Background
Electrocardiographic left bundle branch block (LBBB) may be intrinsic, due to ventricular conduction system disease, or induced by right ventricular pacing. Prior reports clearly delineate the derogatory impact of LBBB on left ventricular (LV) mechanical synchrony and global function, and suggest that the intrinsic and induced varieties are equivalent. This study sought to determine the difference in LV synchrony and global function between intrinsic LBBB and right ventricular apical pacing induced LBBB.
Methods
Ten patients with heart failure, diminished ejection fraction (EF) (33 ± 11%), intrinsic LBBB and an implanted cardiac pacing device were studied. In each patient, separate gated SPECT acquisitions were performed during intrinsic ventricular activation (atrial pacing) and during induced LBBB (atrial and right ventricular pacing). During each condition, LVEF, contraction synchrony (phase standard deviation, PSD), and spatial pattern of activation were measured.
Results
Compared to intrinsic, induced LBBB was associated with decreased EF (30 ± 11% vs 33 ± 11%, P = .007), contraction synchrony (PSD 49.7 ± 23.2° vs 41.6 ± 19.8, P = .02), and a disparate spatial pattern of activation.
Conclusions
Induced LBBB is associated with significantly worse global and regional LV mechanical function than intrinsic LBBB.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Electrocardiographic left bundle branch block (LBBB) may be intrinsic, due to ventricular conduction system disease, or induced by right ventricular pacing. Prior reports clearly delineate the derogatory impact of LBBB on left ventricular (LV) mechanical synchrony and global function, and suggest that the intrinsic and induced forms are equivalent.1,2 However, these reports are based on comparisons between groups of patients, and variation within each could have impaired detection of subtle differences with physiologic significance. We hypothesized that intrinsic and induced forms of LBBB were not equivalent, and that induced LBBB would be associated with diminished LV ejection fraction (LVEF), less LV contraction synchrony, and a disparate spatial pattern of activation. To minimize variation, we compared both LBBB forms within each subject utilizing gated single photon emission computed tomographic (SPECT) imaging.
Methods
The study was approved by the Institutional Review Board of the University of Pittsburgh Medical Center. Each subject had been referred for SPECT scanning for a standard clinical indication.
Subjects
Ambulatory subjects being considered for cardiac resynchronization therapy, who were enrolled met each of the following prospectively defined criteria: 1. chronic New York Heart Association Class III heart failure despite optimal tolerated pharmacotherapy; 2. intrinsic LBBB; 3. atrium in sinus rhythm; and 4. a previously implanted cardiac rhythm management device (pacemaker or defibrillator) incorporating leads terminating in both the right atrial free wall and the right ventricular apical regions. The study cohort was comprised of 10 consecutive subjects: 9 men, age 63 ± 8 years, ischemic etiology in 7.
Study Protocol
Each subject underwent imaging during each of two conditions: 1. atrial pacing, during which LV contraction was dictated by the intrinsic activation wavefront (intrinsic LBBB); 2. atrial and ventricular pacing, during which LV contraction was dictated by the paced activation wavefront (induced LBBB). The absence of fusion with the intrinsic wavefront during ventricular pacing was confirmed using 12 lead electrocardiography. For each condition, QRS duration was measured using the longest among all leads.
Image Acquisition and Analysis
Each subject was injected with 30 mCi of Tc-99m sestamibi. Imaging was performed during atrial pacing first, then repeated during atrioventricular pacing. No additional isotope was injected between imaging sets. Image data were acquired with a dual-headed gamma scintillation camera (Philips Medical Systems Inc., Milpitas, CA, USA) using standard myocardial perfusion imaging parameters (20% acceptance window around 140 keV energy peak, 180° orbit, and 32 steps with 25 seconds of data acquisition per step) and 16 frame gating. Iterative reconstruction was performed using a maximum likelihood expectation maximization algorithm, employing a Butterworth filter with a cut-off frequency of 0.66 and an order of 5.
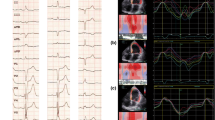
LVEF and contraction synchrony were assayed using Emory Cardiac Toolbox (ECTbx; Syntermed Inc., Atlanta, GA, USA). The efficiency of LV contraction, or synchrony, was determined using phase analysis of LV myocardial contraction (thickening, derived from Tc-99m emission activity). Myocardial contraction (thickening) in a given LV region is directly correlated with Tc-99m emission activity from that region (voxel). Thus, the time-activity curve for each myocardial imaging region represents its contraction profile. We utilize 16 bin gating and therefore, a total of 16 frames are generated per cardiac cycle. A first harmonic Fourier transform is then performed on the 16-frame time-activity curves. The onset of mechanical contraction is considered the point at which the count activity reaches the average count density for each particular voxel.3 Synchrony is quantified by the standard deviation of the phase distribution (PSD). The magnitude of the resulting PSD from among more than 600 LV regions varies inversely with synchrony. Data is represented as a phase histogram which is a visual representation of the dispersion of contraction and polarmap which displays the regional timing of contraction on a typical bulls eye representation of the LV (Figure 1).
These are example phase polarmaps (top) showing the pattern of activation displayed as a typical bullseye representation of the LV with septal to lateral delay during intrinsic LBBB (A) and apical to basal delay during induced LBBB (B). Phase histograms (bottom) depict the cardiac cycle in degrees on the x-axis and the percentage of myocardium at the onset of mechanical contraction on the y-axis with a more narrow phase histogram (indicating more synchronous LV contraction) during intrinsic LBBB (A) than during induced LBBB (B)
Spatial pattern of LV activation was addressed by manipulation of the time-activity data using Segment (Medviso, Lund, Sweden).4 Phase data were compressed into a standard American Heart Association 17 segment LV model.5 We assayed efficiency of activation in two separate spatial planes: 1. apical-basal, which was defined as the delay in activation between the six basal and five apical segments; 2. septal-free wall, which was defined as the delay in activation between four septal segments and four lateral segments.
Analytical Methods
Data are summarized as mean ± standard deviation, unless otherwise stated. Statistical comparison utilized paired t test or a two-way ANOVA, as appropriate. A P value less than .05 was considered statistically significant.
Results
Electrocardiographic QRS duration was significantly longer during atrioventricular pacing (induced LBBB; 176 ± 16 milliseconds) than during atrial pacing (intrinsic LBBB; 144 ± 22 milliseconds, P = .005). EF was lower during induced (30 ± 11%) than intrinsic (33 ± 11%; P = .007) LBBB. PSD was significantly greater during induced (49.7 ± 23.2°) than intrinsic (41.6 ± 19.8; P = .02) LBBB. Compared to induced LBBB, intrinsic LBBB had significantly more septal to lateral delay (46 ± 30° vs 18 ± 30°, P = .03) and less apical to basal delay (8 ± 27° vs 29 ± 22°, P = .02). Table 1 shows the data from each individual patient. Marked differences in spatial pattern of activation resulted in a significant disparity in the region of latest LV mechanical activation (Figure 2).
Average of the ten patients’ activation sequence during intrinsic (A) and induced (B) LBBB displayed as a left ventricular polarmap. Activation timing is represented as degrees according to the color scale seen. Visually, there is a septal to lateral delay during intrinsic LBBB and apical to basal delay during induced LBBB
Discussion
Our data demonstrate that despite their morphologic similarity, intrinsic LBBB and LBBB induced by RV pacing are mechanically dissimilar: RVA pacing exacerbates the mechanical inefficiency caused by intrinsic LBBB. These findings support the notion that intrinsic LBBB is not typically electrically, and thus mechanically, “complete,” and is thus associated with more efficient LV electromechanical activation than RV pacing.6 Our findings add new information to a literature which clearly demonstrates that LBBB is detrimental to LV function, in particular among patients with diminished LV systolic function, and that this detriment has clinical repercussions.7,8
Patients in the study cohort were typical of those referred for cardiac resynchronization, and recent data support an increasing role for SPECT in selecting patients for and optimizing this therapy.9,10 At present, resynchronization is practiced by simultaneously pacing right and left ventricles, with the conceptual goal of more efficient LV activation, and mechanistic studies suggest that the optimal site of LV pacing is in the region of latest mechanical activation.9,11 Observations in the present study raise interesting issues about this practice. First, if RV pacing exacerbates the LV mechanical efficiency associated with intrinsic LBBB, why would it be expected to become advantageous when applied during resynchronization therapy? Comparisons of outcomes during simultaneous RV and LV pacing with those during pacing of the LV alone demonstrate rough equivalency, suggesting that the ameliorative effect of pacing in these patients is attributable primarily to direct LV capture.12-14 It is thus possible that a derogatory effect of RV pacing is masked by a more potent ameliorative effect of LV pacing. If so, the magnitude of benefit from the therapy may be less than that which is possible. Second, in planning surgery for resynchronization therapy, the target for LV lead position is usually determined based on the latest activating region during intrinsic activation, which as we show herein differs significantly from that during RV pacing.
Our data have limitations. First, albeit consecutive, the study cohort is small. Nevertheless, the observations were consistent in most patients, and the strong statistical result given attests to a robust effect. Second, our cohort had significant LV systolic dysfunction. Many patients with intrinsic LBBB have preserved LV systolic function, and this has been associated with more efficient electromechanical activation during RV pacing.15 It would be our expectation that among such patients there would be less disparity in LV mechanical function between intrinsic and induced LBBB. Finally, since this was a small study, it was not randomized. However, a prior study of heart failure patients with LBBB, who underwent scanning with our same protocol reported a very high repeatability of the technique. The average difference in PSD between the two consecutive studies was only 0.58°.16 Our results show a significantly larger change in PSD indicating a true phenomenon and not merely an artifact of the technique.
References
Tanaka H, Hara H, Adelstein EC, Schwartzman D, Saba S, Gorcsan J III. Comparative mechanical activation mapping of RV pacing to LBBB by 2D and 3D speckle tracking and association with response to resynchronization therapy. JACC Cardiovasc Imaging 2010;3:461.
Tops LF, Schalij MJ, Bax JJ. The effects of right ventricular apical pacing on ventricular function and dyssynchrony. Implications for therapy. J Am Coll Cardiol 2009;54:764-76.
Chen J, Garcia EV, Lerakis S, Henneman MM, Bax JJ, Trimble MA et al. Left ventricular mechanical dyssynchrony as assessed by phase analysis of ECG-gated SPECT myocardial perfusion imaging. Echocardiography 2008;25:1186-94.
Soneson H, Ubachs JF, Ugander M, Arheden H, Heiberg E. An improved method for automatic segmentation of the left ventricle in myocardial perfusion SPECT. J Nucl Med 2009;50:205-13.
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the American Heart Association. Circulation 2002;105:539-42.
Rodriguez L, Timmermans C, Nabar A, Beatty G, Wellens HJJ. Variable patterns of septal activation in patients with left bundle branch block and heart failure. J Cardiovasc Electrophysiol 2003;14:135-41.
Wilkoff BL, Cook JR, Epstein AE, Greene L, Hallstrom AP, Hsia H, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: The dual chamber and VVI implantable defibrillator (DAVID) trial. J Am Med Assoc 2002;288:3115-23.
Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003;107:2932-7.
Friehling M, Chen J, Saba S, Bazaz R, Schwartzman D, Adelstein EC, et al. A prospective Pilot study to evaluate the relationship between acute change in left ventricular synchrony after cardiac resynchronization therapy and patient outcome using a single-injection gated SPECT protocol clinical perspective. Circ Cardiovasc Imaging 2011;4:532-9.
Adelstein EC, Saba S. Scar burden by myocardial perfusion imaging predicts echocardiographic response to cardiac resynchronization therapy in ischemic cardiomyopathy. Am Heart J 2007;153:105-12.
Khan FZ, Virdee MS, Palmer CR, Pugh PJ, O’Halloran D, Elsik M, et al. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: The TARGET study: A randomized, controlled trial. J Am Coll Cardiol 2012;59:1509.
Auricchio A, Stellbrink C, Block M, Sack S, Vogt J, Bakker P, et al. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. Circulation 1999;99:2993-3001.
Touiza A, Etienne Y, Gilard M, Fatemi M, Mansourati J, Blanc JJ. Long-term left ventricular pacing: Assessment and comparison with biventricular pacing in patients with severe congestive heart failure. J Am Coll Cardiol 2001;38:1966-70.
Gage RM, Burns KV, Vatterott DB, Kubo SH, Bank AJ. Pacemaker optimization in nonresponders to cardiac resynchronization therapy: Left ventricular pacing as an available option. Pacing Clin Electrophysiol 2012;35:685-94.
Sweeney MO, Prinzen FW. A new paradigm for physiologic ventricular pacing. J Am Coll Cardiol 2006;47:282-8.
Lin X, Xu H, Zhao X, Folks RD, Garcia EV, Soman P, et al. Repeatability of left ventricular dyssynchrony and function parameters in serial gated myocardial perfusion SPECT studies. J Nucl Cardiol 2010;17:811-6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Friehling, M., Ludwig, D.R., Dunn, M. et al. Deterioration of left ventricular ejection fraction and contraction synchrony during right ventricular pacing in patients with left bundle branch block. J. Nucl. Cardiol. 20, 830–834 (2013). https://doi.org/10.1007/s12350-013-9752-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-013-9752-9