL. The ability to identify extracardiac lesions potentially has a significant impact on patient management. On one hand, better visualization and localization of lesions facilitate histological verification of cancer. On the other hand, such findings may render cardiac surgery obsolete in many cases, allowing patients to be switched to other treatment modalities that may be more appropriate for their disease, e.g., chemotherapy or targeted therapy (shown in Supplemental data, Table 1). This is especially the case for lymphoma involving the heart where surgery usually is contraindicated. Our analysis showed prominently increased cardiac 18F-FDG activity (SUVmax and TBRmax) in 7 patients with cardiac DLBCL, 6 lesions localized in the right heart. The imaging pattern could be recognized and distinguished from other malignant cardiac tumors.
Differentiating primary from metastatic malignant heart lesions is another significant clinical challenge. According to our results, 18F-FDG uptake (TBRmax) in primary malignant cardiac tumor is lower than metastases to the heart or lymphoma. This finding might contribute to a better discrimination in clinical unclear constellations.
Finally, and importantly, our study results indicated that 18F-FDG uptake was a useful prognostic variable in patients with histological diagnosis. Kaplan–Meier cumulative overall survival curves showed that patient with malignant tumors categorized by SUVmax ≥ 3.44 or TBRmax ≥ 1.55 was associated with significantly higher mortality as compared to benign tumors (P = .018 and P = .002, respectively). On the Cox regression analysis using relevant variables, including age, gender, tumor size, tumor location, treatment strategy, tumor dignity as determined by the cut-off values of SUVmax 3.44 and the tumor dignity by histopathology, only the cut-off value of SUVmax 3.44 and histopathological diagnosis were independent predictors for death, which has not been previously reported.
Limitations
This was a single-center study of a Chinese expert center cohort with a wide variety of cardiac and other tumor types, and thus the generalizability of our findings may be limited. Also raising potential issues of generalizability, the prevalence of benign lesions, of certain tumor types, and of certain sites of cardiac tumor in our patients differed from those in other published series. For example, frequency of angiosarcoma in our collective was lower than that reported frequency elsewhere,25 as was the prevalence of right 26 or left atrial tumors.27 Another notable difference between our work and earlier work is the substantially higher rate of cases of lymphoma in our sample.27 These differences in disease characteristics may be attributable to either or both of center-specific selection biases, or ethnic composition of the respective cohorts. Finally, our study had a relatively short follow-up. Nonetheless, high mortality was observed (34.2%, 13/38), suggesting a poor prognosis for patients with cardiac tumors and the need for prompt diagnosis and treatment.
New Knowledge Gained
FDG-PET/CT should be performed for the assessment of cardiac tumors; it also appears to be valuable for prognostication.
Conclusion
18F-FDG PET/CT, because of its high accuracy in determining, whether lesions are benign or malignant, should be included in the diagnostic algorithm for cardiac tumors. 18F-FDG uptake, independently predicted survival in cardiac tumors and might serve as a valuable predictive tool for individualized decision-making.
Abbreviations
- 18F-FDG:
-
18Fluorine-fluorodeoxyglucose
- AUC:
-
Area-under-the-curve
- CMR:
-
Cardiac magnetic resonance imaging
- DLBCL:
-
Diffuse large B-cell lymphoma
- PET/CT:
-
Positron emission tomography/computed tomography
- PAS:
-
Periodic acid-schiff
- ROC:
-
Receiver-operating characteristic
- SUVmax :
-
Maximum standardized uptake value
- TBRmax :
-
Maximum tumor-to-background ratio
- VOI:
-
Volume of interest
References
Elbardissi AW, Dearani JA, Daly RC, Mullany CJ, Orszulak TA, Puga FJ, et al. Survival after resection of primary cardiac tumors: A 48-year experience. Circulation 2008;118:S7-15.
Chiles C, Woodard PK, Gutierrez FR, Link KM. Metastatic involvement of the heart and pericardium: CT and MR imaging. Radiographics 2001;21:439-49.
Reynen K. Cardiac myxomas. N Engl J Med 1995;333:1610-7.
Bussani R, De-Giorgio F, Abbate A, Silvestri F. Cardiac metastases. J Clin Pathol 2007;60:27-34.
Hoffmeier A, Sindermann JR, Scheld HH, Martens S. Cardiac tumors–diagnosis and surgical treatment. Dtsch Arztebl Int 2014;111:205-11.
Donisan T, Balanescu DV, Lopez-Mattei JC, Kim P, Leja MJ, Banchs J, et al. In search of a less invasive approach to cardiac tumor diagnosis: Multimodality imaging assessment and biopsy. JACC Cardiovasc Imaging 2018;11:1191-5.
Grebenc ML, Rosado de Christenson ML, Burke AP, Green CE, Galvin JR. Primary cardiac and pericardial neoplasms: Radiologic-pathologic correlation. Radiographics 2000;20:1073-103; quiz 110-1, 112.
Mousavi N, Cheezum MK, Aghayev A, Padera R, Vita T, Steigner M, et al. Assessment of cardiac masses by cardiac magnetic resonance imaging: Histological correlation and clinical outcomes. J Am Heart Assoc 2019;8:e007829.
Barrington SF, Johnson PWM. (18)F-FDG PET/CT in lymphoma: Has imaging-directed personalized medicine become a reality? J Nucl Med 2017;58:1539-44.
Laurens ST, Oyen WJ. Impact of fluorodeoxyglucose PET/computed tomography on the management of patients with colorectal cancer. PET Clin 2015;10:345-60.
Ciarallo A, Marcus C, Taghipour M, Subramaniam RM. Value of fluorodeoxyglucose PET/computed tomography patient management and outcomes in thyroid cancer. PET Clin 2015;10:265-78.
Werner RA, Schmid JS, Higuchi T, Javadi MS, Rowe SP, Markl B, et al. Predictive value of (18)F-FDG PET in patients with advanced medullary thyroid carcinoma treated with vandetanib. J Nucl Med 2018;59:756-61.
Weber WA. Positron emission tomography as an imaging biomarker. J Clin Oncol 2006;24:3282-92.
Rahbar K, Seifarth H, Schafers M, Stegger L, Hoffmeier A, Spieker T, et al. Differentiation of malignant and benign cardiac tumors using 18F-FDG PET/CT. J Nucl Med 2012;53:856-63.
Shao D, Wang SX, Liang CH, Gao Q. Differentiation of malignant from benign heart and pericardial lesions using positron emission tomography and computed tomography. J Nucl Cardiol 2011;18:668-77.
Masuda A, Manabe O, Oyama-Manabe N, Naya M, Obara M, Sakakibara M, et al. Cardiac fibroma with high (18)F-FDG uptake mimicking malignant tumor. J Nucl Cardiol 2017;24:323-4.
Burke A, Tavora F. The 2015 WHO classification of tumors of the heart and pericardium. J Thorac Oncol.
Rinuncini M, Zuin M, Scaranello F, Fejzo M, Rampin L, Rubello D, et al. Differentiation of cardiac thrombus from cardiac tumor combining cardiac MRI and 18F-FDG-PET/CT Imaging. Int J Cardiol 2016;212:94-6.
Nensa F, Tezgah E, Poeppel TD, Jensen CJ, Schelhorn J, Kohler J, et al. Integrated 18F-FDG PET/MR imaging in the assessment of cardiac masses: A pilot study. J Nucl Med 2015;56:255-60.
Liu G, Hu Y, Zhao Y, Yu H, Hu P, Shi H. Variations of the liver standardized uptake value in relation to background blood metabolism: An 2-[18F]Fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography study in a large population from China. Medicine (Baltimore) 2018;97:e0699.
Li X, Samnick S, Lapa C, Israel I, Buck AK, Kreissl MC, et al. 68Ga-DOTATATE PET/CT for the detection of inflammation of large arteries: Correlation with18F-FDG, calcium burden and risk factors. EJNMMI Res 2012;2:52.
Dunet V, Halkic N, Prior JO, Anaye A, Meuli RA, Sempoux C, et al. Detection and viability of colorectal liver metastases after neoadjuvant chemotherapy: A multiparametric PET/CT-MRI study. Clin Nucl Med 2017;42:258-63.
Ezziddin S, Adler L, Sabet A, Poppel TD, Grabellus F, Yuce A, et al. Prognostic stratification of metastatic gastroenteropancreatic neuroendocrine neoplasms by 18F-FDG PET: Feasibility of a metabolic grading system. J Nucl Med 2014;55:1260-6.
Sharma A, Mohan A, Bhalla AS, Vishnubhatla S, Pandey AK, Bal CS, et al. Role of various semiquantitative parameters of 18F-FDG PET/CT studies for interim treatment response evaluation in non-small-cell lung cancer. Nucl Med Commun 2017;38:858-67.
Pun SC, Plodkowski A, Matasar MJ, Lakhman Y, Halpenny DF, Gupta D et al. Pattern and prognostic implications of cardiac metastases among patients with advanced systemic cancer assessed with cardiac magnetic resonance imaging. J Am Heart Assoc 2016;5.
Dhull VS, Sharma P, Mukherjee A, Jana M, Bal C, Kumar R. 18F-FDG PET-CT for evaluation of cardiac angiosarcoma: A case report and review of literature. Mol Imaging Radionucl Ther 2015;24:32-6.
Mkalaluh S, Szczechowicz M, Torabi S, Schmack B, Sabashnikov A, Dib B, et al. Surgical treatment of cardiac tumors: Insights from an 18-year single-center analysis. Med Sci Monit 2017;23:6201-9.
Disclosure
The authors have indicated that they have no financial conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarizes the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
All editorial decisions for this article, including selection of reviewers and the final decision, were made by guest editor Nagara Tamaki, MD.
Funding
This work was supported by National Natural Science Foundation of China [81071177, 81871377, 81571717] and Capital Characteristic Clinical Application Research [Z181100001718071].
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
12350_2019_2022_MOESM1_ESM.docx
Supplementary material 1 Summary for TwitterThis is a retrospectively study for evaluating the diagnostic value of 18F-FDG PET/CT in distinguishing benign versus malignant cardiac tumors, as well as to assess its prognostic value. We analyzed 38 patients with cardiac tumors who underwent 18F-FDG PET/CT and followed for median: 8.5±12.5months. SUVmax and TBRmax (maximum tumor to background ratio) by receive-operating characteristic curve analysis were used to obtain threshold for the diagnosis of malignancy as defined by histology. The cut-off value of SUVmax=3.44, with 100% sensitivity and 92.9% specificity, and the cut-off value TBRmax=1.55, with 95.8% sensitivity and 92.9% specificity. Malignant tumors as categorized by SUVmax≥3.44 or TBRmax≥1.55 was associated with significantly higher mortality as compared to benign tumors with SUVmax<3.44 or TBRmax<1.55 (P=0.018 and P=0.002, respectively). The univariate Cox regression analysis revealed the lesion dignity as determined by the cut-off value of SUVmax (HR 95% CI: 7.834 [1.016-60.411], P=0.048) was an independent predictor for death in patients with cardiac tumors, so was the pathological diagnosis (HR 95% CI: 9.275 [1.201-71.645], P=0.033). 18F-FDG PET/CT should be included in the diagnostic algorithm for cardiac tumors to distinguish the malignancy. 18F-FDG uptake, independently predicted death in cardiac tumors and might serve as a valuable predictive tool. (DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Meng, J., Zhao, H., Liu, Y. et al. Assessment of cardiac tumors by 18F-FDG PET/CT imaging: Histological correlation and clinical outcomes. J. Nucl. Cardiol. 28, 2233–2243 (2021). https://doi.org/10.1007/s12350-019-02022-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-019-02022-1