Abstract
Background
Doxorubicin is a cornerstone in lymphoma treatment, but is limited by dose-dependent cardiotoxicity. Rubidium-82 positron emission tomography (82Rb PET) assesses coronary microvascular function through absolute quantification of myocardial perfusion and myocardial perfusion reserve (MPR). Doxorubicin-induced microvascular injury represents a potential early marker of cardiotoxicity.
Methods and results
We included 70 lymphoma patients scheduled for doxorubicin-based treatment. Cardiotoxicity was evaluated with 82Rb PET myocardial perfusion imaging during rest and adenosine stress before chemotherapy and shortly after the first doxorubicin exposure. Patients with a MPR decline > 20% were defined as having a low threshold for cardiotoxicity. In the 54 patients with complete data sets, MPR was significantly lower after the initial doxorubicin exposure (2.69 vs 2.51, P = .03). We registered a non-significant decline in stress perfusion (3.18 vs 3.02 ml/g/min, P = .08), but no change in resting myocardial perfusion. There were 13 patients with a low cardiotoxic threshold. These patients had a significantly higher age, but were otherwise similar to the remaining part of the study population.
Conclusion
Decreases in MPR after initial doxorubicin exposure in lymphoma patients may represent an early marker of doxorubicin-induced cardiotoxicity. The prognostic value of acute doxorubicin-induced changes in MPR remains to be investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Doxorubicin is the most frequently used anthracycline and a cornerstone in lymphoma treatment. However, doxorubicin treatment is limited by dose-dependent chronic cardiotoxicity that is typically defined as a decline in left ventricular ejection fraction (LVEF) of more than 10 percentage points to below the lower limit of normal.1,2,3 Cardiotoxicity is a particular concern in lymphoma patients where the high cure rates combined with a low median age at the time of diagnosis result in high survivor life expectancies and increased cumulative risk of late chemotherapy-related side effects.
In the initial phase, cardiotoxicity is often asymptomatic and therefore recognized too late to avoid permanent myocardial damage.2 To reduce the risk of cardiotoxic injury, doxorubicin therapy is generally stopped at cumulative doses of 500 mg/m2 due to a sharp increase in heart failure incidence beyond this point.4,5 However, doxorubicin dose alone is not enough to predict the occurrence of heart failure which in some individuals can appear even with low-dose regimens.6,7 Hence, there seems to be an individual threshold for development of cardiotoxic side effects that is determined by a combination of doxorubicin dose and individual patient characteristics.8 Unfortunately, there is no adequate diagnostic tool for identification of patients with a low cardiotoxic threshold or for early detection of doxorubicin-induced cardiotoxicity, and our understanding of the cardiotoxic pathway remains incomplete. Several different pathophysiological mechanisms have been proposed including injury to both cardiomyocytes and non-cardiomyocyte cardiac cells, such as the vascular endothelial cells.9,10,11,12,13 Rubidium-82 positron emission tomography (82Rb PET) has enabled assessment of the coronary microvascular function through absolute quantification of myocardial perfusion and myocardial perfusion reserve (MPR).14 Doxorubicin-induced changes in myocardial perfusion represent a potential early marker of cardiotoxicity that could predict a subsequent decline in cardiac function. Therefore, the objective of our study was to investigate the value of 82Rb PET perfusion imaging for assessment of the acute effects of doxorubicin treatment on the myocardial microcirculation in patients with malignant lymphoma.
Methods
Design and Study Population
The study is a prospective, clinical, single-center imaging study performed at Rigshospitalet, University of Copenhagen. Newly diagnosed lymphoma patients scheduled for doxorubicin-based treatment with curative intent were offered entry into the study. All Hodgkin and non-Hodgkin lymphoma subtypes were accepted, but only patients ≥ 18 years were eligible for study inclusion. Exclusion criteria were pre-existing heart failure of any cause (LVEF < 50%), previous anthracycline therapy or mediastinal radiation therapy, significant renal disease (eGFR < 30 mL/min/1.73 m2), impaired liver function (bilirubin > 20 µmoL/L), and contraindications for 82Rb PET imaging (e.g., adenosine intolerance, bronchospastic lung disease with ongoing wheezing, 2nd or 3rd degree AV block without a functioning pacemaker, systolic blood pressure (SBP) < 90 mmHg, uncontrolled hypertension (SBP > 200 mmHg or diastolic blood pressure > 110 mmHg), use of dipyridamole-containing medication within 48 hours prior to testing, unstable angina pectoris, and sinus node dysfunction).15
From May 5, 2015 to August 30, 2017, 70 consecutive doxorubicin-naive lymphoma patients were included in the study.
Baseline Characteristics
Prior to treatment, patients underwent a standard clinical staging workup including medical history, physical examination, fasting blood samples, virus screening, and, when clinically relevant, ECG, chest x-ray, and lung function testing. Lymphoma subtyping was performed according to the World Health Organization (WHO) classification of malignant lymphomas.16,17 [18F]Fluorodeoxyglucose PET/computed tomography (FDG-PET/CT) was used for disease staging on the basis of the Lugano classification.18 Performance status was evaluated according to the Eastern Cooperative Oncology Group (ECOG) scale. High-risk disease was defined according to relevant prognostic indices. Hypertension was defined as receiving antihypertensive treatment at the time of inclusion. Ischemic heart disease was defined as a verified diagnosis of myocardial ischemia. Similarly, cardiac arrhythmia only included verified episodes of abnormal heart rhythm. Smoking was defined as all types of smoking, including light or intermittent smoking, at the time of inclusion.
82Rb PET Imaging
Acute doxorubicin-induced cardiac effects were evaluated with 82Rb PET imaging prior to chemotherapy (later referred to as ‘baseline’) and up to 3 days after the first doxorubicin exposure (later referred to as ‘post-exposure’). Decreases in MPR of > 20% were considered significant since it has previously been shown that day-to-day biological plus methodological variability in 82Rb PET quantification of myocardial perfusion is of this magnitude.19 For purpose of the analysis, we defined patients with > 20% decrease in MPR as having a low cardiotoxic threshold, while the remaining part of the study population were categorized as high cardiotoxic threshold individuals.
82Rb PET Acquisition
PET myocardial perfusion imaging was performed during rest and pharmacological stress in a single session. Patients were asked not to take caffeine and other methylxanthines for at least 16 hours prior to imaging. Fasting was required for at least 2 hours leading up to the examination. Patients received 1120 megabecquerel (MBq) (± 30%) 82Rb prior to each acquisition supplied from a CardioGen-82 Sr-82/Rb-82 generator (Bracco Diagnostics Inc., Princeton, NJ). Images were acquired ECG-gated in list mode for 7 minutes from the start of the 82Rb infusion on a Siemens Biograph mCT/PET 128-slice scanner (Siemens Helthcare, Knoxville, TN, USA). Patients underwent subsequent adenosine stress for 6 minutes (0.14 mg/kg/min), with 82Rb infusion initiated 2.5 minutes after the start of the adenosine infusion. Low-dose CT for attenuation correction was performed before the rest study, and after the stress study if required. A dedicated ECG-gated CT was obtained for coronary artery calcium score. For dynamic reconstructions, PET images were reconstructed into 21 frames (12 × 10, 3 × 20, 6 × 30 seconds) using 3D ordered subsets expectation-maximization (OSEM) reconstruction (2 iterations, 21 subsets) with point spread function modeling and time of flight information.
82Rb PET Analysis
All analyses were performed using Cedars QPS/QGS® software (v. 2012, Cedars Sinai, USA). Images were automatically reoriented in horizontal long axis (HLA), vertical long axis (VLA), and short axis (SAX), and in polar map format normalized to peak myocardial activity divided into 17 segments as proposed in the standardized guidelines from the American Heart Association.20 Quantification of myocardial perfusion was based on a single-compartment model for 82Rb tracer kinetics.21 MPR was defined as myocardial perfusion during maximal hyperemia divided by myocardial perfusion during rest.22 Due to the prospective nature of our study using paired analyses to investigate the potential cardiac effects of doxorubicin, we did not initially correct rest perfusion values for rate-pressure product (RPP). After the primary analysis, we investigated the effects of RPP correction on our results.
Regional ischemia was evaluated by automatically calculated scores [summed rest score (SRS), summed stress score (SSS), and summed difference score (SDS = SSS-SRS)] using the 17-segment model of the left ventricle.20 Each segment was automatically graded in a semiquantitative manner using a 5-point scale ranging from normal perfusion (= 0) to absent perfusion (= 4) with intermediate scores representing mild (= 1), moderate (= 2), and severe (= 3) perfusion defects.20 The SRS and SSS represent the cumulative scores of all 17 segments (range 0-68) during rest and stress testing. Finally, ECG-gated images were used for assessment of left ventricular volumes and LVEF calculation in both the resting condition and at the peak of adenosine pharmacological action.
Ethical Considerations
The study was prospectively registered with the European Clinical Trials Database (EudraCT, project ID: 2014-002226-13) and approved by The Regional Committee on Health Research Ethics (project ID: H-3-2014-012) and The Danish Medicines Agency (project ID: 2014093488). All subjects provided written informed consent. Participation in the present study did not affect the treatment, response evaluation, or routine follow-up as determined by the treating hematologist. All patients received treatment according to current guidelines or relevant clinical trials.
Statistical Analysis
Data are presented using the median and interquartile range for continuous variables and the median and range for ordinal variables. Categorical variables are described with frequency distributions showing both the percentage value and the total number of observations (no.). Comparison of paired baseline and post-exposure imaging data was done using the Wilcoxon signed rank test. Comparison of low versus high cardiotoxic threshold patients was done using the Mann-Whitney U test. The association between baseline characteristics and cardiotoxic threshold category was investigated using logistic regression for the continuous independent variables and Fisher’s exact test for the categorical independent variables. The association between baseline characteristics and the absolute change in MPR was investigated using the Mann-Whitney U test for the dichotomous independent variables, the Kruskal-Wallis one-way analysis of variance for the categorical independent variables with more than two groups, and the Spearman’s rank correlation coefficient for the continuous independent variables. Two-sided tests were performed and P values < .05 were considered significant. Analyses were performed using R version 3.2.0.23
Results
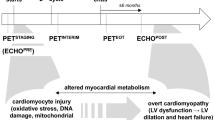
Of the 70 included patients, three canceled their participation in the study prior to baseline imaging. Seven patients were not able to complete both baseline and post-exposure imaging. The remaining 60 patients were all able to comply fully with the study protocol. Median time from doxorubicin exposure to post-exposure imaging was 3 days (interquartile range 2.0-5.5). There were six cases of technical errors during stress testing, thus resulting in 54 complete data sets. Figure 1 provides an overview of the conducted 82Rb PET imaging procedures.
Baseline Characteristics
At the time of inclusion, median age was 61 years with a range of 18-86 years. The vast majority of patients were diagnosed with either diffuse large B-cell lymphoma (DLBCL, n = 43) or Hodgkin lymphoma (HL, n = 17). The study population represented a broad clinical spectrum of patients, with 40% of individuals having stage IV disease and 26% having high-risk disease. Significant cardiac comorbidity was present in only five patients, including four patients with ischemic heart disease and one patient with persistent atrial fibrillation. In contrast, cardiovascular risk factors were quite prevalent with 30% of the study population being hypertensive and 21% being current smokers. Baseline data are presented in Table 1.
Baseline vs Post-exposure 82Rb PET
MPR decreased significantly after the initial doxorubicin exposure (2.69 vs 2.51, P = .03, Figure 2). We also registered a non-significant decline in stress perfusion (3.18 mL/g/min vs 3.02 mL/g/min, P = .08), while there was no change in resting myocardial perfusion. Figure 3 illustrates the absolute and relative MPR changes for each of the individuals in the study population. None of the baseline variables were associated with change in MPR. Similarly, neither initial doxorubicin dose nor calcium score correlated to change in MPR. SSS, SRS, and SDS values did not change from baseline to post-exposure imaging. Resting HR was higher post-exposure (P = .046), while systolic blood pressure was lower after treatment (P = .04). Table 2 provides an overview of the 82Rb PET results.
Myocardial perfusion reserve, rest perfusion, and stress perfusion before (baseline) and after (post-exposure) initial doxorubicin exposure. The blue-shaded part of the figure refers to the left-hand y-axis (stress/rest, no unit). Rest perfusion and stress perfusion values refer to the right-hand y-axis (mL/g/min). MPR, myocardial perfusion reserve; Post-exp, post-exposure
Change in myocardial perfusion from baseline to post-exposure imaging for each of the 54 subjects with complete data sets. The upper part of the figure shows the relative change in myocardial perfusion reserve (MPR) while the lower part of the figure shows absolute changes in MPR, stress perfusion and rest perfusion. Red bars and lines represent individuals with a > 20% decrease in MPR. MPR, myocardial perfusion reserve; LCT, low cardiotoxic threshold; HCT, high cardiotoxic threshold; Post-exp, post-exposure
Low vs High Cardiotoxic Threshold
In total, 13 patients experienced a MPR decline of > 20% and were hence categorized per protocol as having a low cardiotoxic threshold. The remaining 41 patients constituted the high cardiotoxic threshold group. Of these, four patients had a marked increase in MPR of > 45%. The range of change in MPR was − 1.28 to 1.74 corresponding to − 38 to 84%. Patients with a low cardiotoxic threshold had a significantly higher age than patients with a high cardiotoxic threshold (median 67 years vs 57 years, P = .04, Table 3). There were no other significant differences in baseline characteristics between the two groups, although advanced-stage disease appeared to be more frequent in the high cardiotoxic threshold individuals (P = .05). Age was the only factor significantly associated with cardiotoxic threshold status (P = .047). In the low cardiotoxic threshold group, median MPR was 2.69 and 1.94 before and after treatment, respectively. The corresponding values for the high cardiotoxic threshold group were 2.6 and 2.68. There were no differences in rest perfusion at baseline or post-exposure. Stress perfusion was the same in the two groups at baseline, but significantly lower in LCT individuals after doxorubicin exposure (2.54 vs 3.12 ml/g/min; P = .006). Complete 82Rb PET data for both groups can be found in Table s1.
Rate-Pressure Product Correction
RPP-corrected rest perfusion was calculated as (rest flow/RPP) × 10,000. After correction, there was still no difference between baseline and post-exposure rest perfusion (Table 3). RPP-corrected values were markedly higher than non-corrected values since the mean RPP of the study population was below 10,000 bpm × mmHg; as a result of this, the MPR decline was no longer significant (P = 0.5). In comparison of LCT vs HCT patients, RPP-corrected rest perfusion did not differ between the groups. Using RPP correction, post-exposure MPR was still significantly lower in the LCT individuals (P = .001).
Discussion
This paper presents 82Rb PET data on absolute myocardial perfusion before and after the first doxorubicin exposure in patients with malignant lymphoma. To our knowledge, this is the first time that 82Rb PET has been used for detection of potential chemotherapy-induced acute cardiac effects.
The primary finding of our study was a significant decrease in MPR shortly after the initial doxorubicin exposure. The MPR decline was driven by decreased myocardial perfusion during stress and was not paralleled by changes in the SSS, SRS, and SDS scores. We did not find a correlation between change in MPR and baseline characteristics, calcium score, or initial doxorubicin dose.
There are various mechanisms which could explain our observations, including toxic damage to cardiomyocytes or injury to the endothelial cells of the myocardial microcirculation. Traditionally, the pathophysiological explanation of anthracycline-related cardiotoxicity has centered on the generation of reactive oxygen species (ROS) in mitochondria.24,25 A central role of the mitochondria in the cardiotoxic pathway explains why cardiomyocytes—with their high mitochondria content and marked reliance on oxidative phosphorylation—are particularly susceptible to doxorubicin-induced toxicity. Studies of genetic risk factors for doxorubicin cardiotoxicity seem to confirm the pathophysiological role of mitochondrial damage. First, Zhang et al. introduced the Top2β gene (encoding topoisomerase IIβ) as the molecular keystone in doxorubicin cardiotoxicity.26 In mice with functioning Top2β genes compared to mice with deletion of the Top2β gene, the authors demonstrated a doxorubicin-induced repression of oxidative phosphorylation pathways and mitochondrial function along with a decline in cardiomyocyte oxygen consumption rate and a significantly higher ROS formation. More recently, it was demonstrated how mice lacking mitochondrial topoisomerase I (Top1mt), an important regulator of mitochondrial DNA homeostasis, were more susceptible to doxorubicin-induced cardiotoxicity.27 Again, increased ROS formation and decreased mitochondrial oxygen consumption rate were observed. In the present study, the reduction in MPR and stress perfusion could result from toxic damage to cardiomyocyte mitochondria with a resulting decrease in oxidative phosphorylation and thus a reduced metabolic demand. The MPR decline may also be caused by increased ROS production and mitochondrial injury in non-cardiomyocyte cardiac cells including the endothelial cells in the myocardial microcirculation. The endothelium is known to be a central part of several cardiac pathologies with ROS acting as the common denominator,28 and although the cardiac endothelial cells have a lower relative content of mitochondria, they substantially outnumber cardiomyocytes, and thus contribute considerably to the total mitochondrial count.29
Alternatively, the reduction in MPR after doxorubicin exposure might be related to non-mitochondrial injury to the myocardial vasculature. In a preclinical setting, doxorubicin can induce endothelial cell apoptosis9 and impair endothelium-dependent relaxation.10 In addition, reductions in flow-mediated vasodilatation are seen in humans several years post-treatment,30 but also after single doses of doxorubicin in animals.12 Thus, doxorubicin can result in direct doxorubicin-induced vasomotor dysfunction.
The SSS, SRS, and SDS scores did not change after treatment, confirming our perception of doxorubicin-induced cardiotoxicity as a global rather than a regional injury process.31 There was a considerable variation in the doxorubicin effects on MPR, including the 13 patients who experienced a MPR decline > 20%, the predetermined low cardiotoxic threshold cutoff value.
We observed four outliers with a remarkably large (> 45%) increase in MPR from baseline to post-exposure. In three of these four patients, the time-activity curves from the blood pool indicated some degree of detector saturation during the blood pool first-pass uptake phase in the post-exposure scans. This would potentially underestimate the input function and result in a falsely elevated MPR assessment. The large MPR increases in the outliers could also be explained by low MPR values at baseline; this could result from unreported caffeine intake prior to imaging as this would reduce adenosine sensitivity, stress perfusion, and hence MPR. We acknowledge that unreported caffeine intake before post-exposure imaging may similarly have contributed to the MPR decline in the low cardiotoxic threshold group. Still, the low cardiotoxic threshold individuals clearly outnumber the outliers, and the magnitude of their MPR decline seems more biologically plausible than the MPR increases in the outlier group.
Apart from age, a well-known risk factor for doxorubicin cardiotoxicity,24,32 no between-group differences were identified comparing the baseline characteristics of the low and high cardiotoxic threshold groups. This illustrates the difficulty in risk stratification based on pre-exposure variables and emphasizes the need for early detection of doxorubicin-induced cardiotoxicity.
Acute changes in myocardial perfusion could be an early indicator of cardiotoxic injury. At present, we lack evidence that decreases in MPR will translate into chronic ventricular dysfunction. However, the prognostic value of early myocardial perfusion imaging with 82Rb PET is well documented in other settings, including patients with known or suspected myocardial ischemia,33,34,35,36 idiopathic left ventricular dysfunction,37 and hypertrophic cardiomyopathy38. It is thus intriguing to also think of MPR as a potential imaging biomarker for identifying those patients characterized by a low individual cardiotoxic threshold. Such a biomarker could contribute to individualization of both chemotherapy and cardiac follow-up programs.
Our study has some limitations. First of all, we recognize that our primary outcome measure, MPR, is only a proxy of cardiotoxic injury. Whether or not decreases in MPR after chemotherapy is in fact indicative of an increased risk of significant late cardiotoxicity remains to be investigated. Secondly, the study population is rather small and heterogeneous, decreasing our statistical power and precluding subgroup analyses. Thirdly, there was some variation in the time from doxorubicin exposure to post-exposure imaging. This was in part due to logistical reasons (limited scanner capacity, availability of tracer), but also a result of patient-related delays due to disease symptoms or treatment side effects. However, excluding patients with > 7 days between doxorubicin exposure and imaging from the analyses (n = 7) did not affect our results. Finally, it could be argued that rest perfusion values should have been corrected for RPP, although a lack of agreement on this topic does exist39 and previous studies have been conducted without correction.36 From our perspective, RPP correction is not the right approach in a prospective study using paired analysis where each of the study participants serves as their own reference. Furthermore, as we are investigating cardiac effects from doxorubicin exposure, it seems counterintuitive to correct for pre- to post-exposure differences in hemodynamic parameters that may be part of the cardiotoxic response. Most importantly, we found no indications that the MPR decline was driven by increases in rest perfusion as this did not change between baseline and post-exposure imaging and did not differ between LCT and HCT patients, even when RPP correction was applied. RPP correction increased our estimates of rest perfusion, suggesting that we were in fact underestimating rest perfusion when doing uncorrected analysis. From a physiological view point, this hypothesis appears rather difficult to explain.
The strengths of our study include the clinical, prospective design and the broad clinical spectrum of the study population, both of which increase the potential relevance to the daily clinical practice. Furthermore, the use of absolute quantification of myocardial perfusion markedly contributes to the value of the present study, allowing for detection of diffuse changes in myocardial perfusion.
New Knowledge Gained
This is the first study that has applied 82Rb PET myocardial perfusion imaging in the detection of doxorubicin cardiotoxicity. Substantial declines in MPR after the initial doxorubicin exposure may represent an early sign of cardiotoxicity.
Conclusion
Our study provides evidence that changes in myocardial perfusion, as measured by MPR using 82Rb PET, may serve as an early marker of doxorubicin-induced cardiotoxicity that can help identify individuals with a low threshold for cardiotoxicity. Additional prospective clinical studies are warranted to further investigate the predictive value of MPR and the potential implications for patient management.
Abbreviations
- HF:
-
Heart failure
- LVEF:
-
Left ventricular ejection fraction
- MPR:
-
Myocardial perfusion reserve
- PET:
-
Positron emission tomography
- Rb:
-
Rubidium
- SRS:
-
Summed rest score
- SSS:
-
Summed stress score
- SDS:
-
Summed difference score
References
Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 European Society of Cardiology position paper on cancer treatments and cardiovascular toxicity. Eur Heart J 2016;37:2768-801.
Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 2014;27:911-39.
Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015;131:1981-8.
Lefrak E, Pitha J, Rosenheim S, Gottlieb J. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 1973;32:302-14.
Von Hoff DD, Layard MW, Basa P, Davis HLJ, Von Hoff AL, Rozencweig M, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med US 1979;91:710-7.
Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin a retrospective analysis of three trials. Cancer 2003;97:2869-79.
Armenian SH, Sun C, Shannon T, Mills G, Francisco L, Venkataraman K, et al. Incidence and predictors of congestive heart failure after autologous hematopoietic cell transplantation. Blood 2011;118:6023-9.
Oliveira GH, Al-Kindi SG, Caimi PF, Lazarus HM. Maximizing anthracycline tolerability in hematologic malignancies: Treat to each heart’s content. Blood Rev 2016;30:169-78.
Wu S, Ko Y, Teng M, Ko Y, Hsu L, Hsueh C, et al. Adriamycin-induced cardiomyocyte and endothelial cell apoptosis: In vitro and in vivo studies. J Mol Cell Cardiol 2002;34:1595-607.
Murata T, Yamawaki H, Yoshimoto R, Hori M. Chronic effect of doxorubicin on vascular endothelium assessed by organ culture study. Life Sci 2001;69:2685-95.
Kalivendi SV, Kotamraju S, Zhao H, Joseph J, Kalyanaraman B. Doxorubicin-induced apoptosis is associated with increased transcription of endothelial nitric-oxide synthase: Effect of antiapoptotic antioxidants and calcium. J Biol Chem 2001;276:47266-76.
Duquaine D, Hirsch GA, Chakrabarti A, Han Z, Kehrer C, Brook R, et al. Rapid-onset endothelial dysfunction with adriamycin : evidence for a dysfunctional nitric oxide synthase. Vasc Med 2003;8:101-7.
Kotamraju S, Konorev EA, Joseph J, Kalyanaraman B. Doxorubicin-induced apoptosis in endothelial cells and cardiomyocytes is ameliorated by nitrone spin traps and ebselen. Role of reactive oxygen and nitrogen species. J Biol Chem 2000;275:33585-92.
Hagemann CE, Ghotbi AA, Kjær A, Hasbak P. Quantitative myocardial blood flow with Rubidium-82 PET: A clinical perspective. Am J Nucl Mol Imaging 2015;5:457-68.
Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. J Nucl Cardiol 2016;23:606-39.
Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood 2014;117:5019-32.
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375-90.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and Non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol 2018;32:3059-68.
Kitkungvan D, Johnson NP, Roby AE, Patel MB, Kirkeeide R, Gould KL. Routine clinical quantitative rest stress myocardial perfusion for managing coronary artery disease. JACC Cardiovasc Imaging 2017;10:565-77.
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. Circulation 2002;105:539-42.
Lortie M, Beanlands RSB, Yoshinaga K, Klein R, Dasilva JN, DeKemp RA. Quantification of myocardial blood flow with dynamic PET imaging. Eur J Nucl Med Mol Imaging 2007;34:1765-74.
Knudsen A, Christensen TE, Ghotbi AA, Hasbak P, Lebech A, Kjær A, et al. Normal myocardial flow reserve in HIV-infected patients on stable antiretroviral therapy—A cross-sectional study using Rubidium-82 PET/CT. Medicine (Baltimore) 2015;94:e1886.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. 2015.
Volkova M, Russell R. Anthracycline cardiotoxicity: Prevalence, pathogenesis and treatment. Curr Cardiol Rev 2011;7:214-20.
Bugger H, Guzman C, Zechner C, Palmeri M, Russell KS, Russell RR. Uncoupling protein downregulation in doxorubicin-induced heart failure improves mitochondrial coupling but increases reactive oxygen species generation. Cancer Chemother Pharmacol 2011;67:1381-8.
Zhang S, Liu X, Bawa-Khalfe T, Lu L-S, Lyu YL, Liu LF, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med 2012;18:1639-45.
Khiati S, Rosa ID, Sourbier C, Ma X, Rao VA, Neckers LM. Mitochondrial topoisomerase I (Top1mt) is a novel limiting factor of doxorubicin cardiotoxicity. Clin Cancer Res 2014;20:4873-82.
Davidson SM, Duchen MR. Endothelial mitochondria: Contributing to vascular function and disease. Circ Res 2007;100:1128-41.
Davidson SM. Endothelial mitochondria and heart disease. Cardiovasc Res 2010;88:58-66.
Dengel DR, Ness KK, Glasser SP, Williamson EB, Baker KS, Gurney JG. Endothelial function in young adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol 2008;30:20-5.
Glass CK, Mitchell RN. Winning the battle, but losing the war: Mechanisms and morphology of cancer-therapy-associated cardiovascular toxicity. Cardiovasc Pathol 2017;30:55-63.
Chatterjee K, Zhang J, Honbo N, Karliner JS. Doxorubicin cardiomyopathy. Cardiology 2010;115:155-62.
Farhad H, Dunet V, Bachelard K, Allenbach G, Kaufmann PA, Prior JO. Added prognostic value of myocardial blood flow quantitation in rubidium-82 positron emission tomography imaging. Eur Heart J Cardiovasc Imaging 2013;14:1203-10.
Fukushima K, Javadi MS, Higuchi T, Lautam R, Merrill J, Nekolla SG, et al. Prediction of short-term cardiovascular events using quantification of global myocardial flow reserve in patients referred for clinical 82 Rb PET perfusion imaging. J Nucl Med 2011;52:726-32.
Taqueti VR, Everett BM, Murthy VL, Gaber M, Foster CR, Hainer J, et al. Coronary heart disease interaction of impaired coronary flow reserve and cardiomyocyte injury on adverse cardiovascular outcomes in patients without overt coronary artery disease. Circulation 2015;131:528-35.
Ziadi MC, Robert A, Williams KA, Guo A, Ng ME, Chow BJW, et al. Impaired myocardial flow reserve on Rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol 2011;58:740-8.
Wang X, Sun CL, Quiñones-Lombraña A, Singh P, Landier W, Hageman L, et al. CELF4 variant and anthracycline-related cardiomyopathy: A children’s oncology group genome-wide association study. J Clin Oncol 2016;34:863-70.
Olivotto I, Cecchi F, Gistri R, Lorenzoni R, Chiriatti G, Girolami F, et al. Relevance of coronary microvascular flow impairment to long-term remodeling and systolic dysfunction in hypertrophic cardiomyopathy. J Am Coll Cardiol 2006;47:1043.
Van Tosh A, Votaw JR, Cooke CD, Cao JJ, Palestro CJ, Nichols KJ. Relationship of 82 Rb PET territorial myocardial asynchrony to arterial stenosis. J Nucl Cardiol 2018;24:34.
Acknowledgements
The authors would like to gratefully acknowledge the following research funds for their financial support: The Danish Cancer Society; Rigshospitalet Research Fund; Brødrene Hartmanns Fond; Eva og Henry Frænkels Mindefond; Dagmar Marshalls Fond; KV Fonden; Fabrikant Einar Willumsens Mindelegat; LM Byg; and lastly the research funds of the Department of Cardiology and the Department of Haematology, Rigshospitalet. Furthermore, we sincerely thank all the patients who agreed to participate in our study.
Disclosure
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarizes the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Laursen, A.H., Elming, M.B., Ripa, R.S. et al. Rubidium-82 positron emission tomography for detection of acute doxorubicin-induced cardiac effects in lymphoma patients. J. Nucl. Cardiol. 27, 1698–1707 (2020). https://doi.org/10.1007/s12350-018-1458-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-018-1458-6