Abstract
Backgorund
Quantification of myocardial blood flow (MBF) by positron emission tomography (PET) is important for investigation of angina in hypertrophic cardiomyopathy (HCM). Several software programs exist for MBF quantification, but they have been mostly evaluated in patients (with normal cardiac geometry), referred for evaluation of coronary artery disease (CAD). Software performance has not been evaluated in HCM patients who frequently have hyperdynamic LV function, LV outflow tract (LVOT) obstruction, small LV cavity size, and variation in the degree/location of LV hypertrophy.
Aim
We compared results of MBF obtained using PMod, which permits manual segmentation, to those obtained by FDA-approved QPET software which has an automated segmentation algorithm.
Methods
13N-ammonia PET perfusion data were acquired in list mode at rest and during pharmacologic vasodilation, in 76 HCM patients and 10 non-HCM patients referred for evaluation of CAD (CAD group.) Data were resampled to create static, ECG-gated and 36-frame-dynamic images. Myocardial flow reserve (MFR) and MBF (in ml/min/g) were calculated using QPET and PMod softwares.
Results
All HCM patients had asymmetric septal hypertrophy, and 50% had evidence of LVOT obstruction, whereas non-HCM patients (CAD group) had normal wall thickness and ejection fraction. PMod yielded significantly higher values for global and regional stress-MBF and MFR than for QPET in HCM. Reasonably fair correlation was observed for global rest-MBF, stress-MBF, and MFR using these two softwares (rest-MBF: r = 0.78; stress-MBF: r = 0.66.; MFR: r = 0.7) in HCM patients. Agreement between global MBF and MFR values improved when HCM patients with high spillover fractions (> 0.65) were excluded from the analysis (rest-MBF: r = 0.84; stress-MBF: r = 0.72; MFR: r = 0.8.) Regionally, the highest agreement between PMod and QPET was observed in the LAD territory (rest-MBF: r = 0.82, Stress-MBF: r = 0.68) where spillover fraction was the lowest. Unlike HCM patients, the non-HCM patients (CAD group) demonstrated excellent agreement in MBF/MFR values, obtained by the two softwares, when patients with high spillover fractions were excluded (rest-MBF: r = 0.95; stress-MBF: r = 0.92; MFR: r = 0.95).
Conclusions
Anatomic characteristics specific to HCM hearts contribute to lower correlations between MBF/MFR values obtained by PMod and QPET, compared with non-HCM patients. These differences indicate that PMod and QPET cannot be used interchangeably for MBF/MFR analyses in HCM patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Hypertrophic cardiomyopathy (HCM) is the most common genetic cardiovascular disease with a prevalence of ~ 1:500 in the general population.1 Chest pain at rest and/or exertion due to microvascular dysfunction is a common symptom in HCM patients.2 Pathologic studies reveal abnormalities in intramural coronary arterioles and relative reduction of capillary density in the setting of increased left ventricular mass, which contribute to the development of microvascular dysfunction, myocardial ischemia, heart failure, and death in HCM.3,4 Hence, there is a need for imaging modalities that permit quantification of myocardial blood flow (MBF) and assessment of myocardial ischemia in HCM patients.
Positron emission tomography (PET) permits noninvasive quantification of myocardial blood flow (MBF) and hence, is an important clinical tool for the investigation of angina in HCM patients.5 Several methods that implement either a one- or two-compartment model are currently available for measuring myocardial blood flow (MBF). But most of the testing has been performed in patients with normal cardiac geometry, referred for evaluation of coronary artery disease (CAD). It is unknown whether these methods work well in HCM patients, who have LV hypertrophy that can be localized (septum, anterior/lateral/inferior wall, papillary muscles, apex) or generalized.1 Furthermore, HCM patients tend to have hypercontractility of the LV, resulting in narrowing of the left ventricular outflow tract and/or obliteration of the LV cavity during systole, and reduced LV cavity size during diastole (Figure 1). These anatomic features of HCM hearts can affect segmentation and/or sampling of counts in the blood pool and myocardium to obtain input curves for MBF measurements, using automated tools.6 Hence, MBF analysis results obtained using manual segmentation could differ from the results obtained using automated segmentation algorithms.
In this study, we examined the agreement between results of MBF and MFR, obtained using PMod (that permits manual segmentation of the heart) and QPET (that has automated segmentation) in HCM patients who underwent 13N-ammonia PET for clinical indications. Results from HCM patients were compared to a small number of non-HCM patients who underwent 13N-ammonia PET for assessment of CAD (CAD group.)
Methods
Study Subjects
This study was approved by the Institutional Review Board at Johns Hopkins. Patients enrolled in the HCM Registry at Johns Hopkins were retrospectively studied if they fulfilled the standard diagnostic criteria for HCM (left ventricular hypertrophy in the absence of other causes such as uncontrolled hypertension, valvular disease, amyloidosis) and underwent 13N-ammonia PET myocardial perfusion imaging. The cohort was composed of 76 consecutive HCM patients without obstructive CAD, and 10 non-HCM patients referred for evaluation of CAD (with spillover fractions < 0.65), who underwent 13NH3-PET imaging, and had good image quality.
13N-Ammonia Myocardial Perfusion Imaging Protocol
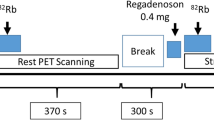
All patients underwent cardiac PET/CT scanning using a GE Discovery VCT PET/CT system (GE Healthcare, Waukesha, Wisconsin). Patients were positioned with the assistance of a computed tomographic (CT) topogram, and a low-dose CT scan was performed for attenuation correction of PET emission data. Motion correction was applied at the time of acquisition to correct misalignment between the PET and the CT. Subsequently, PET images were acquired using a same-day rest/stress protocol as follows: approximately 370 MBq (10 mCi) 13N-ammonia was bolus injected over 30 s at a constant rate (1200 ml·h−1) using a power injector, and a 2-dimensional list-mode PET scan was obtained over 20 min. Approximately 60 min after injection of the rest dose, dipyridamole or regadenoson was administered. Dipyridamole was the drug of choice at the beginning of our PET protocol for HCM patients in 2009, but from June 2011, all HCM patients were administered regadenoson. Dipyridamole (0.56 mg/kg) was infused over a period of 4 min, followed by a second dose of 13N-ammonia (~ 370 MBq; 10 mCi), 4 min after the end of dipyridamole infusion. Regadenoson (0.4 mg/5 ml) was injected as a bolus (over 15-20 s), followed by a 5-ml saline flush, and 13N-ammonia was administered 30 s later. Stress acquisition was begun concomitantly with the second 13N-ammonia injection, with all other parameters being the same as during rest. Heart rate, blood pressure, and a 12-lead electrocardiogram (EKG) were recorded before, during, and after completion of the stress protocol. List-mode data were resampled to create static (4-min prescan delay), EKG-gated (eight bins per cardiac cycle), and 36-frame-dynamic (20 × 6, 5 × 12, 4 × 30, 5 × 60, and 2 × 300 s) images.7
Myocardial Perfusion Imaging Analysis
Attenuation-corrected PET images were reconstructed incorporating the motion-correction parameters, using an iterative algorithm with postprocessing filtering. The CardIQ Physio package (GE Healthcare) was used for analysis of both EKG-gated and nongated static datasets, including oblique reorientation in the transverse planes, definition of the valve plane, quality control of automated contour detection, and software-derived calculation of EKG-gated-LVEF from the rest and stress datasets.8
Myocardial Blood Flow Analysis
Global and regional MBF values in ml/min/g and MFR (myocardial flow reserve) values were obtained with each commercial tool. LV contours and volume of interest (VOI) for the input function were obtained automatically with minimal operator intervention using QPET (version 2012, Cedars-Sinai, Los Angeles, United States), but manual segmentation was performed using PMod (version 3.4, PMOD Technologies Ltd. Zurich, Switzerland). Both programs used the same modified 2-tissue-compartment model developed by Choi et al without metabolite correction.9 This model assumed a constant recovery coefficient of 0.75 based on an assumed uniform myocardial wall thickness of 1 cm. Only the first 2 min of the dynamic acquisition were used for curve fitting and to estimate parameters (MBF and spillover), using a weighted least-squares method. Both software programs corrected for spillover from the LV and RV cavities. Mean-MBF was calculated by time-activity curves and analyzed independently by two expert operators (HY, AT.)
QPET Processing
Left ventricular contours were positioned automatically using a recently described improved QPET algorithm.10 The LV contour was determined from the summed dynamic image data, skipping the first 2 min using the QPET algorithm10 which is based on the original quantitative gated SPECT contour detection principles,11 but takes into consideration, the better valve definition of attenuation-corrected high-resolution PET. The VOI for the LV-input function was automatically placed in the middle of the atrio-ventricular plane and was cylindrical with a 1-cm radius and 2-cm length, with its long axis oriented along the long axis of the heart. Dynamic myocardial samples were obtained from the polar map by analyzing all time frames. Stress-MBF and rest-MBF in ml/min/g were computed for each segment in the polar map. Interpolation was employed, and computation was performed in 17 myocardial segments with equal surface area. Regional flow was computed for each segment. These calculations were then interpolated using a surface-area-weighted bicubic method to determine MBF values for each segment in the polar map. Myocardial flow reserve (MFR) was computed by dividing each stress polar map value by the rest value in each segment. Global rest-MBF, stress-MBF, and MFR were computed within the entire LV myocardium bounded by the atrio-ventricular valve plane. Regional MBF was obtained by dividing the polar map into three regions (left anterior descending artery, left circumflex coronary artery, and right coronary artery) obtained from the standard 17-segment American Heart Association model using the group option in QPET.
PMod Processing
After loading the DICOM files, PMOD automatically generated blood pool and myocardial images from dynamic uptake series by averaging respective frames, from 0 to 36 s for blood pool, and from 2 to 20 min for myocardium. Then, standard reorientation of the heart was performed. The VOI of the LV contour was generated using the EPI/ENDO method with manual adjustment. The VOI for input function was positioned in the same location as for QPET processing (Figure 2). A previously validated tracer kinetic model was fit to the tissue time-activity curves, providing values of regional and global MBF (mL·min−1·g−1). PMod generated a series of polar maps and parameters including MBF at stress, MBF at rest and MFR. The characteristics of QPET and PMod are listed in Supplementary Table 1.
Segmentation of PET images using QPET and PMod. A Representative images of LV segmentation of PET images in an HCM patient, with maximum wall thickness of 3.4 cm, using PMod and QPET softwares. The VOI for LV-input function was placed in atrio-ventricular valve plane, with its long axis oriented along the long axis of the heart. The RV-VOI was placed in right ventricle in the horizontal long axis, in order to correct for spillover in the septum. HLA horizontal long axis, VLA vertical long axis. B CMR image of the same patient showing left ventricular hypertrophy
Transient left Ventricular Cavity Dilation Following Vasodilator Stress
The PET-LVCD-index was obtained by dividing the LV volume during peak vasodilator stress by the LV volume at rest. HCM patients with an index > 1.13 were considered to have LVCD.8
Statistical Analysis
We used SPSS 23.0 (IBM Corp, Somers, NY) for statistical analysis. Variables are presented as mean ± standard deviation. Comparison of stress-MBF, rest-MBF, and MFR obtained using QPET and PMod was performed using the paired t test. A comparison of values obtained between these two methods was evaluated using Pearson correlation or Bland-Altman analysis. Interobserver variability was assessed using Pearson correlation. A value of p < .05 was considered statistically significant.
Results
Patient Characteristics
We studied 76 patients with a clinical diagnosis of HCM. All HCM patients had evidence of asymmetric septal hypertrophy; approximately 50% of HCM patients had evidence of left ventricular outflow tract obstruction—Table 1. All non-HCM patients had normal wall thickness and LVEF.
Global MBF Analysis in HCM
PMod yielded higher values for global stress-MBF and MFR than QPET (Table 2), which resulted in a higher number of HCM patients being classified as having normal stress-MBF (≥ 1.8 mL·min−1·g−1) and MFR (≥ 2.0) values12 by PMod, when compared to QPET (Supplemental Table 2). Next, we examined correlations between SDS scores (which have been validated extensively13,14) and MBF values obtained by QPET and PMod in HCM patients (with SDS ≥ 2) in an attempt to assess which software performed better in HCM. We detected a statistically significant inverse correlation only between MFR values obtained by PMod, and SDS in HCM patients (Supplemental Table 3, Figure 4).
Since high spillover fractions can result in unreliable data, we used the cutoff of 0.65 established by Slomka et al,15 as an indicator of extremely high spillover. PMod resulted in markedly higher values of global stress-MBF in HCM patients with high spillover fractions, which led to higher global stress-MBF values in the population as a whole (Table 2). However, when patients with high spillover fractions were excluded, QPET resulted in higher MFR values than PMod.
We observed fair agreement between QPET and PMod for global rest-MBF, stress-MBF, and MFR using PMod and QPET (Figure 3A, B; Table 2). Correlations between global rest-MBF, stress-MBF, and MFR improved when HCM patients whose data contained high spillover fractions were excluded from the analysis (Figure 3C, D; Table 2). With regard to spillover, we found no difference in spillover fraction values (PMod: 0.34 ± 0.23, QPET 0.31 ± 0.06, p = .25), and modest agreement between PMod and QPET for spillover fractions in HCM patients (r = 0.64, p < .001) (Figure 4).
Correlation and Bland-Altman plots for MBF analysis using QPET and PMod software. A, B. Correlation analysis and Bland-Altman plots for stress-MBF, rest-MBF, and MFR in entire HCM cohort (n = 76). Y-axes in Bland-Altman plots represent difference between PMod and QPET values, and X-axes represent the mean of PMod and QPET values in HCM patients. C, D. Correlation analysis and Bland-Altman plots for QPET and PMod, for stress-MBF, rest-MBF, and MFR values in HCM patients (n = 53) after excluding patients with high spillover fractions
Relationship between SDS and MFR obtained using PMod. A statistically significant inverse correlation (r = − 0.38, p = .004) was present between SDS and MFR values obtained using PMod in HCM patients with SDS ≥ 2 (n = 57). Solid line represents the regression line, and dashed lines represent the 95% confidence interval
Regional MBF Analysis in HCM
PMod yielded higher values for stress-MBF and MFR for the LAD, LCx, and RCA territories, when compared to QPET, but rest-MBF values were similar in the 3 coronary territories (Table 3). The best correlations between QPET and PMod were obtained in the LAD territory, where spillover fraction was the lowest (Table 3). The range for spillover fractions was 0.09-0.69 for the LAD territory, 0.17-0.72 for the LCx territory, and 0.14-0.67 for the RCA territory.
Next, we compared rest-MBF, stress-MBF, and MFR values between PMod and QPET in the hypertrophied and non-hypertrophied regions (identified by echocardiography/CMR), after excluding patients with high spillover fractions. Higher values for stress-MBF and MFR were obtained using PMod, in both non-hypertrophied and hypertrophied walls, but rest-MBF was similar in non-hypertrophied and hypertrophied walls. The best correlations between the two softwares were obtained for rest-MBF in both hypertrophied and non-hypertrophied walls (Table 4).
Global MBF Analysis in non-HCM Patients
In order to assess whether cardiac anatomic characteristics specific to HCM influence MBF analysis obtained by QPET and PMod, we examined agreement between QPET and PMod in 10 non-HCM patients referred for evaluation of CAD, after excluding patients with spillover fractions > 0.65. Excellent agreement was observed between QPET and PMod in the 10 non-HCM patients, in contrast to modest agreement in the HCM cohort. (Supplementary Table 4).
Interobserver Variability
Interobserver agreement was excellent for global MBF and MFR values obtained by QPET and PMod in HCM patients (Supplemental Table 5).
Discussion
Quantification of hyperemic MBF and MFR by PET permits noninvasive assessment of microvascular function and inducible ischemia in HCM patients with angina and arrhythmias. Previous studies have demonstrated that HCM patients who lack obstructive epicardial disease often demonstrate blunting of hyperemic MBF, but preservation of rest-MBF.16,17 Most importantly, HCM patients who have reduction in hyperemic MBF during pharmacologic vasodilatation have been demonstrated to have an increased propensity to develop adverse LV remodeling, heart failure, and death.18 Hence, tools that can reliably compute global and regional blood flow in HCM are needed.5,19
New PET/CT systems permit acquisition in list mode and reformatting of data into dynamic, static, or gated frames as required. These advances aid clinical practice,20 but in order to be utilized for clinical decision-making, the reference limits for different methods need to be determined. This has been accomplished by Slomka et al for patients with normal cardiac geometry, referred for evaluation of obstructive CAD,15 but very limited comparative data are available in the HCM patient population.
Differences in MBF Values Using PMod and QPET in HCM
In this study, we compared global and regional MBF values obtained by QPET and Pmod in HCM patients, utilizing the same two-compartment model. The UCLA model (Choi) that was used for MBF analysis by QPET and PMod assumes a constant recovery coefficient of 0.75, based on an assumed uniform myocardial wall thickness of 1 cm. While this assumption is likely to be inaccurate, particularly for HCM patients who have variability in the degree and location of hypertrophy, it should (in principle) be consistent between software implementations. The aim of our study was to test this assumption and any other differences in software implementations.
Bland-Altman plots revealed modest agreement between the two software programs in HCM patients, but excellent agreement in a small cohort of non-HCM patients, who were referred for evaluation of CAD. This result suggests that cardiac anatomic characteristics specific to HCM patients contribute to differences in MBF values observed using these two softwares.
High Spillover Fractions in HCM
The prevalence of high spillover fractions was higher in the HCM cohort when compared to CAD patients evaluated by Slomka et al15 (30% in HCM, vs 7% in CAD.15) High spillover in HCM could be related to LV hypertrophy, small LV cavity size, and hypercontractility resulting in inclusion of signal from myocardium in the image-derived input function. This would subsequently lead to bad fits of the kinetic modeling curves and result in high spillover fractions. Another possible contributor to high spillover is motion during the PET acquisition, which was not corrected.
In our study, QPET yielded higher global stress-MBF and MFR values when patients with high spillover fractions were excluded. Since VOIs generated by PMod (which permits manual segmentation) would be larger than corresponding QPET-VOIs, the mean-MBF values resulting from PMod would be lower than those generated by QPET, which are thinner. For thinner VOIs, partial volume underestimation would not be as significant, and consequently, the mean-MBF values would be higher while using QPET, compared with PMod.
In practical terms, the high spillover fraction remains a limitation for clinical analysis because it leads to variability in MBF results. Although there is no better spillover correction method in the modeling to avoid such discrepancies, a high spillover fraction provided by quantitative software should raise concern about an overestimation of the calculated MBF value. We found no differences in spillover fractions between PMod and QPET in HCM patients, suggesting that differences in spillover fractions do not underlie differences in MBF values obtained using PMod and QPET, in HCM patients. Another interesting observation was higher correlation between MBF values obtained by QPET and PMod in the RCA territory than in the LCx territory, despite the fact that the RCA territory had the highest prevalence of spillover fractions ≥ 0.65. This result, and persistence of a modest agreement in global and regional MBF/MFR values after excluding patients with high spillover fractions, led us to conclude that high spillover is not the only reason for the modest agreement in MBF/MFR values in HCM patients.
Left Ventricular Hypertrophy and MBF
We observed higher correlations in stress-MBF using PMod and QPET, in hypertrophied walls, when compared to non-hypertrophied walls—this could be due to less partial volume effects in hypertrophied walls, when compared to non-hypertrophied walls.
CAD Patients Demonstrate Better Agreement in MBF Analyses than HCM
The study by Slomka et al15 demonstrated excellent correlation between QPET and PMod in CAD patients. Our results in non-HCM patients were similar to those obtained by Slomka et al15 The modest agreement in MBF values obtained in the HCM cohort are likely related to the cardiac anatomic characteristics, namely, variability in degree and location of LV hypertrophy, small LV cavity size, and hypercontractility of HCM hearts.
Limitations
This is a retrospective study comparing two software programs with several limitations. First, we did not have a phantom or a gold standard for quantification of myocardial blood flow using the software systems. Hence, we are unable to conclude with certainty, whether one software system is more accurate than the other for quantifying MBF in HCM patients. But based on our results of a significant inverse correlation only between PMod-MFR and SDS, we speculate that PMod may perform better than QPET in HCM patients. Second, since stress-MBF ≥ 1.8 mL/min/g and MFR ≥ 2.0 are commonly used as a cutoff to distinguish normal or abnormal myocardial hyperemic flow increases,12 QPET may have overestimated the number of HCM patients with abnormal hyperemic flows. Prospective studies need to be designed in order to ascertain whether MBF/MFR thresholds validated in non-HCM patients are applicable in the HCM patient population.
New Knowledge Gained
The present work compared two software systems for MBF assessment in HCM patients who have abnormal geometry of the LV, variable LV wall thickness, and hypercontractility. Our results indicate that these two software programs cannot be used interchangeably in HCM patients. The observed differences in global and regional MBF values obtained by PMod and QPET could be attributed to cardiac anatomic characteristics specific to HCM, often leading to high spillover fractions and variations in estimated parameters.
Conclusions
PMod and QPET yield significant differences in stress-MBF values and modest agreement between MBF values in HCM patients. Hence, these two software systems cannot be used interchangeably for MBF analyses in HCM patients. Additional studies are needed to establish normal reference ranges for HCM patients, using clinical software programs.
Change history
08 February 2018
The following information is missing from the Funding footnote on the first page of the published article: “This study was partly funded by NIH RO1 HL092985.” The last/corresponding author is incorrectly listed on the first page of the published article: The correct name is Abraham MR.
Abbreviations
- HCM:
-
Hypertrophic cardiomyopathy
- PET:
-
Positron emission tomography
- MBF:
-
Myocardial blood flow
- MFR:
-
Myocardial flow reserve
- LAD:
-
Left anterior descending coronary artery
- RCA:
-
Right coronary artery
- LCx:
-
Left circumflex coronary artery
- LV:
-
Left ventricle
- FDA:
-
Food and drug administration
- CT:
-
Computerized tomography
- MBq:
-
Megabecquerel
- mCi:
-
Millicurie
- EKG:
-
Electrocardiography
- GE:
-
General Electric
- ROI:
-
Region of interest
- LVOTG:
-
Left ventricular outflow tract gradient
- SDS:
-
Summed difference score
- LVCD:
-
Left ventricular cavity dilation
- LVEF:
-
Left ventricular ejection fraction
- SD:
-
Standard deviation
References
Maron BJ. Hypertrophic cardiomyopathy: A systematic review. JAMA 2002;287:1308-20.
Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, et al. Task Force on Clinical Expert Consensus Documents. American College of C and Committee for Practice Guidelines. European Society of C. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol 2003;42:1687-713.
Cecchi F, Olivotto I, Gistri R, Lorenzoni R, Chiriatti G, Camici PG. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med 2003;349:1027-35.
Olivotto I, Cecchi F, Gistri R, Lorenzoni R, Chiriatti G, Girolami F, et al. Relevance of coronary microvascular flow impairment to long-term remodeling and systolic dysfunction in hypertrophic cardiomyopathy. J Am Coll Cardiol 2006;47:1043-8.
Klein R, Renaud JM, Ziadi MC, Thorn SL, Adler A. Beanlands RS and deKemp RA. Intra- and inter-operator repeatability of myocardial blood flow and myocardial flow reserve measurements using rubidium-82 pet and a highly automated analysis program. J Nucl Cardiol 2010;17:600-16.
Czernin J, Muller P, Chan S, Brunken RC, Porenta G, Krivokapich J, et al. Influence of age and hemodynamics on myocardial blood flow and flow reserve. Circulation 1993;88:62-9.
Yalcin H, Valenta I, Yalcin F, Corona-Villalobos C, Vasquez N, Ra J, et al. Effect of diffuse subendocardial hypoperfusion on left ventricular cavity size by 13 N-ammonia perfusion PET in patients with hypertrophic cardiomyopathy. Am J Cardiol 2016;118:1908-15.
Rischpler C, Higuchi T, Fukushima K, Javadi MS, Merrill J, Nekolla SG, et al. Transient ischemic dilation ratio in 82Rb PET myocardial perfusion imaging: Normal values and significance as a diagnostic and prognostic marker. J Nucl Med 2012;53:723-30.
Choi Y, Huang SC, Hawkins RA, Kuhle WG, Dahlbom M, Hoh CK, et al. A simplified method for quantification of myocardial blood flow using nitrogen-13-ammonia and dynamic PET. J Nucl Med 1993;34:488-97.
Slomka PGG, Kavanagh P, Javadi M, Berman D, Bengel F. Evaluation of a new automatic algorithm for quantification of ECG-gated 82Rb cardiac PET. J Nucl Med 2009;50:217P.
Germano G, Kiat H, Kavanagh PB, Moriel M, Mazzanti M, Su HT, et al. Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. J Nucl Med 1995;36:2138-47.
Danad I, Uusitalo V, Kero T, Saraste A, Raijmakers PG, Lammertsma AA, et al. Quantitative assessment of myocardial perfusion in the detection of significant coronary artery disease: Cutoff values and diagnostic accuracy of quantitative [(15)O]H2O PET imaging. J Am Coll Cardiol 2014;64:1464-75.
Shaw LJ, Iskandrian AE. Prognostic value of gated myocardial perfusion SPECT. J Nucl Cardiol 2004;11:171-85.
Giubbini R, Peli A, Milan E, Sciagra R, Camoni L, Albano D, et al. Comparison between the summed difference score and myocardial blood flow measured by 13 N-ammonia. J Nucl Cardiol 2017. https://doi.org/10.1007/s12350-017-0789-z.
Slomka PJ, Alexanderson E, Jacome R, Jimenez M, Romero E, Meave A, et al. Comparison of clinical tools for measurements of regional stress and rest myocardial blood flow assessed with 13 N-ammonia PET/CT. J Nucl Med 2012;53:171-81.
Cannon RO 3rd, Dilsizian V, O’Gara PT, Udelson JE, Tucker E, Panza JA, et al. Impact of surgical relief of outflow obstruction on thallium perfusion abnormalities in hypertrophic cardiomyopathy. Circulation 1992;85:1039-45.
Camici P, Chiriatti G, Lorenzoni R, Bellina RC, Gistri R, Italiani G, et al. Coronary vasodilation is impaired in both hypertrophied and nonhypertrophied myocardium of patients with hypertrophic cardiomyopathy: A study with nitrogen-13 ammonia and positron emission tomography. J Am Coll Cardiol 1991;17:879-86.
Nagueh SF, Bierig SM, Budoff MJ, Desai M, Dilsizian V, Eidem B, et al. American Society of E, American Society of Nuclear C, Society for Cardiovascular Magnetic R and Society of Cardiovascular Computed T. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy: Endorsed by the American Society of Nuclear Cardiology, Society for Cardiovascular Magnetic Resonance, and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 2011;24:473-98.
Camici PG, Rimoldi OE. The clinical value of myocardial blood flow measurement. J Nucl Med 2009;50:1076-87.
Beanlands RS, Ziadi MC, Williams K. Quantification of myocardial flow reserve using positron emission imaging the journey to clinical use. J Am Coll Cardiol 2009;54:157-9.
Acknowledgements
The authors would like to acknowledge help from the Image Response Assessment Team (IRAT) (NIH P30CA006973) with PET image retrieval.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
All authors declare that they have no conflict of interest.
Additional information
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for reuse at meetings and presentations. Search for the article DOI on SpringerLink.com.
Funding
This work was supported by the JTB (John Taylor Babbit) Foundation. Dr. H. Yalçin was supported by a Fulbright Fellowship (Bureau of Educational and Cultural Affairs, United States Department of State). Dr. Zhao was supported by Elite Program of Xiangya Hospital, Central South University, China. Dr. Lu was supported by Taipei Veterans General Hospital-National Yang-Ming University Excellent Physician Scientists Cultivation Program, No. 104-V-A-005.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yalcin, H., Valenta, I., Zhao, M. et al. Comparison of two software systems for quantification of myocardial blood flow in patients with hypertrophic cardiomyopathy. J. Nucl. Cardiol. 26, 1243–1253 (2019). https://doi.org/10.1007/s12350-017-1155-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-017-1155-x