Abstract
Background
We aimed to evaluate the utility of fully automated software registration intended to improve CT attenuation correction (CTAC) map misalignments during cardiac 82Rb PET/CT myocardial perfusion imaging (MPI).
Methods
171 consecutive patients (108 males, mean age 69 years), undergoing both rest-stress 82Rb PET/CT MPI and invasive coronary angiography within 6 months (mean 14 days, range 0-170), were studied. List mode data were automatically processed in batch mode to generate transaxial attenuation corrected slices with four different CTAC alignment correction strategies: (i) no alignment correction (NONE); (ii) manual correction (MANUAL); (iii) automated 6-parameter rigid correction (AUTO); and (iv) targeted use of automated correction only where PET-CTAC alignment was initially judged as incorrect on either stress or rest scan (AUTO for misalignment only). Initial and final registration quality was graded (1-3) by an experienced radiologist (1: satisfactory alignment (<2 mm misalignment), 2: slight misalignment (2-5 mm in any direction), or 3: poor (>5 mm misalignment in any direction). Total perfusion deficit (TPD) and ischemic TPD (ITPD) were computed automatically, and their diagnostic accuracy to detect significant coronary artery disease with each realignment technique was assessed using receiver operating characteristic analysis.
Results
The diagnostic accuracy of ITPD, expressed as area under curve, was .81 ± .03 with no alignment correction (NONE), .83 ± .03 with MANUAL correction, .85 ± .03 with AUTO correction (P < .05 vs. NONE and MANUAL), and .87 ± .03 with the targeted use of AUTO correction (P < .05 vs. NONE, MANUAL and AUTO). Both manual and software corrections increased the percentage of cases with satisfactory PET-CTAC map alignment (P < .05 for all) at rest (from 55% for NONE to 80% for MANUAL and 92% for AUTO) and at stress (from 51% for NONE to 78% for MANUAL and 84% for AUTO).
Conclusion
The diagnostic accuracy of 82Rb PET/CT MPI with automated rigid alignment is improved compared to data with no CTAC scan alignment or with manual alignment. The optimal strategy for diagnostic performance is to apply automatic alignment only in cases which are visually identified as misaligned.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
82Rb PET/CT myocardial perfusion imaging (MPI) offers several important advantages compared to SPECT MPI. Unlike SPECT, the 82Rb PET rest and stress scans can be obtained in one short session (around 30 min). Stress and rest PET allow for a low-injected dose and a low-radiation dose to the patient (less than 4 mSv) especially with the new 3D PET/CT hybrid scanners.1 However, the CT attenuation correction (CTAC) scan required in PET/CT cannot reliably average normal breathing patterns; therefore, spatial misalignment between CT and PET scans may occur, introducing artifacts in the reconstructed PET images.2-4 Several manual methods have been proposed for corrections of such misalignments,3-7 but manual approaches introduce subjective manipulation of the data and increase the test variability, therefore undermining the quantitative advantages of PET MPI. The current clinical practice requires time-consuming visual inspection of stress and rest CTAC-PET scan misalignment, manual correction, and subsequent data re-reconstruction. Several automatic tools have been proposed for the alignment of PET and CTAC maps.6,8-11 If performance of these new tools could be proven to be equal or better than that of experienced observers, the incorporation of such automated methods into routine PET protocols would significantly streamline the clinical application of this important modality.
In this work, we aimed to evaluate the impact of fully automated PET-CTAC alignment on the diagnostic accuracy of PET MPI to detect significant coronary artery disease (CAD), using invasive coronary angiography as the gold standard.
Materials and Methods
The study protocol was approved by the Institutional Review Board. All patients provided written informed consent for the use of their clinical and imaging data for research purposes.
Patient Population
We retrospectively analyzed clinical, scintigraphic, and angiographic data of 171 consecutive patients without history of prior CAD (myocardial infarction, percutaneous coronary interventions, or coronary bypass graft surgery). All patients underwent same day rest/pharmacological stress-gated 82Rb PET MPI on a hybrid PET/CT scanner between 01/2008 and 09/2012 at Cedars-Sinai Medical Center. Adenosine or regadenoson were used as pharmacological stressors in 99 (58%) and 72 (42%) patients, respectively. All patients were referred for pharmacological stress PET MPI followed by invasive coronary angiography according to regular clinical indications. Pretest probability for obstructive CAD was calculated by the Diamond-Forrester criteria.12
82Rb PET/CT Acquisition Protocol and Data Reconstruction
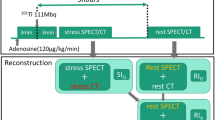
PET imaging protocols have been previously described.13 Briefly, all images were acquired in list mode on a Siemens Biograph-64 TruePoint PET/CT with the True V option.14 The data were acquired in list mode format. Patients were instructed to fast for 6 h and withhold caffeine-containing beverages 24 h before the test and antianginals on the morning of the test. Low-dose helical CTAC was acquired (100 kV, pitch 1.5, 11 mAs, duration 3.4 s). Subsequently, patients were injected with 925-1850 MBq (25-50 mCi) of 82Rb at rest. A 6-min rest imaging acquisition was started simultaneously with the start of the rest 82Rb injection. Immediately after completion of rest imaging, a second CTAC scan was performed for stress PET attenuation correction. The reconstructed slice thickness of CTAC maps was 3 mm. Both rest and stress CTAC maps were acquired at end-expiration breath holds. Pharmacological stress was performed using intravenous adenosine infusion 140 mg/kg/min for 6 min15 or intravenous regadenoson16 bolus. The tracer 925-1850 MBq (25-50 mCi) of 82Rb was injected at 3 min after initiation of adenosine infusion or 10-20 s after the injection of regadenoson followed by the saline solution flush. Patients were instructed to breathe normally during the PET acquisition.
The first 2-3 min of the list file was skipped to allow for blood pool clearance of 82Rb. The next 3-4 min of acquisition data were used to generate sinograms for static images. Emission data were reconstructed in a batch mode using ordered subsets expectation maximization (30 iterations and 2 subsets). CT-based attenuation, scatter, including prompt gamma,17 decay, and random corrections were applied. Gaussian filters with a full-width at half maximum of 8 mm for rest and stress images and a 3-dimensional post-filter (Butterworth filter cutoff frequency 10, order of 5) were used.
Alignment and Image Processing
For clinical reconstruction, the registration quality between PET and CT was estimated by experienced nuclear cardiology technologists using fused images in the Siemens 3D-software on a Leonardo workstation (Syngo 6.0, Siemens Medical Solutions, Hoffman Estates, IL). Misalignments were identified and a manual registration matrix with 3D-translations was applied before the reconstruction, as performed in clinical practice. For the automatic alignment, research software was provided by Siemens Healthcare (Hoffman Estates, IL, USA) as a part of the AutoCardiac package. The software allows CTAC-PET alignment by either rigid or deformable algorithms.8 In our initial evaluation, the rigid method performed better than the deformable method, and thus it was used for all subsequent evaluation.18 The software operates in a fully unsupervised mode, finding initial localization of the heart by template matching with multi-resolution approach and then cropping the volume using the bounding box determined by the templates. Subsequently mutual information similarity measure with Powell (rigid) optimizer was used to register the cropped images.8
All patient images were reconstructed in automated batch mode, with three sets for each patient representing data reconstructed with no alignment (NONE), clinical alignment as described above (MANUAL), and rigid automatic alignment (AUTO).
Subsequently, registration quality was assessed subjectively by an independent radiologist expert in cardiovascular imaging blinded to clinical information and angiographic findings. All data were reviewed in fusion display using soft tissue window settings (width 320, level 50) for the CTAC images in three orthogonal planes. Registration quality was graded on a semi-quantitative scale (1-3) as follows: (1) good alignment with exact superposition of all cardiac borders or <2 mm misalignment at least in one direction; (2) mild misalignment, between 2 and 5 mm at least in one direction; or (3) severe misalignment, >5 mm in at least one direction. Rest and stress alignment were graded separately for each alignment method. We also tested the application of the registration technique targeted only to misaligned cases. In this mode, the original data (no alignment correction) were used if the observer did not detect any misalignment of either stress or rest CTAC, and data processed with the automated method were used only for those cases with misalignment detected by the visual observer. This selective application was based on the grading performed by the independent radiologist expert in cardiovascular imaging as described above. The rationale for assessing the targeted application of automated registration is that some supervision and human decision about alignment is likely to be required in clinical practice.
Transaxial PET perfusion images for each of the three alignment types were then automatically processed using QPET software, Cedars-Sinai Medical Center, Los Angeles, CA.19 The QPET algorithm has increased myocardial sampling (2 times) due to the higher PET resolution and improved frame-to-frame tracking valve plane frame-to-frame. The perfusion defect extent was calculated as the percentage of the total surface area of the left ventricle for which normalized polar map pixels were below the abnormality threshold. The quantitative perfusion variable employed was total perfusion deficit (TPD).20 Stress TPD, rest TPD, ischemic (stress-rest) TPD (ITPD), and the absolute difference between stress and rest were calculated for every patient. Integer thresholds of >3% for ITPD and >5% for TPD were used for calculation of sensitivities and specificities.
Invasive Coronary Angiography
Only patients who did not experience either myocardial infarction or revascularization in the interval between PET/CT MPI and invasive coronary angiography were included. Conventional invasive coronary angiography was performed according to standard clinical protocols, mean time difference 14 ± 27 days, range 0-170 days after the PET/CT MPI. Coronary angiograms were visually analyzed and reported by two experienced interventional cardiologists. A stenosis of ≥ 70% or greater narrowing of the left anterior descending (LAD), left circumflex (LCX) and right coronary artery (RCA), or ≥50% for left main artery was considered as the presence of obstructive CAD. Diagonal lesions were considered as LAD; obtuse marginal and ramus arteries were considered as LCX; posterior descending and postero-lateral branches were considered as RCA.
Statistical Analysis
Statistical analyses were performed with Analyze software (version 3.71, Analyze-it software Ltd). All continuous variables were described as mean ± SD. Student two-sample t tests were used to compare differences in continuous variables. A Chi-square test was used to compare differences across groups for categorical variables. A 2-tailed P < .05 was considered statistically significant. Receiver operating characteristic (ROC) analysis was performed and areas under curve (AUC) were compared using the DeLong & Delong method. AUC was the primary end-point in this study. McNemar’s test was used to compare proportions of misaligned data.
Results
Baseline patient characteristics and data regarding angiographic severity of CAD are detailed in Table 1. ROC analysis for each of the registration methods using ITPD (stress TPD—rest TPD) is shown in Figure 1. We first evaluated the results separately for the patients with CTACs initially judged as misaligned at either stress or rest (n = 111) (Figure 1A) and for patients with CTACs initially judged as correctly aligned (n = 60) (Figure 1B). The AUC using the AUTO method was significantly improved for misaligned cases (n = 111) compared to using NONE or MANUAL realignment methods (both P values <.05) (Figure 1A). However, there was no significant change in the AUC in the cases judged as correctly aligned (n = 60) with the AUTO method compared to no correction (P < .07) (Figure 1B). Finally, when the study group was analyzed as a whole (n = 171) (i.e., both aligned and misaligned groups) (Figure 1C), the AUTO methods were both found to have a higher AUC than the MANUAL method. Furthermore, the AUC with the AUTO registration method applied only to misaligned cases (.87 ± .03) was found to be higher to that for the AUTO method (.85 ± .03) applied indiscriminately to all cases (P < .05). In Figure 2, we show sensitivities and specificities for detection of ≥70% stenosis for both TPD and ITPD measures.
Detection of coronary artery disease by the ischemic total perfusion deficit, (ITPD) for the PET/CT data with no CTAC alignment (NONE), manual registration of CTAC (MANUAL), and fully automatic CTAC alignment (AUTO). (A) ROC curves for the subset of patients with CTAC initially misaligned on stress or rest (n = 111), as judged by the experienced observer. Comparisons of areas under ROC curves (AUC) for AUTO vs. NONE and AUTO vs. MANUAL were significant (P < .05). (B) ROC curves for the detection of the disease in a subset of cases originally judged with as correctly aligned at both stress and rest (n = 60). None of the AUC comparisons were significant. (C) Results for all cases (n = 171) combined including selective application of the automatic PET/CTAC alignment (purple) only when observer detected misalignment
The sensitivity and specificity of ischemic total perfusion defect (ITPD) and total perfusion defect (TPD) for the detection of significant CAD from PET/CT data with no CTAC alignment (NONE), manual registration (MANUAL) and automatic alignment (AUTO). Sensitivity and specificity values are also given for the selective application of the AUTO technique to misaligned cases only. Error bars represent 95% CI
In total, 45% of rest and 49% stress CTAC scans were judged to be initially misaligned by the experienced observer before any correction method was applied. In total, 111 out of 171 CTAC scans were judged as initially misaligned by the experienced observer on either stress or rest scan. The proportion of well-aligned cases for the AUTO (stress 84%; rest 92%) was higher than that for the MANUAL (stress 80%, and rest 78%) and NONE (stress 52%, and rest 55%) (P < .0001 for all). The results of subjective alignment evaluation are shown in Figure 3.
The quantitative results were highly correlated for all methods. The Pearson correlation of ITPD obtained by the MANUAL registration was .944, (95% CI .925-.958). The correlation between the AUTO and MANUAL was .952 (95% CI .935-.964).
The originally misaligned CTAC scan could lead to either false-positive or in some cases false-negative findings. In Figures 4 and 5, we demonstrate case examples of the automatic registration effects on the image quantification.
False-positive nuclear scan due to the CTAC registration error resolved by the automatic correction in a 44-year-old male presenting with shortness of breath. PET and CTAC images fused before alignment (A) and after rigid alignment (B). Misalignment is indicated by the white arrow head (A). Stress perfusion PET images before alignment (C) and after alignment (D) and polar maps are shown with the white arrow indicating the perfusion artifact due to misalignment. Quantitative ITPD was 11% (C right), when no correction for alignment was performed (ITPD after manual verification was also 11%) compared to a normal scan (ITPD 1.8%) after automatic registration (D right). Invasive coronary angiography did not show significant coronary artery disease
False-negative scan due to registration error resolved by automatic CTAC map alignment in a 60-year-old male presenting with atypical chest pain and shortness of breath. PET and CTAC fused before (A) and after alignment (B). Misalignment is indicated by the white arrow head (A). Corresponding stress perfusion images before (C) and after alignment (D) and polar maps are shown with the white arrow indicating the perfusion defect which becomes more apparent after alignment. Quantitative ITPD was 0% with no correction (C right) compared to a 5% ITPD with automatic PET/CTAC alignment correction (D right). Rest scan was normal. Note the slight misalignment at the apex on PET-CTAC fusion images (on the opposite side of the apex where the reversible defect was located) (A), which reduces the relative activity in the lateral wall and therefore masks the apical defect. Invasive angiography revealed 80% left anterior descending (LAD) artery stenosis, which was treated with drug-eluting stent
Discussion
To our knowledge, this is the first study of PET-CTAC misregistration utilizing an objective standard of invasive angiography. In previous studies, only false-positive artifacts have been demonstrated.2,4 In our study, we have also demonstrated for the first time that false-negative errors due to incorrect PET-CTAC scan registration do also occur. We also demonstrate that automatic registration can be superior to manual PET-CTAC scan alignment. These conclusions are based on the objective comparison with angiographic findings. As a secondary finding, we have used visual alignment expert judgment to illustrate that if the registration algorithm is applied only to cases visually identified as misaligned, the diagnostic accuracy can be further improved as compared to manual correction by an experienced human operator. Furthermore, automatic registration can potentially result in slight misregistration and produce false-negative results when applied to cases with no initial misalignment. In Figure 2, we see 2 patients who were reclassified as false negative after automatic registration. Significant angiographic disease was mis-classified for one of these patients due to a subtle misalignment after automatic registration. In the other patient, no misalignment was observed and false-negative results occurred due to a very small change (<.1%) in ITPD—falling just below the threshold. Nonetheless, the invasive fractional flow reserve test, which was performed in this case, was normal (>0.80).
Although the overall AUC for the detection of CAD improved significantly with the automatic alignment and especially when this alignment was applied selectively, there was not a clear incremental improvement in sensitivity and specificity results (Figure 2). In particular, the specificity (although increasing) remains relatively low as compared to previous reports with PET, even with automatic alignment. There are likely several reasons for this. First, unlike previous reports of diagnostic performance for PET, we did not include the low likelihood population, and second, all the cases in our study were consecutive patients already selected to undergo coronary angiography for clinical purposes. Therefore, the relatively low specificity observed is likely due to the referral bias and due to the overall limitations of angiographic gold standard.21 Future evaluation of the CTAC map registration techniques could include the prospective evaluation in low likelihood populations. Additionally, although the study was well powered to detect a difference in overall diagnostic performance as defined by the AUC of ROC analysis, it was not powered to statistically compare individual sensitivity or specificity values in a dichotomous model with McNemar’s test as this would have required much larger sample size.22
We observed PET-CTAC misalignment in 45% rest studies and 49% of stress studies, and similar incidences were reported in previous studies. Lautamaki et al found misalignment in 60% of rest and 67% of stress 82Rb PET/CT studies.23 Gould et al reported 40% of the low-dose CTAC scans were mis-registered with PET.2 Martinez et al reported misalignment of 50% of stress or rest cases (n = 56). The large number of mis-registered studies has been observed despite the use of end-expiration breath-hold position for the CT acquisition as recommended for the minimization of registration problems.24
Recently, Zaidi et al studied the same PET-CTAC registration algorithms in a different application—proposing the use of CT coronary calcium scans for the attenuation correction.25 They found that a deformable registration resulted in the best correlation of the relative perfusion and absolute blood flow as compared to studies performed with standard CTAC. In our preliminary study, we did not observe the superiority of deformable registration,18 and therefore, we focused on evaluation of the rigid registration. However, we applied the algorithm to a different kind of data (low-dose CTAC scans), and we used an objective external standard (invasive coronary angiography).
As an alternative to the software registration, technically sophisticated CT acquisition approaches have been proposed for the prevention of the PET-CTAC misalignment, attempting to replicate the free-breathing mode of the PET acquisition during the CT scan. Pan et al proposed a cine CT of over one breath cycle per couch position.26 Gould et al subsequently reported that the software alignment was still needed for that method2 and proposed the use of rest-only cine-CT data and a manual CTAC-PET registration.27 Other variations of this approach have been put forward, such as respiratory matched CT acquisitions.28,29 Nevertheless, most of these novel techniques are associated with significantly increased radiation doses to the patient and may further complicate the already demanding stress/rest PET MPI protocols. For example, cine CT are associated with radiation doses of at least 2.7 mSv, even for the specialized low-dose protocols,2,30 compared to the 1.7 mSv dose from a standard 40 mCi Rb-82 stress scan,31 and 1.2 mSv for the low-dose 3D PET/CT acquisitions.32 It seems therefore, that currently, the most practical solution is the standard low-dose CTAC scan coupled with automatic registration—as validated in this work, especially in view of the increased scrutiny of the radiation doses incurred by patients during imaging procedures.33
This work has several clinical implications. In clinical practice, the check for the misalignment must be performed by the technologist who is acquiring the MPI study. This procedure adds a heavy burden to a busy technologist who needs to take care of the patient, start and monitor the rest/stress isotope injections and acquisitions, and also reconstruct the data—all typically within the 30-40 min. The manual correction of PET-CTAC misalignment is time consuming, as it requires careful data review in multiple orientations, with interactive image blending. Furthermore, multiple datasets may need to be subsequently re-reconstructed—for example, myocardial flow data and perfusion scans can be both affected by CTAC misalignment. These requirements during busy clinical workflow may compromise the accuracy of the manual adjustments. The additional check for the alignment during routine clinical reporting is also performed by the reporting physician, but it disrupts the clinical workflow since the study must be sent back for re-reconstruction if misaligned. These circumstances could explain the relatively high-rate of PET-CTAC misalignments in routine clinical studies as retrospectively judged by the by the expert. As demonstrated, the realignment may be needed in up to two thirds of patient scans for either stress or rest datasets. There is a considerable inter-observer variability in the manual alignment,4 and thus the availability of a reliable tool, integrated with the image reconstruction and able to objectively align the PET and CTAC data, would simplify the clinical logistics of PET MPI and provide more reproducible results. The routine use of automated registration may subsequently lead to more accurate diagnostic results for 82Rb PET/CT.
Our study has several limitations. The analysis is retrospective and comes from a single center and a single type of scanner. It is possible that in other centers the manual PET-CTAC map alignment results would be different. However, our frequency of the misalignments seems to be similar to previously published studies. Furthermore, the automated registration and the quantitative analysis will be reproducible across different centers. In this study, we used retrospectively visual evaluation of alignment by a single expert to check both the original clinical alignment and the automatic alignment. Such evaluation can be subjective and associated with variability. In addition, the expert was not blinded to whether the registration was applied and therefore the visual decision about the classification of alignment could be biased. However, the primary conclusion of this work is based on the objective comparison of the quantitative perfusion measures with the invasive angiographic findings, and therefore is not affected by the visual evaluation of the misalignment for different methods. The expert was fully blinded to the clinical information and angiographic results; thus, a potential human bias would not affect the ROC analysis. In this study, we focused on the PET perfusion images which are the primary images reviewed in the clinical practice of PET MPI, and we did not analyze myocardial flow or gated datasets. Further studies need to be conducted to validate the effect of PET-CTAC image registration on the quantitative myocardial blood flow and flow reserve results. The effect of CTAC scan misalignment on gated parameters, such as ejection fraction and volumes, is likely to be small—since the primary effect is the count distribution change, rather than the change of the left ventricular contours; furthermore, PET-CTAC scan registration is not currently routinely feasible for the reconstruction of gated data due to time constraints. We acknowledge that our results are applicable to the particular automatic registration software utilized and conclusions might not be applicable to other algorithms.
Conclusion
Automated registration software significantly improves PET-CTAC alignment. Furthermore, the diagnostic accuracy of 82Rb PET/CT MPI with automatic rigid alignment is improved compared to data with no CTAC alignment or with manual alignment. The optimal strategy for diagnostic performance is to apply automatic alignment only in cases in which PET-CTAC misalignment is visually identified.
New Knowledge Gained
This is the first study objectively evaluating the effect of CTAC misalignment on the accuracy of CAD detection by PET with invasive angiography used as a reference standard. We have found that automatic PET-CTAC alignment results in higher AUC than achieved by manual alignment. We also found that the optimal strategy for diagnostic performance is to apply automatic alignment only when PET-CTAC misalignment is visually identified. Finally, we have found that PET-CTAC misalignment can lead to false-negative as well as false-positive diagnostic results.
References
Nakazato R, Berman DS, Alexanderson E, Slomka P. Myocardial perfusion imaging with PET. Imaging Med 2013;5:35-46.
Gould KL, Pan T, Loghin C, Johnson NP, Guha A, Sdringola S. Frequent diagnostic errors in cardiac pet/ct due to misregistration of ct attenuation and emission pet images: A definitive analysis of causes, consequences, and corrections. J Nucl Med 2007;48:1112-21.
Loghin C, Sdringola S, Gould KL. Common artifacts in pet myocardial perfusion images due to attenuation-emission misregistration: Clinical significance, causes, and solutions. J Nucl Med 2004;45:1029-39.
Slomka PJ, Le Meunier L, Hayes SW, W Acampa, M Oba, GG Haemer, et al. Comparison of myocardial perfusion 82Rb pet performed with CT- and transmission CT-based attenuation correction. J Nucl Med 2008;49:1992-8.
Gould KL, Johnson NP, Bateman TM, Beanlands RS, Bengel FM, Bober R, et al. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol 2013;62:1639-53.
Martinez-Moller A, Souvatzoglou M, Navab N, Schwaiger M, Nekolla SG. Artifacts from misaligned ct in cardiac perfusion PET/CT studies: Frequency, effects, and potential solutions. J Nucl Med 2007;48:188-93.
Koepfli P, Hany TF, Wyss CA, Namdar M, Burger C, Konstantinidis A, et al. CT attenuation correction for myocardial perfusion quantification using a PET/CT hybrid scanner. J Nucl Med 2004;45:537-42.
Bond S, Kadir T, Hamill J, Casey M, Platsch G, Burckhardt D, et al. (2008) Automatic registration of cardiac PET/CT for attenuation correction. Paper presented at: Nuclear Science Symposium Conference Record, 2008. NSS’08. IEEE; 19-25 October 2008; Dresden, Germany.
Marinelli M, Positano V, Tucci F, Neglia D, Landini L. Automatic PET-CT image registration method based on mutual information and genetic algorithms. Sci World J 2012;2012:067-567.
Khurshid K, McGough RJ, Berger K. Automated cardiac motion compensation in PET/CT for accurate reconstruction of pet myocardial perfusion images. Phys Med Biol 2008;53:5705-18.
Alessio AM, Kinahan PE, Champley KM, Caldwell JH. Attenuation-emission alignment in cardiac PET/CT based on consistency conditions. Med Phys 2010;37:1191-200.
Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med 1979;300:1350-8.
Nakazato R, Berman DS, Dey D, Le Meunier L, Hayes SW, Fermin JS, et al. Automated quantitative Rb-82 3d PET/CT myocardial perfusion imaging: Normal limits and correlation with invasive coronary angiography. J Nucl Cardiol 2012;19:265-76.
Jonsson C, Odh R, Schnell PO, Larsson SA (2007) A comparison of the imaging properties of a 3- and 4-ring biograph pet scanner using a novel extended nema phantom. Paper presented at: Nuclear Science Symposium Conference Record, 2007. NSS ‘07. IEEE; Oct. 26 2007-Nov. 3 2007.
Cerqueira MD, Verani MS, Schwaiger M, Heo J, Iskandrian AS. Safety profile of adenosine stress perfusion imaging: Results from the adenoscan multicenter trial registry. J Am Coll Cardiol 1994;23:384-9.
Hendel RC, Bateman TM, Cerqueira MD, Iskandrian AE, Leppo JA, Blackburn B, et al. Initial clinical experience with regadenoson, a novel selective a2a agonist for pharmacologic stress single-photon emission computed tomography myocardial perfusion imaging. J Am Coll Cardiol 2005;46:2069-75.
Esteves FP, Nye JA, Khan A, Folks RD, Halkar RK, Garcia EV, et al. Prompt-gamma compensation in Rb-82 myocardial perfusion 3d PET/CT. J Nucl Cardiol 2010;17:247-53.
Slomka P, Diaz-zamudio M, Dey D, Brodov Y, Choi D, Germano G, et al. Automatic alignment of PET/CT attenuation correction maps. J Nucl Med 2014;55(Suppl 1):1763.
Germano G, Kavanagh PB, Slomka PJ, Van Kriekinge SD, Pollard G, Berman DS. Quantitation in gated perfusion SPECT imaging: The cedars-sinai approach. J Nucl Cardiol 2007;14:433-54.
Slomka PJ, Nishina H, Berman DS, Akincioglu C, Abidov A, Friedman JD, et al. Automated quantification of myocardial perfusion SPECT using simplified normal limits. J Nucl Cardiol 2005;12:66-77.
Miller TD, Hodge DO, Christian TF, Milavetz JJ, Bailey KR, Gibbons RJ. Effects of adjustment for referral bias on the sensitivity and specificity of single photon emission computed tomography for the diagnosis of coronary artery disease. Am J Med 2002;112(4):290-7.
Eng J. Sample size estimation: How many individuals should be studied? Radiology 2003;227(2):309-13.
Lautamaki R, Brown TL, Merrill J, Bengel FM. CT-based attenuation correction in (82)Rb-myocardial perfusion PET-CT: Incidence of misalignment and effect on regional tracer distribution. Eur J Nucl Med Mol Imaging 2008;35:305-10.
Le Meunier L, Maass-Moreno R, Carrasquillo JA, Dieckmann W, Bacharach SL. PET/CT imaging: Effect of respiratory motion on apparent myocardial uptake. J Nucl Cardiol 2006;13:821-30.
Zaidi H, Nkoulou R, Bond S, Baskin A, Schindler T, Ratib O, et al. Computed tomography calcium score scan for attenuation correction of n-13 ammonia cardiac positron emission tomography: Effect of respiratory phase and registration method. Int J Cardiovasc Imaging 2013;29:1351-60.
Pan T, Mawlawi O, Luo D, Liu HH, Pai-chun M, Mar MV, et al. Attenuation correction of PET cardiac data with low-dose average CT in PET/CT. Med Phys 2006;33:3931-8.
Gould KL, Pan T, Loghin C, Johnson NP, Sdringola S. Reducing radiation dose in rest-stress cardiac PET/CT by single poststress cine ct for attenuation correction: Quantitative validation. J Nucl Med 2008;49:738-45.
Cook RA, Carnes G, Lee TY, Wells RG. Respiration-averaged ct for attenuation correction in canine cardiac PET/CT. J Nucl Med 2007;48:811-8.
Alessio AM, Kohlmyer S, Branch K, Chen G, Caldwell J, Kinahan P. Cine CT for attenuation correction in cardiac PET/CT. J Nucl Med 2007;48:794-801.
Xia T, Alessio AM, De Man B, Manjeshwar R, Asma E, Kinahan PE. Ultra-low dose CT attenuation correction for PET/CT. Phys Med Biol 2012;57:309-28.
Senthamizhchelvan S, Bravo PE, Lodge MA, Merrill J, Bengel FM, Sgouros G. Radiation dosimetry of 82Rb in humans under pharmacologic stress. J Nucl Med 2011;52:485-91.
Slomka PJ, Berman DS, Germano G. Low-dose 3d (82)Rb PET. J Nucl Cardiol 2012;19:1110-2.
Einstein AJ, Weiner SD, Bernheim A, Kulon M, Bokhari S, Johnson L, et al. Multiple testing, cumulative radiation dose, and clinical indications in patients undergoing myocardial perfusion imaging. JAMA 2010;304:2137-44.
Acknowledgments
This research was supported in part by a research grant from Siemens Medical Solutions. Yafim Brodov is a Michael Kogan Save a Heart Foundation fellow in cardiac imaging. Manish Motwani is supported by the Dowager Countess Eleanor Peel Trust (Rothwell-Jackson Travelling Fellowship [UK]) and the Dickinson Trust Scholarship (UK). Cedars-Sinai Medical Center receives royalties for the quantitative assessment, a portion of which is distributed to some of the authors (DB, GG, PS). We thank Arpine Oganyan and Vikash Singh for their help in proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
See related editorial, doi:10.1007/s12350-015-0083-x.
Rights and permissions
About this article
Cite this article
Slomka, P.J., Diaz-Zamudio, M., Dey, D. et al. Automatic registration of misaligned CT attenuation correction maps in Rb-82 PET/CT improves detection of angiographically significant coronary artery disease. J. Nucl. Cardiol. 22, 1285–1295 (2015). https://doi.org/10.1007/s12350-014-0060-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-014-0060-9