Abstract
Objectives
We examined whether regional improvement in stress myocardial blood flow (sMBF) following angiography-guided coronary revascularization depends on the existence of a perfusion defect on positron emission tomography (PET).
Background
Percent stenosis on coronary angiography often is the main factor when deciding whether to perform revascularization, but it does not reliably relate to maximum sMBF. PET is a validated method of assessing sMBF.
Methods
19 patients (79% M, 65 ± 12 years) underwent PET stress before and after revascularization (17 PCI, 2 CABG). Pre- and post-revascularization sMBF for each left ventricular quadrant (anterior, septal, lateral, and inferior) was stratified by the presence or absence of a baseline perfusion defect on PET and whether that region was revascularized.
Results
Intervention was performed on 40 of 76 quadrants. When a baseline perfusion defect existed in a region that was revascularized (n = 26), post-revascularization flow increased by 0.6 ± 0.7 cc/min/g (1.2 ± 0.4 vs 1.7 ± 0.8, P < 0.001). When no defect existed but revascularization was performed (n = 14), sMBF did not change significantly (1.7 ± 0.3 vs 1.5 ± 0.4 cc/min/g, P = 0.16). In regions without a defect that were not revascularized (n = 29), sMBF did not significantly change (2.0 ± 0.6 vs 1.9 ± 0.7, P = 0.7).
Conclusions
When a stress-induced perfusion defect exists on PET, revascularization improves sMBF in that region. When there is no such defect, sMBF shows no net change, whether or not intervention is performed in that area. PET stress may be useful for identifying areas of myocardium that could benefit from revascularization, and also areas in which intervention is unlikely to yield improvement in myocardial blood flow.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Current practice guidelines for the management of coronary artery disease (CAD) employ both measurements based on anatomy (percent stenosis) and physiology (fractional flow reserve [FFR]) to guide coronary revascularization.1,2 However, physiologic assessment using FFR during angiography is performed in only 6% of percutaneous coronary interventions (PCI) in the United States.3,4 Consequently, percent stenosis on coronary angiography most often is the main determinant when deciding whether to proceed with either PCI or coronary artery bypass grafting (CABG) in patients with CAD.
The main purpose of mechanical revascularization is to improve myocardial blood flow such that morbidity and mortality are reduced. Coronary angiography frequently is unable to adequately characterize the hemodynamic impact of coronary stenoses. In particular, percent stenosis does not predict or reliably relate to maximum coronary blood flow capacity or coronary flow reserve (CFR).5 - 7 Results from the COURAGE, FAME, and FAME 2 trials demonstrate that the use of FFR improves outcomes compared to percent stenosis-based PCI or optimal medical therapy alone.8 - 10 It is plausible; therefore, that when epicardial vessels are targeted for revascularization based solely on visually determined percent stenosis, intervention may not lead to a significant improvement in flow capacity. In addition, intervening on lesions that are not flow limiting may pose an unfavorable risk/benefit relationship.
Non-invasive myocardial perfusion imaging by positron emission tomography (PET) offers high-spatial resolution and is the gold standard for assessment of myocardial blood flow (MBF) under both resting and hyperemic conditions.11 - 16 As such, cardiac PET may be useful for identifying potential improvement (or the lack thereof) in coronary blood flow in patients being evaluated for coronary revascularization.17
We hypothesized that regional improvement in stress myocardial blood flow (sMBF) following angiography-guided coronary revascularization depends on the existence of a stress-induced perfusion defect on PET.
Methods
The study was approved by the Ochsner Medical Center Institutional Review Board. Patients who underwent PET stress before and after coronary revascularization (PET 1 and PET 2, respectively) were identified through a hospital database query at the Ochsner Heart and Vascular Institute. Only patients whose left heart catheterization was triggered by PET 1 and who were subsequently revascularized prior to PET 2 were included. PET 2 was obtained when clinically indicated according to appropriate use criteria, modeling what would be encountered in typical practice. Due to the potential confounding resulting from the wide variety of techniques utilized for CTO intervention, such as beta-radiation, cutting balloons, and retrograde approach via collateral vessels, we excluded patients with intervention on chronic total occlusions (CTOs).
Image Acquisition and Reconstruction
Patients were instructed to fast for 4 h and to abstain from caffeine, theophylline, and cigarettes for 24 h prior to image acquisition. Cardiac imaging was performed using list-mode 2D acquisition on an Attrius PET scanner (Positron, Westmont, IL) with Ge-68 rod source attenuation correction. Rest emission data were obtained in list mode over 7 min beginning immediately upon intravenous injection of 35 to 50 mCi (1295 to 1850 MBq) at 50 ml/min of generator-produced Rb-82 (Bracco Diagnostics, Monroe Township, NJ). The first two minutes of the emission images were binned to form the arterial input data. The final five minutes of the emission images were binned to form the myocardial uptake data. Hyperemia was achieved with intravenous infusion of either dipyridamole [142 mcg/(kg min)] or regadenoson (.4 mg). For stress imaging, the same dose of radiotracer was given as for the rest acquisition, and emissions images were binned similarly. Rod source transmission scan containing 28 million counts for segmented attenuation correction was performed prior to the resting emission scan and after the stress emission scan on all studies.18 Images were reconstructed with filtered back projection, with a Butterworth filter order of 5 and 0.04 cycles/mm, corresponding to a cutoff of 0.16 for the input pixel dimensions of 2 × 2 × 2.559 mm, displayed with image dimensions of 256 × 256. Fused emission and transmission images were optimized by shifting as needed. Corrected image sets were oriented into short-axis, vertical, and horizontal long-axis tomographic slices as well as polar and Mercator projection images.19 - 21 Three-dimensional topographic views of the left ventricle were reconstructed on an Ultra 60 workstation (Sun Microsystems/Oracle, Santa Clara, CA) running CARDIAC version 4.66, reflecting relative regional activity distribution at rest and after stress. These 3D topographic views were then divided into fixed sections consisting of anterior, septal, lateral, and inferior quadrants as previously described17,18,22 - 24 and depicted in Figure 1.
Absolute Myocardial Flow
Arterial input functions were determined by the peak integrated activity over an approximately 2.5 mm diameter manually selected area, typically in the left atrium or thoracic aorta, acquired during the first 2 min after each radiotracer injection. Integrated myocardial activity on late images was determined from topographical LV maps. For each radial segment of every short-axis slice, integrated arterial input and integrated myocardial uptake were used to compute absolute myocardial flow using an established model.25 Partial volume corrections were based on the quantitative phantom studies specific for the Attrius PET scanner.26 Absolute rest and stress myocardial blood flow (sMBF; expressed in cc/min/g), as well as CFR, for each quadrant were calculated using FDA-approved software (CFRQuant™, Positron, Westmont, IL).
Image Analysis
The size, location, and severity of perfusion defects were quantified by the percent of the topographic maps with relative activity <60% of maximal activity (100%). A significant stress-induced perfusion defect was defined as ≥10% change in size from the resting scan in a contiguous area of left ventricular (LV) myocardium with activity equal to or below 60% of maximum. This threshold for reaching significance (size and relative change) is a visually obvious defect that is not due to small deviations/variations in radiotracer uptake or reconstruction and not secondary to stress-induced LV conformational changes. This method has been described extensively and has been validated.19 - 23,27 - 30
Correlation between anatomy, coronary revascularization, and regional myocardial territories
Revascularization was defined as either percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). Each epicardial vessel was assigned to a major myocardial quadrant (or quadrants) based on the typical perfusion pattern for the given vessel. Figure 2 summarizes these relationships.27
Stratification
Significant stress-induced perfusion defects affecting ≥10% of the LV myocardium were identified on PET 1 and assigned to one or more quadrant(s). Contiguous defects involving two or more quadrants were assigned only to those quadrants in which the defect affected ≥5% of the LV myocardium. An investigator blinded to PET data reviewed angiographic and/or operative data to determine which vessels were revascularized. This analysis led to four distinct quadrant categories or “types” as outlined below and depicted in Table 1.
-
(1)
“Matched Revascularization” (MR)—a perfusion defect was present in the quadrant, and there was revascularization of an artery corresponding to that quadrant. For example, a perfusion defect in the anterior and septal quadrants and revascularization of the left anterior descending (LAD) artery.
-
(2)
“Unmatched revascularization” (UR)—no perfusion defect was present in the quadrant, but there was revascularization of an artery corresponding to that quadrant. For example, no perfusion defect in the anterior or septal quadrants, and revascularization of the LAD.
-
(3)
“Matched no revascularization”(MNR)—no perfusion defect was present in the quadrant, and there was no revascularization of an artery corresponding to that quadrant. For example, no perfusion defect in the anterior or septal quadrants and the LAD was not revascularized. This group represents an internal control.
-
(4)
“Unmatched no revascularization”(UNR)—a perfusion defect was present in the quadrant, but there was no revascularization of an artery corresponding to that quadrant. For example, a perfusion defect in the anterior and septal quadrants, but revascularization of the LAD was not performed.
Examples of matched (MR) and unmatched revascularization (UR) are shown in Figures 3 and 4, respectively.
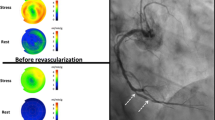
Example of matched revascularization. A Perfusion images at stress showing a significant defect in the septal and anterior walls prior to percutaneous intervention of a mid-LAD lesion. Relative uptake PET perfusion images are scaled by color, with white representing 100% maximum relative uptake, red being the next highest, and the color gradations from red to yellow, green and blue indicating progressively lower relative myocardial perfusion. A generic coronary map is superimposed on the relative perfusion images to show arterial distributions. B Perfusion images at stress of the septal and anterior walls in the same patient after revasclarisation of the mid-LAD lesion. Average sMBF in cc/min/g and CFR are displayed for each quadrant. Each quadrant is considered "matched" as the continious defect comprised 20% of the myocardium with ≥5% in more than one quadrant and each quadrant was revascularized. The defects in the septal and anterior quadrant improved by .85 and 1.13 cc/min/g, respectively: yielding a net increase in sMBF of 1.98 cc/min/g. sMBP Stress myocardial blood flow, CFR coronary flow reverse, LAD Left anterior descending, LCx Left circumflex, RI Rambus intermedius, D1 First diagonal branch, D1 Second diagonal branch
Example of unmatched revascularization. A Perfusion images at stress of the septal, anterior and lateral walls prior to CABG for left main disease. The green zone in the basilar septum represents the membranous septum and is excluded from flow measurements. There are no significant defects present (greater than 10 % of the myocardium). There is a small defect (green region) in the D2 distribution located in the anterior quadrant quantified as 7 % of the myocardium. A generic coronary map is superimposed on the relative perfusion images to show arterial distributions. B Perfusion images at stress of the septal and anterior walls in the same patient after CABG (LUMA-LAD and SVG-OM1). Average sMBF in cc/min/g and CFR are displayed for each quadrant. the blue zone in the basilar septum represents the membranous septum and is excluded from flow measurements. Each quadrant is considered “unmatched” as there were no perfusion defects ≥10% of the myocardium and each quadrant received mechanical revascularization. The defect in the anterior quadrant improved as did sMBF by .14 cc/min/g. However, septal sMBF was unchanged and lateral wall sMBF decreased from 1.30 to 1.18 cc/min/g yielding no net increase in stress flow
Statistical Methods
All statistical testing was performed using SPSS software version 14.0 (IBM SPSS, Armonk, NY). The normality of variables’ distributions was assessed using histograms, Q–Q plots, and the Shapiro–Wilk test. Results are expressed as mean ± standard deviation (SD) or as median and interquartile range [IQR], as appropriate. The change in regional sMBF and CFR before and after revascularization was assessed for each quadrant category using paired Student t-tests for normally distributed variables or the related samples Wilcoxon signed-rank test for variables with a non-normal distribution. For all tests, two-sided P < 0.05 was considered statistically significant.
RESULTS
Nineteen patients (79% male, 65 ± 12 years) underwent PET stress before and after revascularization (17 PCI, 2 CABG). Baseline clinical characteristics are shown in Table 2, and indications for PET scanning are shown in Table 3. The characteristics of the patients’ PET scans (for both PET 1 and PET 2) are summarized in Table 4, and patients’ medication use is shown in Table 5.
In all patients, the indication for angiography was PET 1. The median time between PET 1 and revascularization was 15 days (interquartile range [IQR], 12 to 26). Revascularization was performed in 40 of the 76 total regions (anterior, n = 13; septal, n = 13; lateral, n = 8; inferior, n = 6). The median time between revascularization and PET 2 was 153 days (IQR 107 to 257).
First, changes in average global sMBF and CFR for the entire LV myocardium were calculated for all 19 patients. Following intervention, neither global average sMBF nor global average CFR changed significantly, though there were trends in the direction of improvement (1.6 ± 0.5 vs 1.8 ± 0.7, P = 0.19; and 1.6 ± 0.5 vs 1.8 ± 0.6, P = 0.11, respectively).
Next, the myocardial quadrants were analyzed following stratification as described in the Methods. The types of quadrants observed in each patient are depicted in Table 6. There were 13 patients with MR and MNR ± UNR quadrants, 4 patients with UR and MNR ± UNR quadrants, and 2 patients with both MR and UR ± MNR or UNR quadrants.
As shown in Figures 5 and 6, when a baseline stress-induced perfusion defect existed in a quadrant that was then revascularized (“matched revascularization”; n = 26), post-revascularization sMBF and CFR increased by 0.6 ± 0.7 cc/min/g (1.2 ± 0.4 vs 1.7 ± 0.8; median increase 0.5, IQR 0.1 to 1.0; both Student t test and Wilcoxon signed-rank test P < 0.001) and 0.5 ± 0.6 (1.3 ± 0.4 vs 1.8 ± 0.7; median increase 0.4, IQR −0.1 to 1.0; t test P = 0.001, signed-rank test P = 0.002), respectively. Following matched revascularization, the average stress-induced perfusion defect size decreased from 20% to 8% of the entire LV myocardium (P < 0.001). Furthermore, average minimal stress flow and CFR within the defects improved from 0.59 to 0.96 cc/min/g (P = 0.05) and from 0.77 to 1.13 (P = 0.02), respectively.
The effect of revascularization on myocardial quadrant stress flow, stratified by the presence or absence of a baseline perfusion defect in that quadrant. Each line represents change in sMBF in a myocardial quadrant. The bold black line represents the average ± SD of the group. A sMBF pre- and post-revascularization of quadrants with a baseline perfusion defect showing an improvement of 0.6 ± 0.7 cc/min/g (1.1±0.4 vs 1.7±0.8, P < 0.001). B sMBF pre- and post-revascularization of quadrants without a baseline defect (1.7 ± 0.3 vs 1.5 ± 0.4 cc/min/g, P = 0.16). C Pre- and post-sMBF in quadrants without a baseline perfusion defect which not revascularized (2.0 ± 0.6 vs 1.9 ± 0.7, P = 0.7). sMBF Stress myocardial blood flow
The effect of revascularization on myocardial quadrant CFR, stratified by the presence or absence of a baseline perfusion defect in that quadrant. Each line represents change in CFR in a myocardial quadrant. The bold black line represents the average ± SD of the group. A CFR pre- and post-revascularization of regions with a baseline perfusion defect showing an improvement of 0.5 ± 0.6 (1.3 ± 0.4 vs 1.8 ± 0.7, P = 0.001). B CFR pre- and post-revascularization of regions without a baseline defect (1.4 ± 0.2 vs 1.5 ± 0.3 cc/min/g, P = 0.11). C Pre- and post-CFR in regions without a baseline perfusion defect which were not revascularized (2.0 ± 0.7 vs 1.9 ± 0.6, P = 0.6). CFR Coronary blood flow
As depicted in Fig. 6, when no defect existed but revascularization was performed (“unmatched revascularization”; n = 14), neither sMBF nor CFR showed a significant change (1.7 ± 0.3 vs 1.5 ± 0.4 cc/min/g, P = 0.16; and 1.4 ± 0.2 vs 1.5 ± 0.3, P = 0.11, respectively). There was no significant change in the average stress-induced perfusion defect size (4% vs. 10% of the myocardium, P = 0.41). There also was no significant change in the average minimal stress flow or CFR within the defects (1.18 vs 0.84 cc/min/g, P = 0.11; and 0.98 vs 0.97, P = 0.93, respectively).
In quadrants without a defect that were not revascularized (“matched no revascularization”; n = 29), neither sMBF nor CFR changed significantly (2.0 ± 0.6 vs 1.9 ± 0.7, P = 0.7; and 2.0 ± 0.7 vs 1.9 ± 0.6, P = 0.6).
Only seven quadrants with defects were not intervened upon (unmatched no revascularization). All of these quadrants were supplied by arteries that were found to be chronically occluded. Due to the small number of such regions, related statistical analysis was not performed.
There was higher baseline sMBF in “unmatched revascularization” quadrants compared to “matched revascularization” quadrants (1.7 ± 0.3 vs 1.2 ± 0.4, P < 0.001), despite there being no difference in angiographic percent stenosis between the two groups (85 ± 14% vs. 91 ± 9%, P = 0.43).
Some observations were made with regard to ECG abnormalities and symptoms of angina reported during stress testing. Five of the nineteen patients had either a ventricular paced rhythm or underlying left bundle branch block (LBBB), which prohibited ECG conclusions in these patients. Of the remaining 14 patients, 5 had ST-depression >1 mm from baseline during PET 1, and 4 had ST-depression >1 mm from baseline during PET 2. There were 2 and 3 patients who reported angina during PET 1 and PET 2, respectively.
Discussion
To our knowledge, this is the first study to report regional changes in myocardial blood flow following coronary revascularization. The principal finding is that regional improvement in myocardial blood flow following revascularization is predicated upon the prior existence of a stress-induced perfusion defect in that region. When regions with a baseline stress-induced defect were revascularized, myocardial blood flow improved. When no such defect was present, revascularization did not improve regional blood flow, regardless of the severity of the angiographic stenosis. Thus, our data suggest that PET stress imaging may be used to identify which areas of myocardium may benefit from revascularization, and also in which areas intervention is unlikely to yield improvement in myocardial blood flow.
While it is often used for determining stenosis severity, visual analysis of CAD by coronary angiography can be a poor discriminator of physiological impact. Hypothetically, revascularization should improve myocardial blood flow when discrete stenoses are targeted, but would have less of a beneficial effect on tissue supplied by vessels with diffuse disease or visually overestimated lesions. Several studies support these claims. Firstly, the FAME trial demonstrated a lower one-year combined endpoint of death, non-fatal MI, and repeat revascularization in patients undergoing FFR-guided revascularization compared to revascularization guided by angiography alone. Similarly, in the COURAGE and STICH trials, in which revascularization was guided by visual assessment of angiographic coronary anatomy, optimal medical therapy plus mechanical revascularization (using PCI and CABG, respectively), did not achieve better outcomes versus optimal medical therapy alone.8,31 Furthermore, these concepts have been corroborated invasively with post-PCI FFR data, and have been linked with outcomes data presented by Johnson et al.32. Finally, several studies have demonstrated that approximately 38 to 50% of lesions that undergo FFR-guided PCI show post-PCI FFR values <0.9, despite angiographically “successful” PCI.33,34 These post-PCI data validate the notion that in the presence of diffuse disease, revascularization does not necessarily improve myocardial blood flow. Our study’s integration of absolute myocardial blood flow with PET allows for the characterization of the combined negative impact of discrete stenosis, diffuse disease, and microvascular disease with the positive impact of arterial remodeling and collateralization. A well-established definition of a stress-induced perfusion defect on PET stress was used in this study. This definition correlates with invasively measured FFR and indicates the presence of a physiologically discrete lesion.35,36
The first observation gleaned from the current study is that in regions with a significant baseline stress-induced perfusion defect that underwent revascularization (“matched revascularization”), sMBF increased by 0.6 ± 0.7 cc/min/g. While some regions showed larger improvement than others, our use of non-parametric statistics minimized the effect of potential outliers that could have exaggerated the magnitude of average change in myocardial blood flow, and still the difference was highly significant. In fact, the potential outliers did not have a large effect: the median improvement following a matched intervention was not very different from the mean.
Our data quantitatively confirm the widespread assumption that mechanical revascularization is effective at improving flow capacity. The caveat to this assumption evident from our study is that the potential for improvement in flow depends on the existence of a baseline perfusion defect that correlates anatomically with angiographic focal stenosis. These findings raise the question whether PET-guided revascularization could improve outcomes congruent with those observed in FAME, beckoning the need for a prospective study.
The imperfect correlation between angiographic anatomy and physiology has been described extensively. This discordance has led to the hypothesis that some patients in the COURAGE, STICH, and FAME groups undergoing “angiographic percent stenosis” guided revascularization received interventions on lesions, which, although angiographically severe, did not negatively impact coronary flow. An alternative hypothesis states that although lesions may appear amenable to PCI, the target vessel’s disease may be more diffuse than discrete—rendering focal intervention ineffective. In addition, invasively derived data presented by van de Hoef et al. demonstrate only modest correlations between FFRs ranging between 0.66 and 0.80 and “coronary flow capacity,”37 a new framework introduced by Johnson and Gould utilizing absolute stress flow and CFR to redefine “ischemia,”38 thus validating both of the above hypotheses. Thus, intervention based only on angiographic appearance may produce negligible improvement in flow.36 These hypotheses also are supported by our analysis of myocardial flow and angiographic percent stenosis. In our study, the average sMBF prior to intervention in the “unmatched revascularization” quadrants was significantly higher than that in the “matched revascularization” quadrants, despite similar percent stenosis on angiography. Furthermore, in the “unmatched revascularization” group, revascularization of angiographically high-grade lesions yielded no improvement in sMBF or CFR. Indeed, our data suggest potential worsening in sMBF following “unmatched revascularization,” a finding that was not statistically significant but warrants further research.
Regions without a defect that were not revascularized (“matched non-revascularization” quadrants), which make up an internal control group, displayed essentially no change between PET 1 and PET 2. This finding is consistent with the previously demonstrated high reproducibility of PET as a tool to accurately measure coronary physiology.17 It further supports our finding that improvement in sMBF is dependent upon a preexisting PET defect and is not somehow artifactual in nature. Finally, the global average sMBF and CFR did not show a significant change after revascularization, which is not surprising as the average baseline stress-induced defect comprised only ~20% of the total LV myocardium.
The major focus of this study was to determine the impact of revascularization on regional absolute myocardial flow in each of the four myocardial quadrants. The software utilized, although not common, has been extensively validated in hundreds of publications over the past ~25 years.5,14,17 - 24 This software’s use of topographic polar quadrants, rather than a 17-segment “bullseye” polar map, has 2 major advantages. First, there is less spatial distortion in the topographic quadrants than in the standard “bullseye” polar map.39 Second, coronary vessels are aligned with the quadrants more than in “bullseye” polar maps, such that precise assignment of epicardial vessels to myocardial territory is simplified.14,27 This study theoretically could have been performed using more commonly used software with 17 segment “bullseye” polar maps, but for the reasons addressed above, our approach is simplified and quite elegant.
The focus of the present study was to demonstrate the impact of regional revascularization on regional myocardial blood flow based on PET perfusion imaging. However, upon reviewing the presented data, many questions emerge that are outside the scope of this study, such as the impact of changes in patient lifestyle and/or medication use, in addition to events that occurred both during and after PET 1 and intervention (e.g., ECG changes, angina, outcomes, etc.). In order to address these issues, we must first take into account the methodology used in this study for assessing changes in myocardial flow with revascularization utilized a quadrant-based analysis, not a patient-based analysis. In addition, the purpose was not to define/redefine the term “ischemia,” “ischemic thresholds,” or to associate changes in flow with subsequent clinical outcomes. While these are extremely important questions, our sole intent was to remain focused on the basic question of whether discrete perfusion defects predict response to revascularization.
One possible concern is that changes in lifestyle and/or medication use that occurred over the course of the study could confound the findings. Although it is well known that PET defects can improve with lifestyle changes and medical therapy,40 one must recognize that these confounders influence the entire myocardium as a whole, not individual quadrants. In other words, for any given individual, any individual myocardial quadrant(s) could receive mechanical therapy, whereas lifestyle and medical therapy changes could only occur in a whole individual (i.e., in all four quadrants together). As shown in Table 6, all patients had at least two quadrant “types,” and 5 patients had 3 quadrant types. Furthermore, as one can see in Table 5, medical therapy at the time of PET 1 and PET 2 showed no significant difference that could explain our findings. Regardless, in order for lifestyle and medication differences over time to differentially influence a particular quadrant type, one would have to propose that medication use and lifestyle changes in an individual could impact selected quadrants of myocardium while having no impact on other myocardial quadrants in the same individual. Although theoretically possible, this is unlikely.
These same principles also apply to additional findings, such as ECG changes and angina that occurred during the diagnostic testing or symptomatic improvement in symptoms post-PCI. It is impossible to assign ECG changes, angina, or symptomatic improvement to quadrant types, as all patients had multiple quadrant types. However, when performing a small sub-analysis on symptomatic patients who only had MR + MNR quadrants (n = 5) vs. patients who only had UR + MNR quadrants (n = 4), there was definite improvement in symptoms (100% vs 25%). Furthermore, our intent was to focus solely on the impact of revascularization on regional absolute myocardial blood flow. Clinical findings such as ST depressions, angina, and possibly outcomes, although extremely interesting and of clinical importance, are less relevant to the questions posed in the current study. We do not wish to downplay the relevance of these factors, but the following discussion of the definition of “ischemia” may be helpful.
Concept of Ischemia
In this discussion, we have intentionally avoided using the term “ischemia” when describing absolute myocardial flow and perfusion abnormalities. The definition of ischemia, although seemingly obvious, is elusive when attempting to quantify it with myocardial blood flow.41 Published data have demonstrated a wide range of cutoffs defining “ischemic” flow (i.e., 0.91 to 2.5 cc/min/g), depending on the definition used.13 In addition, as noted above, the recent introduction of the concept of coronary flow capacity (CFC) has redefined the concept of ischemia by incorporating low flow thresholds derived from perfusion abnormalities, ECG changes, and angina, and in doing so has expanded the definition of ischemia beyond that of simple “reversibility.”38
By conventional perfusion imaging definitions, all “matched revascularization” quadrants in our study would be considered ischemic by virtue of the existence of a stress-induced defect. However, in these quadrants, pre-revascularization sMBF ranged from 0.28 to 2.11 cc/min/g; therefore, several of these values may not be considered “ischemic” depending on which definition is used. Regardless of whether or not one labels these regions as “ischemic,” our data indicate that regional absolute myocardial flow improved after matched revascularization. On the other hand, while the “unmatched revascularization” quadrants did not contain any relative perfusion defect, the pre-revascularization sMBF ranged from 1.05 to 2.09 cc/min/g, which again could be considered “ischemic” by some definitions, but in these quadrants absolute myocardial flow did not significantly improve after revascularization.
Thus, without attempting to redefine “ischemia,” establish a new absolute “threshold” for ischemia, or identify clinical signs of ischemia, our study demonstrates that a baseline reversible perfusion defect on PET predicts improvement in absolute myocardial flow following revascularization, and that the lack of such a defect predicts no improvement from revascularization. Perhaps once a standardized definition of “ischemia” is established and validated, our findings could help define the patient population that would benefit from mechanical revascularization.
Limitations
The limitations of this study include its retrospective design and the small sample of patients. There are two potential sources of selection bias. Patients underwent coronary revascularization triggered by the initial PET study. However, this bias parallels real-world clinical practice in that patients often are referred for revascularization procedures based on the abnormal functional studies. Furthermore, only patients with clinical indications underwent a second PET, which reflects a second level of selection bias. While this bias is unavoidable in our retrospective design, our results could also suggest a correlation between symptoms and the “unmatched revascularization” group. There is a need for a prospective study in which these biases are eliminated.
In order to perform this analysis, we chose to divide the myocardium into four myocardial quadrants that correlate with distributions of coronary arteries. There is potential overlap of effects between adjacent quadrants. However, although such overlap would be expected to bias the results toward the null hypothesis, still a significant difference was found. There was some variation in time between revascularization and PET 2, but given the typical natural history of coronary artery disease (i.e., progressive worsening), a long delay between these time points would be expected to render areas that underwent “matched intervention” less likely to show an average benefit, and yet a benefit was seen.
At the time of PET 2, angiography was not routinely performed. As such, it is unknown whether the frequency of restenosis preferentially disadvantaged the “unmatched intervention” group.
Lastly, the same modality was used to identify defects and to follow response to intervention (or non-intervention). While this may appear to be a potential limitation of the study, PET imaging is the gold standard for measurement of myocardial blood flow, to which any other comparator would be inferior.
Conclusion
To our knowledge, this is the first study to report changes in regional myocardial blood flow following coronary revascularization. PET stress imaging may be used to identify areas of myocardium that could benefit from revascularization, and also areas in which intervention is unlikely to yield improvement in myocardial blood flow. When a stress-induced perfusion defect exists on PET, revascularization improves sMBF and CFR in that region. When an area of myocardium shows no such baseline defect, sMBF and CFR show no net change whether or not intervention is performed in that area. A prospective trial testing these findings is warranted.
Abbreviations
- PET:
-
Position emission tomography
- CAD:
-
Coronary artery disease
- PCI:
-
Percutaneous coronary intervention
- CABG:
-
Coronary artery bypass grafting
- CTO:
-
Chronic total occlusion
- sMBF:
-
Stress myocardial blood flow
- CFR:
-
Coronary flow reserve
- FFR:
-
Fractional flow reserve
- COURAGE:
-
Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation study
- FAME:
-
Fractional Flow Reserve versus Angiography for Multivessel Evaluation study
- FAME 2:
-
Fractional Flow Reserve versus Angiography for Multivessel Evaluation study 2
References
Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:e652–735.
Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:e44–122.
Dattilo PB, Prasad A, Honeycutt E, Wang TY, Messenger JC. Contemporary patterns of fractional flow reserve and intravascular ultrasound use among patients undergoing percutaneous coronary intervention in the United States: Insights from the National Cardiovascular Data Registry. J Am Coll Cardiol. 2012;60:2337–9.
Kleiman NS. Bringing it all together: integration of physiology with anatomy during cardiac catheterization. J Am Coll Cardiol. 2011;58:1219–21.
Gould KL. Does coronary flow trump coronary anatomy? JACC Cardiovasc Imaging. 2009;2:1009–23.
Meijboom WB, Van Mieghem CA, van Pelt N, Weustink A, Pugliese F, Mollet NR, et al. Comprehensive assessment of coronary artery stenoses: computed tomography coronary angiography versus conventional coronary angiography and correlation with fractional flow reserve in patients with stable angina. J Am Coll Cardiol. 2008;52:636–43.
White CW, Wright CB, Doty DB, Hiratza LF, Eastham CL, Harrison DG, et al. Does visual interpretation of the coronary arteriogram predict the physiologic importance of a coronary stenosis? New Engl J Med. 1984;310:819–24.
Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, et al. Optimal medical therapy with or without PCI for stable coronary disease. New Engl J Med. 2007;356:1503–16.
De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. New Engl J Med. 2012;367:991–1001.
Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’t Veer M, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. New Engl J Med. 2009;360:213–24.
Araujo LI, Lammertsma AA, Rhodes CG, McFalls EO, Iida H, Rechavia E, et al. Noninvasive quantification of regional myocardial blood flow in coronary artery disease with oxygen-15-labeled carbon dioxide inhalation and positron emission tomography. Circulation. 1991;83:875–85.
Bergmann SR, Herrero P, Markham J, Weinheimer CJ, Walsh MN. Noninvasive quantitation of myocardial blood flow in human subjects with oxygen-15-labeled water and positron emission tomography. J Am Coll Cardiol. 1989;14:639–52.
Bol A, Melin JA, Vanoverschelde JL, Baudhuin T, Vogelaers D, De Pauw M, et al. Direct comparison of [13N]ammonia and [15O]water estimates of perfusion with quantification of regional myocardial blood flow by microspheres. Circulation. 1993;87:512–25.
Gould KL. Positron emission tomography in coronary artery disease. Curr Opin Cardiol. 2007;22:422–8.
Muzik O, Beanlands RS, Hutchins GD, Mangner TJ, Nguyen N, Schwaiger M. Validation of nitrogen-13-ammonia tracer kinetic model for quantification of myocardial blood flow using PET. J Nucl Med. 1993;34:83–91.
Sdringola S, Johnson NP, Kirkeeide RL, Cid E, Gould KL. Impact of unexpected factors on quantitative myocardial perfusion and coronary flow reserve in young, asymptomatic volunteers. JACC Cardiovasc Imaging. 2011;4:402–12.
Gould KL, Johnson NP, Bateman TM, Beanlands RS, Bengel FM, Bober R, et al. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol. 2013;62:1639–53.
Xu EZ, Mullani NA, Gould KL, Anderson WL. A segmented attenuation correction for PET. J Nucl Med. 1991;32:161–5.
Demer LL, Gould KL, Goldstein RA, Kirkeeide RL, Mullani NA, Smalling RW, et al. Assessment of coronary artery disease severity by positron emission tomography. Comparison with quantitative arteriography in 193 patients. Circulation. 1989;79:825–35.
Gould KL, Martucci JP, Goldberg DI, Hess MJ, Edens RP, Latifi R, et al. Short-term cholesterol lowering decreases size and severity of perfusion abnormalities by positron emission tomography after dipyridamole in patients with coronary artery disease. A potential noninvasive marker of healing coronary endothelium. Circulation. 1994;89:1530–8.
Gould KL, Ornish D, Scherwitz L, Brown S, Edens RP, Hess MJ, et al. Changes in myocardial perfusion abnormalities by positron emission tomography after long-term, intense risk factor modification. JAMA. 1995;274:894–901.
Hicks K, Ganti G, Mullani N, Gould KL. Automated quantitation of three-dimensional cardiac positron emission tomography for routine clinical use. J Nucl Med. 1989;30:1787–97.
Gould KL. Coronary artery stenosis and reversing atherosclerosis. London New York: Arnold; Co-published by Oxford University Press; 1999.
Gould KL. PET perfusion imaging and nuclear cardiology. J Nucl Med. 1991;32:579–606.
Yoshida K, Mullani N, Gould KL. Coronary flow and flow reserve by PET simplified for clinical applications using rubidium-82 or nitrogen-13-ammonia. J Nucl Med. 1996;37:1701–12.
Johnson NP, Sdringola S, Gould KL. Partial volume correction incorporating Rb-82 positron range for quantitative myocardial perfusion PET based on systolic-diastolic activity ratios and phantom measurements. J Nucl Cardiol. 2011;18:247–58.
Nakagawa Y, Nakagawa K, Sdringola S, Mullani N, Gould KL. A precise, three-dimensional atlas of myocardial perfusion correlated with coronary arteriographic anatomy. J Nucl Cardiol. 2001;8:580–90.
Gould KL, Nakagawa Y, Nakagawa K, Sdringola S, Hess MJ, Haynie M, et al. Frequency and clinical implications of fluid dynamically significant diffuse coronary artery disease manifest as graded, longitudinal, base-to-apex myocardial perfusion abnormalities by noninvasive positron emission tomography. Circulation. 2000;101:1931–9.
Sdringola S, Nakagawa K, Nakagawa Y, Yusuf SW, Boccalandro F, Mullani N, et al. Combined intense lifestyle and pharmacologic lipid treatment further reduce coronary events and myocardial perfusion abnormalities compared with usual-care cholesterol-lowering drugs in coronary artery disease. J Am Coll Cardiol. 2003;41:263–72.
Sdringola S, Patel D, Gould KL. High prevalence of myocardial perfusion abnormalities on positron emission tomography in asymptomatic persons with a parent or sibling with coronary artery disease. Circulation. 2001;103:496–501.
Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction. New Engl J Med. 2011;364:1607–16.
Johnson NP, Tóth GG, Lai D, Zhu H, Açar G, Agostoni P, et al. Prognostic value of fractional flow reservelinking physiologic severity to clinical outcomes. J Am Coll Cardiol. 2014;64:1641–54.
Bech GJ, Pijls NH, De Bruyne B, Peels KH, Michels HR, Bonnier HJ, et al. Usefulness of fractional flow reserve to predict clinical outcome after balloon angioplasty. Circulation. 1999;99:883–8.
Pijls NH, Klauss V, Siebert U, Powers E, Takazawa K, Fearon WF, et al. Coronary pressure measurement after stenting predicts adverse events at follow-up: a multicenter registry. Circulation. 2002;105:2950–4.
Johnson NP, Gould KL. Clinical evaluation of a new concept: resting myocardial perfusion heterogeneity quantified by markovian analysis of PET identifies coronary microvascular dysfunction and early atherosclerosis in 1,034 subjects. J Nucl Med. 2005;46:1427–37.
Johnson NP, Kirkeeide RL, Gould KL. Is discordance of coronary flow reserve and fractional flow reserve due to methodology or clinically relevant coronary pathophysiology? JACC Cardiovasc Imaging. 2012;5:193–202.
van de Hoef TP, Echavarria-Pinto M, van Lavieren MA, Meuwissen M, Serruys PW, Tijssen JG, et al. Diagnostic and prognostic implications of coronary flow capacity: A comprehensive cross-modality physiological concept in ischemic heart disease. JACC Cardiovasc Interv. 2015;8:1670–80.
Johnson NP, Gould KL. Integrating noninvasive absolute flow, coronary flow reserve, and ischemic thresholds into a comprehensive map of physiological severity. JACC Cardiovasc Imaging. 2012;5:430–40.
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging. 2002;18:539–42.
Sdringola S, Loghin C, Boccalandro F, Gould KL. Mechanisms of progression and regression of coronary artery disease by PET related to treatment intensity and clinical events at long-term follow-up. J Nucl Med. 2006;47:59–67.
Bober RM, Jahangir E. What is ischemia and how should this be defined based on modern imaging? Prog Cardiovasc Dis. 2015;57:537–54.
Acknowledgments
The authors are grateful to Dr. Christopher White for his thoughtful critique of the manuscript.
Disclosures
C.D. Thompson: none. R.M. Bober: Bracco Inc., consultant; research funding. D.P. Morin: none.
Author information
Authors and Affiliations
Corresponding author
Additional information
See related editorial, doi:10.1007/s12350-016-0479-2.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bober, R.M., Thompson, C.D. & Morin, D.P. The effect of coronary revascularization on regional myocardial blood flow as assessed by stress positron emission tomography. J. Nucl. Cardiol. 24, 961–974 (2017). https://doi.org/10.1007/s12350-016-0442-2
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-016-0442-2