Abstract
Background
Rubidium-82 (82Rb) PET imaging has been proposed for routine myocardial blood flow (MBF) quantification. However, few studies have investigated the test-retest repeatability of this method. The aim of this study was to optimize same-day repeatability of rest MBF imaging with a highly automated analysis program (FlowQuant) using image-derived input functions and dual spillover corrections (SOC).
Methods
Test-retest repeatability of resting left-ventricle (LV) MBF was measured in patients (n = 27) with suspected coronary artery disease (CAD) and healthy volunteers (n = 9). The effects of scan-time, reconstruction, and quantification methods were assessed with correlation and Bland-Altman repeatability coefficients.
Results
Factors affecting rest MBF included gender, suspected CAD, and SOC (P < .001). Significant test-retest correlations were found using all analysis methods tested (r > 0.79). The best repeatability coefficient for same-day MBF was 0.20 mL/minute/g using a 6-minute scan-time, iterative reconstruction, SOC, resting rate-pressure-product (RPP) adjustment, and left atrium input function. This protocol was significantly less variable than standard protocols using filtered back-projection reconstruction, longer scan-time, no SOC, or LV input function.
Conclusion
Absolute MBF can be measured with good repeatability using FlowQuant analysis of 82Rb PET scans with a 6-minute scan time, iterative reconstruction, dual SOC, RPP-adjustment, and an image-derived input function in the left atrium cavity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Conventional myocardial perfusion imaging (MPI) with SPECT or PET is known to have reduced sensitivity in patients with multivessel coronary artery disease (CAD).1,2 The extent and severity of disease can be underestimated when MPI is measured relative to the “most normal” territory, which may also have reduced perfusion if supplied by a stenotic vessel. Advances in rubidium-82 (82Rb) PET imaging have led to the possibility of routine quantification of myocardial blood flow (MBF),3 which increases the ability to detect multi-vessel disease (MVD).4 82Rb has been validated against nitrogen-13-ammonia and microspheres, and has been shown to measure rest and stress MBF accurately.5,6 We recently reported inter- and intra-operator variability for MBF measurement using 82Rb PET and a highly automated analysis program.7 However, while test-re-test data exist for flow quantification using 13N-ammonia8,25 and 15O-water9,22,23 there are limited data regarding the test-retest repeatability using 82Rb PET in patient studies.5,10 The purpose of this study was to optimize the test-retest repeatability of resting 82Rb PET MBF quantification using the FlowQuant program.
Materials and Methods
Patient Cohort and Preparation
Thirty-six subjects were included in this study. Patients (n = 27) with suspected CAD and healthy normal volunteers (n = 9) underwent two successive rest scans and one dipyridamole stress scan. The scans were carried out in a single visit allowing for 82Rb decay between scans. Patients were instructed to refrain from consuming caffeine for 12 hours, beta-blockers for 24 hours, and eating for at least 4 hours prior to the scan.
Each patient gave written informed consent under a cardiac PET registry study approved by the Ottawa Heart Institute Research Ethics Board.
Image Acquisition Protocol
Three 82Rb PET scans were completed in one imaging session using a standard protocol as described previously by Klein et al.11 Subjects were purposely not repositioned between scans to limit body motion, changes in heart orientation, and resting hemodynamics. In brief, following a scout scan to confirm patient positioning, a low-dose (0.4 mSv) and fast helical (1.5 seconds) CT scan was performed at normal end-expiration for attenuation correction of both resting 82Rb scans. Tracer activity (10 MBq/kg) was administered from a 82Sr/Rb generator11 intravenously over a 30-second interval using a custom infusion system,12 limiting the coincidence dead time to less than 50 % to ensure accurate measurement of the first-pass bolus activity. Dynamic rest data (scan 1) were acquired over 10 minutes using a Discovery RX PET-VCT scanner (GE Healthcare, Waukesha, MI) in 3D-mode with 15 time intervals (9 × 10, 3 × 30, 1 × 60, 1 × 120, and 1 × 240 seconds). Immediately following the end of scan 1, a second rest 82Rb study (scan 2) was acquired to assess repeatability. Dipyridamole (0.14 mg/kg/minute for 5 minutes) was infused to induce hyperemic stress, and the same 82Rb PET imaging protocol was repeated. A second low-dose CT scan was performed for attenuation correction of the stress scan data. After Fourier rebinning (FORE) of the 3D scan data, dynamic images were reconstructed using filtered back-projection (FBP) with 12-mm Hann filter, and ordered subsets expectation maximization (OSEM) with four iterations, 24 subsets, and 8-mm Hann filter.
Image Analysis
Image data were processed using FlowQuant v2.2 (University of Ottawa Heart Institute, ON) as reported previously.7 In brief, dynamic images were reoriented automatically into short-axis views and the left ventricle (LV) myocardial time-activity-curves (TAC) were measured in 568 polar map sectors, C myo(t). Blood regions were placed automatically within the left ventricle and atrium cavities to sample the arterial input function, C cav(t).
Standard clinical protocol settings (10 minute scan, FBP reconstruction) were used as a baseline for comparison. Protocol changes were investigated using eight variations of the following three settings: (1) 6 minute scan, (2) OSEM reconstruction, and (3) dual spillover correction (SOC) and adjustment for the resting rate-pressure-product (RPP). The RPP was calculated as heart-rate × systolic blood pressure. Rest MBF values were adjusted for the patient-specific RPP as MBFadj = MBF/RPP × RPPavg, where the average RPP value across all the study subjects was 7,426 bpm × mmHg.
Quantification of MBF
Kinetic modeling
The one-tissue-compartment distribution-volume (1 comp DV) model of tracer uptake in myocardial tissue was used to describe the 82Rb tracer kinetics13
where C t (t) is the true myocardial tissue activity concentration, K 1 is the 82Rb uptake rate in mL/minute/g, and C cav(t) is the concentration of arterial blood measured in the cavity region as a function of time, and \( \otimes \) is the convolution operator.7 Distribution volume (DV) is the ratio of tissue and blood tracer concentrations after the compartments reach equilibrium. At equilibrium, the net exchange of tracer is zero and DV becomes
DV was set to a constant value (specific for each scan) determined by fitting the unconstrained model to the normal uptake region (>75 % max) within the polar map.
Uptake, K 1, and MBF (mL/minute/g) are related by
where
The 82Rb extraction function, E(MBF), is a model-specific term that accounts for nonlinear tracer extraction and the permeability surface-area product, PS (mL/minute/g) as a function of MBF.14,15 The PS function was previously determined in human studies,13,16 as
making K 1
The myocardial image concentration in each polar map sector was modeled as
where FBV is the estimated fractional blood volume, and (1 − FBV) was used to correct for regional partial-volume losses in the myocardium.17 The parameters K 1, k 2, and FBV were estimated with Eqs. (1) and (7) in each sector of the LV myocardium, using weighted nonlinear least squares minimization comparing the modeled with the measured myocardium TACs.
Dual SOC
Spillover from the myocardium into the LV cavity reduces the quantitative accuracy of the image-derived arterial input function. To improve the accuracy, a technique was developed to estimate a pure blood signal for use as input to the kinetic model instead of C cav(t). The proposed method models the myocardium and cavity TACs as mixed partial-volume fractions of pure blood, C b(t) and pure myocardium C t (t) signals.16 The myocardium and blood pool regions are
and
where C cav(t) is the mixed blood signal in the cavity, and β is the partial-volume fraction of pure blood signal in the cavity region of interest. Equations (8) and (9) can be combined to generate the pure blood signal, C b(t), from the measured functions, C myo(t) and C cav(t),
First, the parameters K 1, k 2, FBV, and β were estimated using the average LV polar map data C myo(t), using weighted nonlinear least squares minimization with Eqs. (1), (8), and (10) . Then, in a second step, using the β value estimated above, the corresponding pure blood signal C b(t) from Eq. (10) was substituted in place of C cav(t) to estimate regional MBF in each polar map sector with Eqs. (1) and (7).
Statistical Analysis
Student’s t-test and multiple factor ANOVA (with interactions) were used to analyze the segmental MBF data where appropriate. Bonferroni correction was applied to account for multiple t-tests. The F-test was used to compare the test-retest variance between scans. Correlation plots (Pearson’s r 2) were used to show agreement between datasets. Bland-Altman plots were used to compare test-retest differences, to calculate the repeatability coefficient (RPC = 1.96 × standard deviation (SD) of differences), and to calculate the coefficient of variation (CV, SD/mean × 100 %).18 Differences were considered statistically significant when P < .05.
Results
Demographics
The subject demographics and hemodynamics are summarized in Tables 1 and 2. The age range of study subjects was 27-76 years: 45-76 for CAD patients, and 27-60 for normal volunteers. The difference between male and female RPP was significant, both in scan 1 and scan 2 (P < .05). The difference in RPP between CAD patients and normal volunteers was not significant for either scan 1 or scan 2 (P = .1). Scan 1 to scan 2 RPP differences were also not statistically significant (P > .3).
Protocol Effects on MBF
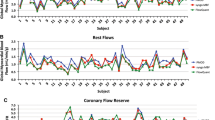
Table 3 summarizes scan 1 and scan 2 MBF results for the eight imaging protocols evaluated. Multifactor ANOVA showed differences in male vs female (P < .01) and normal vs CAD patients (P < .05) MBF values, as well as a systematic decrease in MBF (P < .01) using the dual-SOC. Other parameters (reconstruction method, scan length, scan number, and RPP adjustment) did not have a significant effect on the mean MBF values (P > .18). There were significant interactions of gender and disease status with RPP (P < .01).
The population SD values shown in Table 3 were not significantly different between test-retest scans, reconstruction methods, scan length, or between population subgroups, i.e., gender and disease status. After RPP adjustment, the mean MBF remained essentially unchanged, but the population sample SD values decreased significantly (P < .01) on average from 0.26 to 0.17 mL/minute/g for scan 1, and from 0.33 to 0.21 mL/minute/g for scan 2. Therefore, individual RPP values accounted for a significant fraction (~1/3) of the population variability in resting MBF.
Figure 1 shows a box-plot of resting scan 1 MBF by gender with and without RPP adjustment (using 6-minute scans with OSEM reconstruction and dual-SOC, OSEM-6-SOC). The mean MBF was ~50 % higher in females vs males in scan 1 (P < .001) and scan 2 (P = .005). However, after adjusting for the resting RPP, the difference was no longer statistically significant.
Notched box-plot comparison of MBF values between male and female subjects, with and without RPP adjustment using 6-minute scan-time, OSEM reconstruction and dual-SOC. Each bar shows the sample median (red line) and inter-quartile range, with the notches representing 95 % confidence intervals on the median value. Outliers (+ symbols) are flow values >1.5 × inter-quartile range. Resting mean MBF values (circles) are significantly higher in females vs males before adjustment for RPP (0.97 vs 0.65 mL/minute/g; P < .001)
Test-Retest Repeatability
Table 4 shows the repeatability results for all eight protocol variations. MBF values generally showed good correlation between repeat scans (r 2 = 0.52-0.78), and was typically reduced after adjusting for changes in the RPP (r 2 = 0.37-0.66). On average, the OSEM-6-SOC-RPPadj method had the lowest RPC value (0.27 mL/minute/g) and CV (18 %), with no outliers greater than three SD from the mean. The SOC tended to improve the RPC and CV for OSEM reconstructions, but the opposite was observed for FBP.
Separating the OSEM-6-SOC results into normal and CAD patient groups, the RPC values were 0.20 and 0.31 mL/minute/g respectively (P = .3). RPP-adjusted normal and CAD patient RPC values were 0.19 and 0.27 mL/minute/g respectively (P = .7). The test-retest RPC was significantly smaller for reconstruction method OSEM-6-SOC vs FBP-6-SOC (P = .005) and for SOC OSEM-6-SOC vs OSEM-6 (P = .04), but not for scan length or RPP adjustment (P > .3). Using the preferred OSEM-6-SOC protocol, RPC in males was significantly better than females (0.21 mL/minute/g vs 0.40 mL/minute/g) before RPP adjustment (P < .01) but not after (0.21 mL/minute/g vs 0.33 mL/minute/g) (P = .07).
Figure 2 shows the correlation and Bland-Altman plot of test-retest differences using the optimal OSEM-6-SOC-RPPadj protocol. The plot also demonstrates the narrow population range of mean MBF values after RPP-adjustment (Table 3), but with similar test-retest repeatability compared to the unadjusted values (RPC = 0.26 vs 0.28; P = NS).
Discussion
This study evaluated the test-retest repeatability of global resting MBF measurements with 82Rb PET. We assessed the effect of scan length, image reconstruction method, dual-SOC, RPP correction, gender, arterial input function and CAD on mean blood flow, population variance, and test-retest repeatability.
A number of the myocardial time-activity curves (TACs) showed increasing blood activity in the last frame of the scan (6-10 minutes). This included points sampled in the LV myocardium, base, atrium, and cavity. Shortening the length of the data used in the scan from 10 to 6 minutes appeared to eliminate the noisy blood pool and myocardium signal observed at this late time-point. Shortening the scan could also reduce the likelihood or severity of patient motion, which may be more common in the final 4-minute time-frame. Our results show that a 6- vs 10-minute scan improved repeatability.
Patient body motion is a potential concern between and during scans. To minimize radiation dose, a single rest CT attenuation correction scan was used to correct both rest 82Rb PET scans. Patient translational motion (x, y, z) between scans was corrected manually using the standard ACQC PET-CT alignment and attenuation correction software. Small body movements within scans (i.e., between dynamic frames) were observable in a majority of cases, mostly in the final 4-minute frame. Intra-scan motion correction was not one of the goals of this study, but it is important to recognize that patient body and internal organ motion may decrease the repeatability and accuracy of absolute MBF quantification.
Hemodynamic differences between males and females were associated with significant changes in the resting MBF measurements (~0.3 mL/minute/g). After adjusting scan 1 MBF values for these differences in the rest RPP, the mean change between male and female subjects was no longer significant as shown in Figure 1. However, when scan 1 and scan 2 data were pooled, a significant gender difference of 0.1 mL/minute/g remained even after RPP-adjustment. Previous studies have reported that myocardial spillover effects (e.g., FBV) may bias PET measurements of MBF.19 The size of the LV cavity, thickness of the myocardium, and therefore, the variable activity spillover between blood pool and myocardium, could potentially affect the kinetic modeling accuracy of MBF measurements in men vs women. In the present study, male and female subjects did have significantly different fractional blood volumes (FBV = 0.33 vs 0.37; P < .001), and LV short-axis dimensions (56.4 vs 46.8 millimeters; P < .001), but the myocardium-to-blood spillover fractions (b) were not significantly different between genders (0.86 vs 0.87; P > .5). Adjustment of the MBF values for differences in FBV in addition to RPP eliminated the residual gender difference in MBF (0.77 mL/minute/g in males and females), suggesting that a small bias in estimation of the FBV may account for the residual differences in MBF. Interestingly, an inverse adjustment for LV dimension (MBF × individual subject ÷ population average LV dimension) in addition to RPP, also reduced the residual gender difference to ~0.05 mL/minute/g (P = .24), suggesting that a small physiologic effect of heart size on MBF may also be possible. To our knowledge, this effect has not been reported previously in CAD patients, and may warrant further investigation.
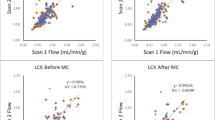
A small cylindrical region near the base of the LV cavity was used to sample the input function in the present study; however, the use of a left atrium (LA) input has also been reported.20 For comparison, two methods with the lowest RPC were used in the present study (Table 4: FBP-10-RPPadj and OSEM-6-SOC-RPPadj); MBF was also evaluated with a larger LA region to define the blood input function C cav(t). The mean MBF using the optimal protocol OSEM-6-SOC-RPPadj (0.85 ± 0.33 mL/minute/g) was slightly higher compared to the LV input function (P < .05), but not significantly different from the original clinical standard value (in Table 3: FBP-10-RPPadj = 0.83 mL/minute/g). The mean MBF using FBP-10-RPPadj with the LA input was also similar at 0.87 ± 0.29 mL/minute/g.
Figure 3 shows the correlation and Bland-Altman results for the most repeatable protocol (OSEM-6-SOC-RPPadj) using the LA input function. There were significant reductions in RPC (0.21 and 0.20 mL/minute/g) and CV (13 % and 12 %) using both OSEM-6-SOC and OSEM-6-SOC-RPPadj with the LA compared to the LV cavity input function values shown in Figure 2 (P > .05). However, the RPC (0.27 and 0.26 mL/minute/g) and CV (16 % and 15 %) using FBP-10 and FBP-10-RPPadj did not improve with the LA compared to the LV cavity input function values.
Optimized test-retest repeatability in the present study was 12 % using the LA blood input region and the OSEM-6-SOC-RPPadj protocol (Figure 3). Previously, we determined that the intra-operator variability for quantification of absolute MBF using the FlowQuant program was approximately 6 %.7 Since all test-retest studies were processed by the same operator in the present study, we can conclude that approximately half of the total variabilities may be attributed to operator interactions (required in ~10 % of cases), and the remaining half to the true test-retest differences in measured MBF values. The test-retest repeatability results presented in this study may not be exactly the same as those obtained using other flow quantification programs. However, we have demonstrated recently21 that very similar MBF (Δ < 4-12 %) and flow reserve (Δ < 8 %) values are obtained using FlowQuant compared with two commercial software implementations of the same 1-tissue-compartment model of rubidium kinetics,13 suggesting that the same physiologic and gender effects may be generalizable to MBF measurements made using the same or similar software programs.
Table 5 shows the present study results using the LA input with the SOC-6-OSEM protocol in comparison to previous articless that have reported rest MBF test-retest repeatability. Manabe et al10 reported an RPC value of 0.19 mL/minute/g in young healthy subjects, similar to the present study value (25 % of the mean MBF) measured in normal subjects and CAD patients. Other studies8,22–24 reported similar RPC values in either young, healthy volunteers and in older CAD patients using several PET tracers including 15O-water, 13N-ammonia and 82Rb. El Fakhri et al5 reported a coefficient of reproducibility of 0.45 mL/minute/g for combined rest and stress data. The repeatability of resting 82Rb MBF in this study using the OSEM-6-minutes-SOC protocol is among the lowest reported in the literature.
Adjusting the individual MBF values to the population average RPP can alter the population variance significantly. In the present study, the normal range of resting MBF was influenced heavily by a single patient with a very high resting RPP. Following adjustment for the individual scan-specific RPP, this resting MBF outlier was corrected to a value within the population mean ± 2 SD. Similarly, the test-retest RPCadj and CVadj showed incremental improvement compared to non-adjusted values. Previous studies5,10,22,23 found an increase in population variance and RPC with RPP adjustment. However, both Manabe et al10] and Chareonthaitawee et al24 reported a similar decrease in CV when RPP adjustment was applied. This suggests that measurements of heart-rate and blood pressure must be performed carefully to avoid introducing additional variability into the adjusted MBF values. In the present study, heart-rate and blood pressure measurements were performed immediately before and after tracer injections, and the average values were used to calculate the scan-specific RPP.
Optimizing the repeatability of resting MBF is also expected to have important effects on myocardial flow reserve (MFR) measured as the ratio of stress/rest MBF. While the present study was unable to incorporate a second stress scan with the clinical standard administration of aminophylline, the single stress scan was paired with both rest scans to simulate single-session MFR measurements. Table 6 shows the MFR results for the two optimal LV and associated LA input protocols. Similar to the rest MBF results, the most repeatable MFR results were obtained using OSEM-6-SOC-RPPadj with the LA input (RPC = 0.52) as expected. The test-retest variance was significantly lower than the next most repeatable protocol FBP-10 (P = .04), also using the LA input. Figure 4 shows the MFR correlation and Bland-Altman plots using the optimal OSEM-6-SOC-RPPadj protocol. Neglecting the added variability that may be expected from repeated stress scans, the RPC values measured in the present study (22-24 %) are much smaller than what has been reported previously in the literature as shown in Table 7.
The present study used a 30-second constant-activity-rate uniform infusion of 82Rb to optimize test-retest repeatability, and to limit PET system dead-time losses during the tracer first-pass through the heart and lungs. Our previous investigations suggest that the precision of MBF estimates may be further improved by using even longer infusion intervals, e.g., 1-4 minutes, together with the one-tissue-compartment model.26 This type of slow-bolus-controlled infusion may be particularly advantageous for MBF imaging on 3D PET systems with limited dynamic range, where it is essential to maintain quantitative accuracy at the peak count-rates encountered during the first-pass transit of activity through the circulatory system. Future investigations may help in determining potential trade-offs between accuracy and precision, using protocols optimized for absolute MBF quantification vs relative MPI for routine clinical use.
Statistical iterative reconstruction methods such as OSEM used in the present study have become the de facto standard for relative MPI with PET and SPECT. However, there are some important nonlinear effects that can potentially limit the accuracy of MBF quantification using OSEM or other statistical-based methods. Compared to analytic methods such as FBP, which have a stable and predictable linear behavior, the reconstructed resolution and contrast recovery with OSEM can vary spatially and dynamically, depending on the local count-statistics and convergence properties of the particular algorithm implementation. The accuracy of MBF measurements using a particular imaging instrument, iterative reconstruction code, and tracer kinetic analysis should always be validated for the intended use against an appropriate gold-standard.5,6,13
Limitations
A single stress scan was used to evaluate the effect of resting flow values on MFR repeatability, removing a significant source of physiologic variability that may be expected from repeated stress measurements. Repeatability of stress MBF measurements will be influenced by heterogeneity of the physiologic response to dipyridamole stress and amplified by decreased tracer extraction at higher flows. These effects should be investigated further to optimize the measurement of stress MBF and stress/rest flow reserve with rubidium PET.
Conclusion
Resting blood flow was measured with good repeatability in the LV myocardium using 82Rb PET; 95 % of test-retest differences were within 0.2 mL/minute/g. The optimal imaging protocol used a 6-minute scan, OSEM iterative reconstruction, dual-SOC, and image-derived input function using a blood region located in the left atrium cavity. Further prospective studies are warranted to evaluate the test-retest repeatability using this protocol at rest and stress.
References
Ziadi M, Beanlands R. The clinical utility of assessing myocardial blood flow using positron emission tomography. J Nucl Cardiol 2010;17:571-81.
Ziadi M, deKemp R, Williams K, Guo A, Renaud J, Chow B, et al. Does quantification of myocardial flow reserve using rubidium-82 positron emission tomography facilitate detection of multivessel coronary artery disease? J Nucl Med 2012;19(4):670–80.
Parkash R, deKemp R, Ruddy T, Kitsikis A, Hart R, Beauschene L, et al. Potential utility of rubidium 82 PET quantification in patients with 3-vessel coronary artery disease. J Nucl Cardiol 2004;11:440-9.
Ziadi MC, deKemp RA, Williams KA, Guo A, Chow BJ, Renaud JM, et al. Imparied myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol 2011;58:740-8.
El Fakhri G, Kardan A, Sitek A, Dorbala S, Abi-Hatem N, Lahoud Y, et al. Reproducibility and accuracy of quantitative myocardial blood flow assessment with 82Rb PET: Comparison with 13N-ammonia PET. J Nucl Cardiol 2009;50:1062-71.
Lautamäki R, George R, Kitagawa K, Higuchi T, Merrill J, Voicu C, et al. Rubidium-82 PET-CT for quantitative assessment of myocardial blood flow: Validation in a canine model of coronary artery stenosis. Eur J Nucl Med Mol Imaging 2009;36:576-86.
Klein R, Renaud J, Ziadi M, Thorn S, Adler A, Beanlands R, et al. Intra- and inter-operator repeatability of myocardial blood flow and myocardial flow reserve measurements using rubidium-82 and a highly automated analysis program. J Nucl Cardiol 2010;17:600-16.
Schindler T, Zhang X, Prior J, Dadenas J, Dahlbom M, Sayre J, et al. Assessment of intra- and interobserver reproducibility of rest and cold pressor test-stimulated myocardial blood flow with 13N-ammonia and PET. Eur J Nucl Med Mol Imaging 2007;34:1178-88.
Kaufmann P, Gnecchi-Ruscone T, Yap J, Rimoldi O, Camici P. Assessment of the reproducibility of baseline and hyperemic myocardial blood flow measurements with 15O-labeled water and PET. J Nucl Med 1999;40:1848-56.
Manabe O, Yoshinaga K, Katoh C, Naya M, deKemp R, Tamaki N. Repeatability of rest and hyperemic myocardial blood flow measurements with 82Rb dynamic PET. J Nucl Med 2009;50:68-71.
Klein R, Adler A, Beanlands R, deKemp R. Precision-controlled elution of a 82Sr/82Rb generator for cardiac perfusion imaging with positron emission tomography. Phys Med Biol 2007;52:659-73.
Ruby-Fill™ Product Monograph. Rubidium Rb82 generator diagnostic radiopharmaceutical (myocardial imaging). Kirkland: Jubilant DraxImage Inc.
Lortie M, Beanlands R, Yoshinaga K, Klein R, DaSilva J, deKemp R. Quantification of myocardial blood flow with 82Rb dynamic PET imaging. Eur J Nucl Med Mol Imaging 2007;34:1765-74.
Renkin E. Transport of potassium-42 from blood to tissue isolated mammalian skeletal muscles. Am J Physiol 1959;197:1205-10.
Crone C. Permeability of capillaries in various organs as determined by use of the indicator diffusion method. Acta Physiol Scand 1963;8:292-305.
Klein R, Katoh C, Yoshinaga K, Tamaki N, Adler A, Beanlands R, et al. Evaluation of myocardium to blood pool spillover correction in quantification myocardial blood flow with 82Rb PET [Poster]. Montreal: World Molecular Imaging Congress; 2009.
Hutchins G, Caraher J, Raylman R. A region of interest strategy for minimizing resolution distortions in quantitative myocardial PET studies. J Nucl Med 1992;3:1243-50.
Altman D, Bland J. Measurement in medicine: The analysis of method comparison studies. Statistician 1983:307-17.
Slomka P, Alexanderson E, Jácome R, Jiménez M, Romero E, Meave A, et al. Comparison of clinical tools for measurements of regional stress and rest myocardial blood flow assessed with 13N-ammonia PET/CT. J Nucl Med 2012;53:171-81.
Hove J, Iida H, Kofoed K, Freiberg J, Holm S, Kelbaek H. Left atrial versus left ventricular input function for quantification of the myocardial blood flow with nitrogen-13 ammonia and positron emission tomography. Eur J Nucl Med Mol Imaging 2004;31:71-6.
deKemp R, Slomka PJ, Declerck J, Pan XB, Klein R, Nakazato R, et al. Reproducible imaging of myocardial flow reserve with rubidium-82 PET—A multicentre multi-vendor comparison study. Eur Heart J 2011;13(suppl):A76. [abstract].
Wyss C, Koepfli P, Mikolajczyk K, Burger C, von Schulthess G, Kauffmann P. Bicycle exercise stress in PET for assessment of coronary flow reserve: Repeatability and comparison with adenosine stress. J Nucl Med 2003;44:146-54.
Jagathesan R, Kaufmann P, Rosen S, Rimoldi O, Turkeimer F, Foale R, et al. Assessment of the long-term reproducibility of baseline and dobutamine-induced myocardial blood flow in patients with stable coronary artery disease. J Nucl Med 2005;46:212-9.
Chareonthaitawee P, Kaufmann P, Rimoldi O, Camici P. Heterogeneity of resting and hyperemic myocardial blood flow in healthy humans. Cardiovasc Res 2001;50:151-61.
Nagamachi S, Czernin J, Kim A, Sun K, Bottcher M, Phelps M, et al. Reproducibility of measurements of regional resting and hyperemic myocardial blood flow assessed with PET. J Nucl Med 1995;37:1626-31.
deKemp R, Klein R, Lortie M, Beanlands RS. Constant-activity-rate infusions for myocardial blood flow quantification with 82Rb and 3D PET. IEEE Nucl Sci Symp Conf Rec 2006;6:3519-21.
Acknowledgments
The authors would like to thank Judy Etele for enrolling the study subjects, and May Aung and Kimberly Gardner for performing the PET scans. This study was supported by an Imaging in Cardiovascular Therapeutics grant from the Ontario Research Fund (RE02038), and a Molecular Function and Imaging Program grant from the Heart and Stroke Foundation of Ontario (PRG6242). Matthew Efseaff was supported in part by an Ontario Graduate Scholarship.
Conflict of interest
Robert deKemp, Ran Klein, and Rob Beanlands are consultants for Jubilant DRAXimage. Robert deKemp and Ran Klein share revenues from the sale of FlowQuant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Efseaff, M., Klein, R., Ziadi, M.C. et al. Short-term repeatability of resting myocardial blood flow measurements using rubidium-82 PET imaging. J. Nucl. Cardiol. 19, 997–1006 (2012). https://doi.org/10.1007/s12350-012-9600-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-012-9600-3