Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) has emerged as an independent risk factor for cardiovascular diseases. However, there were few studies evaluating the condition of myocardial glucose metabolism in patients with NAFLD. Therefore, the aim of this study was to investigate the association between NAFLD and myocardial glucose uptake assessed by using 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) and whether or not alteration of myocardial glucose uptake could be an indicator linking to cardiac dysfunction in NAFLD individuals.
Methods and Results
A total of 743 asymptomatic subjects (201 with NAFLD, 542 without NAFLD) were retrospectively studied. The ratio of maximum myocardium FDG uptake to the mean standardized uptake value of liver (SUVratio) was calculated to estimate myocardial glucose uptake by using 18F-FDG PET. The diagnosis of fatty liver and fatty liver grading was confirmed by unenhanced CT according to diagnostic criterion of previous studies. The myocardial geometric and functional data were obtained by echocardiogram. Myocardial glucose uptake was significantly lower in individuals with NAFLD compared with those without fatty liver (P < .001). When analysis of association trend was performed, SUVratio quartiles showed correlated inversely and strongly with liver steatosis (P < .001). NAFLD patients with lower myocardial glucose uptake were more likely to have higher proportion of increased LV filling pressure (P < .05). A significant relationship between myocardial SUVratio and E/e’ ratio was presented in the trend analysis (P < .05). Moreover, multivariate regression analysis showed that myocardial glucose uptake was independently associated with NAFLD after adjusting for clinical important factors (all P < .001).
Conclusions
The presence of NAFLD in otherwise healthy subjects is closely associated with decreased myocardial glucose uptake assessing by 18F-FDG PET imaging. Furthermore, the NAFLD individuals with lower myocardial glucose uptake are more likely to have high risk of having impaired diastolic heart function.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is defined as fat accumulation in hepatocytes more than 5% of total liver weight with the absence of excessive alcohol consumption or other causes of steatosis. NAFLD is the most common chronic liver disease in the western world and in rapidly developing countries, with estimated prevalence of 19% in the United States and of up to 32.5% worldwide.1,2 A considerable proportion of patients with NAFLD develops into progressive liver fibrosis or cirrhosis, and nearly 5% of NAFLD patients die from liver-related diseases.3
Growing evidence supports that patients with NAFLD have higher risk of cardiovascular diseases (CVD), such as coronary artery disease, vascular inflammation or stroke than non-NAFLD control patients.4,5 Moreover, the leading cause of death in patients with NAFLD is CVD rather than liver-associated complications,6 highlighting the significance of identifying and addressing CVD in patients with NAFLD.
Previous studies have shown a convincing association between NAFLD and cardiac abnormalities, including systolic and diastolic dysfunction, altered left ventricular (LV) geometry and epicardial adipose tissue thickness.7,8,9,–10 The mechanisms of the relationship between NAFLD and cardiac complications still remain uncertain.11,12 Some studies previously demonstrated that patients with fatty liver displayed insulin resistance in myocardium,13,14 which might lead to alteration in myocardial fatty acids and glucose metabolism,15 resulting in myocardial structure abnormality and, eventually, cardiac dysfunction. Therefore, it is possible to hypothesize that patients who develop hepatic steatosis are more likely to present abnormal myocardial metabolism before the occurrence of structural or functional abnormality. Moreover, it is possible to take alteration in myocardial metabolism as an early indicator to predict the future cardiovascular events in individuals with NAFLD. However, to the best of our knowledge, there were few studies evaluating the condition of myocardial energy metabolism in patients with NAFLD. Furthermore, the precise relation between NAFLD and myocardial glucose uptake has not been properly explored.
In the present study, we thus enrolled a cohort of asymptomatic adults to investigate the association between NAFLD with alteration in myocardial glucose uptake assessed by using 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET).
Materials and Methods
Patients
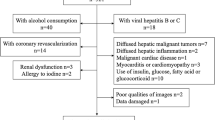
From December 1, 2011 to November 30, 2017, a total of 3750 consecutive asymptomatic subjects who were performed FDG PET/computed tomography (CT) for health examination at our institute were assessed retrospectively. Among this population, we exclude individuals from further analysis if they met any of the following criteria: (1) with history of alcohol intake exceeded 140 g/week; (2) had viral hepatitis B or C; (3) with history of CVD, including coronary artery stenosis ≥ 50%, myocardial infarction, heart failure or cardiovascular revascularization; (4) use of medications that might affect myocardial or hepatic glucose uptake within 6 months; (5) history of malignancy or metastasis; (6) poor qualities of PET/CT images that were unavailable for evaluation; (7) data damaged or data missed. Finally, a total of 743 subjects were included in the present study.
Clinical and Laboratory Measurements
Anthropometric data measured from all participants were height, weight, and blood pressure (BP). Body mass index (BMI) was calculated as weight (kg)/height2 (m2). The age and clinical history of the participant were obtained prior to the PET examination. Positive or negative smoking habits were grouped according to they ever or never smoked before. Diabetes was defined as a fasting glucose > 126 mg/dl or taking hypoglycemic medications. Hypertension was defined as systolic BP ≥ 140 mm Hg, diastolic BP ≥ 90 mmHg, or use of antihypertensive medication. Laboratory tests included fasting blood glucose (FBG), total cholesterol (TC), serum triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and r-glutamy1 transferase (r-GGT).
Echocardiographic Examination
All echocardiographic images were performed at standard views using a Vivid 9 GE ultrasound systems equipped with a M5S probe (1.7 -3.4 Hz). The left atrial (LA) diameter and LV diameter were measured by the parasternal long axis, and then the left ventricular M curve was obtained. LV end-systolic volume (LVESV), LV end-diastolic volume (LVEDV), LV posterior wall thickness (LVPWT) were measured and LV ejection fraction (LVEF) and stroke volume (SV) were calculated. Early diastolic peak velocity (E) and late diastolic peak velocity (A) were measured at the apical four-chamber view. The early diastolic velocity (e′) and late diastolic velocity (a′) were measured at the mitral annulus wall, and then E/A, e′/a′ and E/e′ were calculated, respectively. Cutoff value for increased LV filling pressure is average E/e′ ratio > 14 as guided by American Society of Echocardiography.16
18F-FDG PET/CT Protocol
All patients were required to fast for at least 6 h, and the serum glucose levels were < 110 mg/dl before administration of 18F-FDG. PET images were acquired approximately 1 hour later by a hybrid PET/CT scanner (GEMINI TF 64, Philips, Netherlands) after intravenous injection of 3.7 MBq/kg of 18F-FDG. A low-dose unenhanced CT scan was performed from the skull base to the middle of thigh, with the following parameters: 120 kvp, 80 mAs, pitch of 0.829, reconstruction thickness and interval of 5.0 mm, for precise anatomical localization and attenuation correction, and was followed by a three-dimension mode PET scan which matched the CT section thickness. The PET images were obtained using the ordered subset expectation maximization (OSEM) method (33 subsets per iteration). All collected data were transferred into Philips Extend Brilliance Workstation (EBW) 3.0 to reconstruct PET, CT, and PET/CT fusion images, respectively.
Image Analysis
All PET and CT imaging data measurement and analysis were finished on the Philips EBW. The presence of fatty liver was confirmed on the transverse unenhanced CT images by a single radiologist with 12 years of experience who was blinded to the clinical and laboratory data. A 3-cm diameter region of interest (ROI) was drawn over the right lobe of liver on transverse CT images, avoiding any visible vessel or lesions in either image. Identical size ROI was also placed at the spleen. The mean hepatic and splenic attenuation (Hounsfield units, HU) were respectively calculated by averaging of three ROI values on different transverse sections. Fatty liver was diagnosed when the hepatic attenuation was 1 HU less than that of spleen and the attenuation ratio of liver to spleen was less than 1.0.17,18 The degree of hepatic steatosis has been previously described,19 and the same standards, including mild, moderate and severe grades, were used in the present study.
The measurement and analysis of PET images were performed by an experienced nuclear physician who was blinded to CT, echocardiographic, anthropometric, and laboratory data. For semiquantitative analysis, a volume of interest was set on the transverse PET images to identify the maximum standardized uptake value (SUV) of left ventricular myocardium (SUVmyo) within an inner edge.19 We also calculated the mean SUV of liver (SUVliv) by averaging of three ROI values along the periphery of the right lobe, avoiding any obvious great vessel or local lesions. To reduce variability, the myocardium FDG uptake to the mean SUV of liver ratio (SUVratio) was calculated to estimate myocardial glucose uptake.
Statistical Analysis
Data analyses were performed with IBM SPSS Statistics (version 23.0, IBM Corp., Armonk, NY, USA). The independent t test was used to compare the differences between patients with and without NAFLD in continuous variables which was described as means ± standard deviations. The χ2 test was used to compare categorical data which were expressed as frequency and percentage. The differences of CT number, SUVmyo, SUVliv, and SUVratio in patients with and without NAFLD were compared by independent samples t test or Mann–Whitney U test. To assess the relationship between myocardium FDG uptake (SUVmyo) as a function of hepatic steatosis (HU), spearman correlation analysis was performed. The correlation coefficient for the correlation between these two variables was also calculated. High myocardium FDG uptake to the mean SUV of liver ratio was defined as the highest quartile of SUVratio. The trend analyses between myocardium FDG uptake (SUVratio), left ventricular diastolic function (E/e′ ratio), and fatty liver were performed by linear-by-linear association. Multivariate logistic regression analysis with NAFLD as a dependent variable was conducted to evaluate whether SUVratio quartiles were its independent factors after adjusting for clinically important factors. A P-value of less than .05 was considered statistically significant.
Results
General Characteristics of Subjects
The clinical and laboratory characteristics for study population with and without NAFLD are shown in Table 1. In our sample of 743 study participants, 61.64% (458/743) were male and 38.36% (285/743) were female with an average age of 51.36 ± 11.32 years (range 27-92 years). Among them, NAFLD was present in 201 (27.05%) subjects and absent in 542 (72.95%) subjects. Although the age and incidence of smoking had no significant differences between the two groups, NAFLD patients were more likely to be men, had higher BMI, and had a significantly higher prevalence of obesity (62.69% vs. 25.09%), diabetes (15.92% vs. 7.93%), hypertension (22.39% vs. 14.94%) and metabolic syndrome (6.47% vs. 1.85%) than the subjects without NAFLD. Compared with control group, laboratory findings showed significantly higher TC, TG, LDL-C, AST, and ALT in patients with NAFLD.
Echocardiographic Characteristics of Subjects
The echocardiographic characteristics for patients with and without NAFLD are illustrated in Table 2. Compared with control group, patients with NAFLD showed no significant difference in cardiac geometry, including LA diameter, LV diameter, LVESV, LVEDV, and LVPWT. Although LV systolic function reflected by LVEF and SV was similar between two groups, patients with NAFLD showed impaired LV diastolic function. The mitral E velocity and E/A ratio were significantly decreased, whereas A velocity and E/e′ ratio were obvious higher in patients with NAFLD (P all < .001).
The proportion of increased LV filling pressure (E/e′ ratio > 14) in patients with NAFLD was much higher than that of control group (19.61% vs. 4.24%). In mild, moderate and severe degree of fatty liver, the proportion of increased LV filling pressure showed a stepwise increase with the values of 9.76%, 28.85%, and 50.00%, respectively (Figure 1). When the proportion of individuals with NAFLD was stratified by quartiles of E/e′ ratio, a stepwise increase was showed with the increments of quartiles of E/e′ ratio (Figure 2). The linear-by-linear association analysis presented a strong positive relationship between liver steatosis and LV filling pressure (P < .001).
The proportion of increased LV filling pressure in individuals with and without NAFLD. The proportion of increased LV filling pressure (E/e′ ratio > 14) in patients with NAFLD was much higher than that of control group (P < .001). In mild, moderate, and severe degree of fatty liver, the proportion of increased LV filling pressure showed a stepwise increase
The association between liver steatosis and LV filling pressure. The proportion of individuals with NAFLD stratified by quartiles of E/e′ ratio showed a stepwise increase following the increments of quartiles of E/e′ ratio. The trend analysis presented a strong positive relationship between liver steatosis and LV filling pressure (P < .001)
Association of Myocardial FDG Uptake with NAFLD and E/e′ Ratio
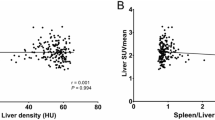
The liver and myocardium FDG uptake of subjects with and without NAFLD are illustrated in Table 3. The results showed that SUVliv had no difference between patients with and without NAFLD (P = .088). However, participants with NAFLD showed significant decreases in SUVmyo and SUVratio compared with control group (P all < .001). Spearman correlation analysis exhibited a strong negative relationship between SUVmyo and hepatic steatosis (HU), with a correlation coefficient of 0.3 (P < .001).
The proportion of high SUVratio in patients without NAFLD was significantly higher than that of NAFLD patients (28.78% vs. 12.44%, P < 0.001). In mild, moderate, and severe degree of fatty liver, the proportion of high SUVratio showed a stepwise decrease with the values of 14.63%, 9.62%, and 7.69%, respectively (Figure 3). When the proportion of individuals with NAFLD was stratified by quartiles of SUVratio, a stepwise decrease was showed with the increments of quartiles of SUVratio (Figure 4). When analysis of association trend was performed, SUVratio quartiles showed correlated inversely and strongly with liver steatosis (P < .001). In addition, patients with low quartiles of SUVratio were more likely to have increased E/e′ ratio than that of patients with high quartiles of SUVratio (P < .05) (Figure 5). The association of SUVratio quartiles with E/e′ ratio quartiles showed a significant relationship (P = .027) (Figure 6).
The proportion of high SUVratio in individuals with and without NAFLD. The proportion of high SUVratio in patients without NAFLD was significantly higher than that of NAFLD patients (28.78% vs. 12.44%, P < .001). In mild, moderate and severe degree of fatty liver, the proportion of high SUVratio showed a stepwise decrease
The association between liver steatosis and myocardial SUVratio. The proportion of individuals with NAFLD stratified by quartiles of SUVratio showed a stepwise decrease following the increments of quartiles of SUVratio. The trend analysis presented correlated inversely and strongly relationship between liver steatosis and myocardial SUVratio (P < .001)
The proportion of increased LV filling pressure in individuals stratified by quartiles of SUVratio. Patients with low quartiles of SUVratio were more likely to have higher proportion of increased LV filling pressure than that of patients with high quartiles of SUVratio (Q1 and Q2 vs. Q3 and Q4, P < .05)
Multivariate Logistic Regression Analysis for NAFLD According to Quartiles of SUVratio
Multivariate logistic regression analysis with NAFLD as a dependent variable was conducted to determine whether SUVratio quartiles were its independent factors after adjusting for clinically important factors. The results are summarized in Table 4. After adjusting of age and gender, the odds ratio (OR) for NAFLD risk was 8.452 (95% CI 5.061, 14.116) in the lowest quartile (Q1) of SUVratio compared to the highest quartile (Q4). Furthermore, SUVratio quartiles were independently associated with NAFLD after adjusting for all factors including age, gender, smoking, FBG, ALT, LDL-C, BMI, obese, metabolic syndrome, hypertension, diabetes, TC, TG, and HDL-C (adjusted OR for Q1 vs. Q4 of SUVratio = 7.351, 95% CI 4.051, 13.340).
Discussion
Our study evaluated the association between NAFLD and myocardial glucose uptake in a cohort of asymptomatic subjects. Myocardial glucose uptake was significantly lower in individuals with NAFLD compared with those without fatty liver. Furthermore, the present study demonstrated that NAFLD patients with lower myocardial glucose uptake were more likely to have higher proportion of increased LV filling pressure. This suggests that otherwise healthy subjects with NAFLD may be at risk of having impaired myocardial glucose metabolism and diastolic heart dysfunction.
In general, free fatty acids (FFA), glucose, and lactate are main energy sources for healthy heart. FDG is an analog of glucose, and thus physiological myocardial FDG uptake can be observed in normal heart. Previous studies reported that 18FDG uptake in normal heart showed variable myocardial glucose uptake because the affection of substrate metabolism, glucose load condition, and so on.20,21,–22 However, there were few studies to explore the condition of myocardial glucose metabolism in patients with NAFLD. Although previous study revealed that fasting myocardial glucose uptake was markedly decreased in patients with T2 diabetes mellitus (DM),23 the relationship between NAFLD and myocardial glucose uptake still remains unclear. In order to reduce the influence of other factors on myocardial FDG uptake, we excluded patients with CVD, using related medications and with history of malignancy. In addition, we used the average FDG uptake in the liver as a background comparator to reduce variability. Although there were contradictory results about the effect of hepatic steatosis on liver FDG uptake,24,25,–26 this study showed that the mean SUV of liver had no statistical difference between patients with and without NAFLD. Thus, the myocardium FDG uptake to the mean SUV of liver ratio (SUVratio) was calculated to estimate myocardial glucose uptake. Our study demonstrated that a stepwise decrease of myocardial glucose uptake was observed following the increments of degree of fatty liver. In agreement with previous study,19 the trend analysis in our study also presented a strong and inverse relationship between liver steatosis and myocardial glucose uptake.
However, the underlying mechanisms of the alteration of myocardial glucose uptake in patients with NAFLD still remain uncertain. Previous study reported that glucose was transported into cardiac myocytes by members of glucose transporters (GLUTs), mostly via insulin-sensitive GLUT4.27 It has been confirmed that patients with NAFLD are closely related to decreased insulin sensitivity at the whole body, and NAFLD is the most significant explanatory variable for myocardial insulin resistance.13 In addition, reduced insulin sensitivity is linked to impaired activity of the glucose transporter GLUT4 and increased amount of the circulating FFA.13,28 Therefore, based on the above mechanisms, impaired myocardial glucose uptake may be observed in patients with NAFLD, which is consistent with finding of the present study. In addition to impaired cardiac efficiency in insulin-resistant heart,29 decreased coronary flow reserve (CFR) was also found in patients with NAFLD in previous studies.30,31 Although the complex interplay between insulin resistant, epicardial fat-related adipokines and liver histology may be considered as common factors in promoting impaired myocardial glucose uptake and decreased CFR in NAFLD patients, its relationship still needs further study to explore. Multivariate regression analysis of this study showed that myocardial glucose uptake was independently associated with NAFLD after adjusting for clinical important factors, including age, gender, smoking, FBG, ALT, LDL-C, BMI, obese, metabolic syndrome, hypertension, diabetes, TC, TG, and HDL-C. However, limited by lacking of application software, several factors were not analyzed in this study, such as visceral adipose tissue and subcutaneous adipose tissue. Kim et al23 reported that visceral adiposity was significantly associated with the alteration of myocardial glucose uptake in patients with T2DM, but its influence on NAFLD patient needs to be confirmed by further study.
Recently, NAFLD has emerged as an independent risk factor for cardiovascular diseases, which leads to coronary artery disease, myocardial structure abnormality, cardiac dysfunction and, eventually, liver-related cardiovascular events.4,8,32,33 In fact, the pathogenic mechanisms linking NAFLD to cardiovascular complications are very complex, including the roles of genetic factors, atherosclerosis, inflammatory cytokines, insulin resistance, and so on.11,34,35 Although the cardiac geometry and LV systolic function in our study was similar between two groups, patients with NAFLD showed impaired LV diastolic function, including decreased E/A ratio and increased E/e′ ratio. Interestingly, patients with lower myocardial glucose uptake were more likely to have higher proportion of increased LV filling pressure than that of patients with higher myocardial glucose uptake. Our findings demonstrated that 12.9% of patients with low myocardial glucose uptake had increased E/A ratio, while only 6.63% had impaired diastolic heart function in patients with high myocardial glucose uptake. In patients with severe fatty liver and low myocardial glucose uptake, the proportion of LV diastolic dysfunction was even as high as 50%. Moreover, a significant relationship between myocardial SUVratio and E/e′ ratio was presented in the trend analysis. Those findings suggested that lower myocardial glucose uptake might be a possible indicator to link the strong relationship between hepatic steatosis and impaired diastolic heart function, which is considered as an independent predictor of mortality in patients with normal LV ejection fraction.36 Consistent with the results of the present study, some studies reported that patients with hepatic steatosis, as well as hepatic fibrosis were both significantly associated with LV diastolic dysfunction.19 Furthermore, those with steatohepatitis even had worsened alterations in right ventricular function compared with non-NAFLD counterparts.37 Because of the strong association of myocardial glucose uptake with NAFLD and diastolic heart function, it is important to assess the condition of myocardial glucose metabolism in patients with NAFLD for identifying those at high risk of future liver-related cardiac mortality. In addition, understanding the myocardial glucose metabolic consequences of NAFLD is important in evaluating potential mechanisms for disease progression and determining the optimal time to choose appropriate therapeutic strategies.
This study had several limitations. First, the cross-sectional study nature of present study did not allow to determine causal relationships among hepatic steatosis, myocardial glucose uptake, and heart function. Second, we did not perform liver biopsy to confirm the diagnosis of fatty liver and fatty liver grading because of the invasive nature of the procedure. However, the diagnostic criterion for fatty liver used in this study has high sensitivity and specificity according the results of previous studies.38,39,–40 Third, some other factors, such as visceral adipose tissue, subcutaneous adipose tissue and FFA, were not included in the analysis. Fourth, although we tried to perform echocardiography closely to PET/CT, echocardiography was not synchronous with PET/CT. Fifth, although fasting myocardial FDG uptake was required and the serum glucose levels of individuals were under control (< 110 mg/dl) before administration of 18FDG, it was hard for us to accurately estimate the real state of myocardial insulin sensitivity. Finally, the molecular mechanisms linking NAFLD to myocardial glucose uptake did not fully investigated in this study, therefore possible related mechanisms would be explored in further studies.
New Knowledge Gained
This study demonstrates that the presence of NAFLD in otherwise healthy subjects is closely associated with decreased myocardial glucose uptake. When analysis of association trend was performed, myocardial glucose uptake showed correlated inversely and strongly with liver steatosis. Furthermore, NAFLD individuals with lower myocardial glucose uptake are more likely to have high risk of having impaired diastolic heart function. In addition, myocardial glucose uptake is independently associated with NAFLD after adjusting for clinically important factors.
Abbreviations
- BMI:
-
Body mass index
- CT:
-
Computed tomography
- CVD:
-
Cardiovascular diseases
- FDG:
-
Fluorodeoxyglucose
- FFA:
-
Free fatty acids
- LV:
-
Left ventricular
- NAFLD:
-
Non-alcoholic fatty liver disease
- OR:
-
Odds ratio
- PET:
-
Positron emission tomography
- SUV:
-
Standardized uptake value
References
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73-84.
Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol 2013;178:38-45.
Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol 2018;68:268-79.
Haddad TM, Hamdeh S, Kanmanthareddy A, Alla VM. Nonalcoholic fatty liver disease and the risk of clinical cardiovascular events: A systematic review and meta-analysis. Diabetes Metab Syndr 2017;11(Suppl 1):S209-16.
Lee HJ, Lee CH, Kim S, Hwang SY, Hong HC, Choi HY, et al. Association between vascular inflammation and non-alcoholic fatty liver disease: Analysis by (18)F-fluorodeoxyglucose positron emission tomography. Metabolism 2017;67:72-9.
Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol 2008;49:608-12.
Sert A, Aypar E, Pirgon O, Yilmaz H, Odabas D, Tolu I. Left ventricular function by echocardiography, tissue doppler imaging, and carotid intima-media thickness in obese adolescents with nonalcoholic fatty liver disease. Am J Cardiol 2013;112:436-43.
Alp H, Karaarslan S, Eklioglu BS, Atabek ME, Altin H, Baysal T. Association between nonalcoholic fatty liver disease and cardiovascular risk in obese children and adolescents. Can J Cardiol 2013;29:1118-25.
Fotbolcu H, Yakar T, Duman D, Karaahmet T, Tigen K, Cevik C, et al. Impairment of the left ventricular systolic and diastolic function in patients with non-alcoholic fatty liver disease. Cardiol J 2010;17:457-63.
Fracanzani AL, Pisano G, Consonni D, Tiraboschi S, Baragetti A, Bertelli C, et al. Epicardial adipose tissue (EAT) thickness is associated with cardiovascular and liver damage in nonalcoholic fatty liver disease. PLoS ONE 2016;11:e0162473.
Pais R, Giral P, Khan J-F, Rosenbaum D, Housset C, Poynard T, et al. Fatty liver is an independent predictor of early carotid atherosclerosis. J Hepatol 2016;65:95-102.
Santoro N, Caprio S, Giannini C, Kim G, Kursawe R, Pierpont B, et al. Oxidized fatty acids: A potential pathogenic link between fatty liver and type 2 diabetes in obese adolescents? Antioxid Redox Sign 2014;20:383-9.
Lautamaki R, Borra R, Iozzo P, Komu M, Lehtimaki T, Salmi M, et al. Liver steatosis coexists with myocardial insulin resistance and coronary dysfunction in patients with type 2 diabetes. Am J Physiol Endocrinol Metab 2006;291:E282-90.
Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients 2013;5:1544-60.
Masoud WG, Ussher JR, Wang W, Jaswal JS, Wagg CS, Dyck JR, et al. Failing mouse hearts utilize energy inefficiently and benefit from improved coupling of glycolysis and glucose oxidation. Cardiovasc Res 2014;101:30-8.
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277-314.
Boyce CJ, Pickhardt PJ, Kim DH, Taylor AJ, Winter TC, Bruce RJ, et al. Hepatic steatosis (fatty liver disease) in asymptomatic adults identified by unenhanced low-dose CT. AJR Am J Roentgenol 2010;194:623-8.
Park YS, Park SH, Lee SS, Kim DY, Shin YM, Lee W, et al. Biopsy-proven nonsteatotic liver in adults: Estimation of reference range for difference in attenuation between the liver and the spleen at nonenhanced CT. Radiology 2011;258:760-6.
Lee YH, Kim KJ, Yoo ME, Kim G, Yoon HJ, Jo K, et al. Association of non-alcoholic steatohepatitis with subclinical myocardial dysfunction in non-cirrhotic patients. J Hepatol 2017. https://doi.org/10.1016/j.jhep.2017.11.023.
Hasegawa S, Kusuoka H, Uehara T, Yamaguchi H, Hori M, Nishimura T. Glucose tolerance and myocardial F-18 fluorodeoxyglucose uptake in normal regions in coronary heart disease patients. Ann Nucl Med 1998;12:363-8.
Tamaki N, Yonekura Y, Konishi J. Myocardial FDG PET studies with the fasting, oral glucose-loading or insulin clamp methods. J Nucl Med 1992;33:66-8.
van den Brom CE, Bulte CS, Loer SA, Bouwman RA, Boer C. Diabetes, perioperative ischaemia and volatile anaesthetics: consequences of derangements in myocardial substrate metabolism. Cardiovasc Diabetol 2013;12:42.
Kim G, Jo K, Kim KJ, Lee YH, Han E, Yoon HJ, et al. Visceral adiposity is associated with altered myocardial glucose uptake measured by (18)FDG-PET in 346 subjects with normal glucose tolerance, prediabetes, and type 2 diabetes. Cardiovasc Diabetol 2015;14:148.
Abikhzer G, Alabed YZ, Azoulay L, Assayag J, Rush C. Altered hepatic metabolic activity in patients with hepatic steatosis on FDG PET/CT. AJR Am J Roentgenol 2011;196:176-80.
Abele JT, Fung CI. Effect of hepatic steatosis on liver FDG uptake measured in mean standard uptake values. Radiology 2010;254:917-24.
Keramida G, Potts J, Bush J, Dizdarevic S, Peters AM. Hepatic steatosis is associated with increased hepatic FDG uptake. Eur J Radiol 2014;83:751-5.
Aerni-Flessner L, Abi-Jaoude M, Koenig A, Payne M, Hruz PW. GLUT4, GLUT1, and GLUT8 are the dominant GLUT transcripts expressed in the murine left ventricle. Cardiovasc Diabetol 2012;11:63.
Kouidhi S, Berrhouma R, Rouissi K, Jarboui S, Clerget-Froidevaux MS, Seugnet I, et al. Human subcutaneous adipose tissue Glut 4 mRNA expression in obesity and type 2 diabetes. Acta Diabetol 2013;50:227-32.
Mazumder PK, O’Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, et al. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes 2004;53:2366-74.
Yilmaz Y, Kurt R, Yonal O, Polat N, Celikel CA, Gurdal A, et al. Coronary flow reserve is impaired in patients with nonalcoholic fatty liver disease: Association with liver fibrosis. Atherosclerosis 2010;211:182-6.
Yilmaz Y, Kurt R, Gurdal A, Alahdab YO, Yonal O, Senates E, et al. Circulating vaspin levels and epicardial adipose tissue thickness are associated with impaired coronary flow reserve in patients with nonalcoholic fatty liver disease. Atherosclerosis 2011;217:125-9.
Wong VWS, Wong GLH, Yip GWK, Lo AOS, Limquiaco J, Chu WCW, et al. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut 2011;60:1721-7.
Lee SH, Yun SJ, Kim DH, Jo HH, Park YS. Severity of nonalcoholic fatty liver disease on sonography and risk of coronary heart disease. J Clin Ultrasound 2017;45:391-9.
Faasse S, Braun H, Vos M. The role of NAFLD in cardiometabolic disease: An update. F1000Res 2018;7:170.
Abenavoli L, Peta V. Role of adipokines and cytokines in non-alcoholic fatty liver disease. Rev Recent Clin Trials 2014;9:134-40.
Aljaroudi W, Alraies MC, Halley C, Rodriguez L, Grimm RA, Thomas JD, et al. Impact of progression of diastolic dysfunction on mortality in patients with normal ejection fraction. Circulation 2012;125:782-8.
Sunbul M, Kivrak T, Durmus E, Akin H, Aydin Y, Ergelen R, et al. Nonalcoholic steatohepatitis score is an independent predictor of right ventricular dysfunction in patients with nonalcoholic fatty liver disease. Cardiovasc Ther 2015;33:294-9.
Lee SW, Park SH, Kim KW, Choi EK, Shin YM, Kim PN, et al. Unenhanced CT for assessment of macrovesicular hepatic steatosis in living liver donors: Comparison of visual grading with liver attenuation index. Radiology 2007;244:479-85.
Pickhardt PJ, Park SH, Hahn L, Lee SG, Bae KT, Yu ES. Specificity of unenhanced CT for non-invasive diagnosis of hepatic steatosis: Implications for the investigation of the natural history of incidental steatosis. Eur Radiol 2012;22:1075-82.
Limanond P, Raman SS, Lassman C, Sayre J, Ghobrial RM, Busuttil RW, et al. Macrovesicular hepatic steatosis in living related liver donors: Correlation between CT and histologic findings. Radiology 2004;230:276-80.
Author Contributions
KT drafted the manuscript and contributed to the data analysis. XZ and JL contributed to clinical data acquisition and PET images analysis. MZ contributed to the data Statistical analysis. HL contributed to echocardiographic images analysis. TL contributed to CT images analysis. LW contributed to the study design and manuscript drafting. All authors read and approved the final version of the manuscript.
Disclosure
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tang, K., Zheng, X., Lin, J. et al. Association between non-alcoholic fatty liver disease and myocardial glucose uptake measured by 18F-fluorodeoxyglucose positron emission tomography. J. Nucl. Cardiol. 27, 1679–1688 (2020). https://doi.org/10.1007/s12350-018-1446-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-018-1446-x