Abstract
Background
Although vasodilator stress myocardial perfusion imaging (MPI) is increasingly performed with exercise, adenosine A2A receptor agonists have not been studied with exercise.
Objectives
To determine the safety of administering regadenoson during exercise and, secondarily, to evaluate image quality, patient acceptance, and detection of perfusion defects.
Methods
Patients requiring pharmacologic MPI received a standard adenosine-supine protocol (AdenoSup, n = 60) and were then randomized (2:1) in a double-blind manner to low-level exercise with bolus intravenous injection of regadenoson (RegEx, n = 39) or placebo (PlcEx, n = 21).
Results
Adverse events occurred in 95%, 77%, and 33% of patients receiving AdenoSup, RegEx, and PlcEx, respectively. Peak heart rate was 13 beats per minute (bpm) and 21 bpm greater following RegEx compared to that following PlcEx and AdenoSup, respectively (P = .006 and <.001). Change from baseline in mean systolic blood pressure (SBP), change from baseline to nadir SBP, and percentage of patients with a decline in SBP by ≥20 mm Hg showed no important differences between RegEx and PlcEx. No occurrences of 2nd degree or higher AV block were observed following RegEx or PlcEx; one patient developed 2nd degree AV block following AdenoSup. The mean heart-to-liver and heart-to-gut ratios were improved on RegEx vs AdenoSup: 0.85 (0.34) vs 0.65 (0.26), P < .001 and 1.1 (0.36) vs 0.97 (0.34), P < .001, respectively. Compared to AdenoSup, 70% of patients felt RegEx was much or somewhat better.
Conclusions
Combining regadenoson with low-level exercise is feasible, well tolerated, and associated with fewer side effects compared to AdenoSup.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Maximizing the diagnostic utility of myocardial perfusion imaging is predicated on the ability to obtain the highest quality images. To this end, a number of testing strategies have been evaluated utilizing vasodilators as myocardial stress agents. A recent review of 10 studies published over 13 years utilizing different exercise protocols with adenosine concluded that low-level exercise during adenosine MPI appears to decrease adverse effects and improve patient acceptance, and image quality, and may increase the sensitivity for detecting perfusion defects.1 Additionally Thomas et al2 found that amongst outpatients referred for non-invasive cardiac evaluation, use of a test protocol which combined adenosine with low-level exercise was a better predictor of cardiac event-free survival than testing with adenosine alone.
Although vasodilators are combined with exercise in approximately 17% of MPI studies in the United States3 and, indeed, combination testing is recommended by the American Society of Nuclear Cardiology practice guidelines,4 the Food and Drug Administration (FDA) labeled indications for adenosine and dipyridamole do not include use with exercise.5,6 In a 2005 editorial on the future of selective adenosine A2A receptor agonists,7 Miller suggested that modifications of the usage protocol that improve testing efficiency and/or diagnostic yield of the new selective agents, such as augmentation of demand stress, should ideally be supported by evidence-based modifications to their FDA labeling. This is the first study to evaluate the safety of combining a selective adenosine A2A receptor agonist (regadenoson), with low-level exercise on pharmacologic MPI. The objectives of this study were to evaluate safety, patient acceptance and various aspects of image quality as exploratory endpoints.
Adenosine directly and dipyridamole indirectly interact with all four known adenosine receptors subtypes (A1, A2A, A2B, and A3).8-12 Regadenoson (CVT-3146), a selective adenosine A2A receptor agonist, is a novel short-acting pharmacological stress agent that has been studied in two phase 3 randomized, double-blind clinical trials enrolling more than 2,000 patients worldwide.13 The affinity of regadenoson for human adenosine A2A receptors exceeds that for adenosine A1 receptors by >9-fold and its affinity for A2B and A3 receptors is minimal.14
Methods
Patients
To be enrolled in the study, patients must have been ≥18 years of age, required a clinically indicated adenosine pharmacologic stress SPECT MPI, and were anticipated to be able to exercise sufficiently to perform the study protocol- specified low-level exercise. Female patients who were pregnant, breastfeeding, or of childbearing potential were not included. The primary exclusion criteria were as follows: (1) History of coronary revascularization by either percutaneous coronary intervention or coronary artery bypass graft, or documented history of acute myocardial infarction or unstable angina within 3 months; (2) Change within 7 days of adenosine–supine MPI of medications that may affect the rate-pressure product or anticipated changes in such medications during the study; (3) Uncontrolled hypertension (i.e., >200/120 mm Hg); (4) Known hypertrophic cardiomyopathy with obstruction or severe aortic stenosis; (5) Decompensated congestive heart failure or cardiac transplantation; (6) A history of sick sinus syndrome or greater than 1st degree AV block, except in patients who had a functional artificial pacemaker or in whom these conditions occurred due to a temporary condition that now no longer exists; (7) Asthma or other bronchospastic reactive airway disease; and (8) Current use of dipyridamole, aminophylline use within 24 hours, or theophylline use within 48 hours.
Study Design
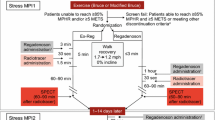
In this multicenter study (3 sites), subjects requiring pharmacologic MPI based on clinical criteria received adenosine infusion (Astellas Pharma, Inc.), 140 mcg/kg/minute over 6 minutes in the supine position (AdenoSup), following enrollment and were then randomized (2:1) in a double-blind manner to a novel protocol (RegEx) consisting of 4 minutes of low-level exercise (1.7 mph at 0% grade) with bolus intravenous injection of 400 mcg regadenoson at 1.5 minutes and Tc99m sestamibi at 2 minutes or matching placebos (PlcEx). “PlcEx” consisted of blinded study treatment and placebo tracer given in conjunction with the same exercise regimen as used for the RegEx protocol (Figure 1). Perfusion imaging was performed as part of the AdenoSup protocol and for those randomized to RegEx. For those randomized to PlcEx, the patient was placed under the camera at the appropriate time, but as only placebo tracer was administered, no imaging was performed. All were blinded to whether imaging was performed except the nuclear technologist.
Testing Protocol. All patients received adenosine while supine as a 6-minute intravenous infusion. In Session 2, patients were randomized (2:1) in double-blind fashion to Regadenoson during low level exercise for 4 minutes with bolus intravenous injection (400 mcg) at 1.5 minutes and Tc99m sestamibi at 2 minutes, or to matching placebo with low level exercise. Adenosup, adenosine supine; MPI, myocardial perfusion imaging; RegEx, regadenoson during exercise; PlcEx, placebo during exercise
Prior to randomization, patients were stratified based on the presence of reversible perfusion defects, defined as two or more segments with a stress score > rest score and a stress score ≥2 on a 5-category scale, as interpreted by a board-certified nuclear cardiologist at each site. The 5-category scale, used both for stratifying patients and for evaluation of perfusion defects on study, was as follows: 0 = normal; 1 = mild reduction in tracer uptake, not definitely abnormal; 2 = moderate reduction in uptake, definitely abnormal; 3 = severe reduction in uptake; 4 = absent uptake.
The primary objective was to assess the overall safety of regadenoson in patients undergoing low-level exercise by comparing hemodynamic, cardiac rhythm, and adverse effects of the 3 protocols. In addition, patient acceptance was determined by comparing patient comfort and test protocol preference using questionnaires. Three blinded expert readers independently interpreted randomly presented perfusion scans at a nuclear core lab (Services NucMed, Montreal, Canada). Readers were blinded not only to treatment assignment, but also to type of radioisotope tracer. Image quality was compared between AdenoSup and RegEx by computation of heart-to-liver and heart-to-gut ratios and the readers’ visual assessment of overall image quality and image quality with respect to subdiaphragmatic interference specifically. A 17-segment MPI model was used by the core lab readers to compare the extent of the perfusion defect between RegEx and AdenoSup quantitatively and also qualitatively with side-by-side visual comparison.15 For the subjects who underwent dual isotope imaging, we addressed the possibility of residual counts from the rest thallium scan by obtaining a 60-second planar view of the thorax and abdomen immediately after the thallium-201 SPECT imaging using the same technetium window that was going to be used for the AdenoSup Tc99m study. Radiotracer uptake counts (average counts per pixel) from the AdenoSup Tc99m study could be adjusted by the core laboratory by subtracting decay-adjusted counts obtained from the 60-second planar view. However, the extent of the correction was found to be so minor (1% to 2% in most and ≤3% in all subjects) that no adjustment was required.
Patients were required to abstain from methylxanthine-containing foods and beverages for 12 hours prior to receiving study drug and adenosine. The protocol was approved by an Institutional Review Board and all patients provided written informed consent.
Imaging Protocols
Nuclear imaging was performed using either a dual isotope protocol or a 2-day Tc99m (technetium) sestamibi protocol at the investigators’ discretion. However, men with body weight >220 pounds and body mass index >30 kg/m2 and women with body weight >200 pounds and body mass index >30 kg/m2 were to undergo the 2-day protocol. The single photon emission computed tomography (SPECT) imaging was standardized for image acquisition and transmittal in accordance with the American Society of Nuclear Cardiology guidelines.16 The protocol required an extra ~8 mSv radiation to the patients in the study arm, and none to the patients in the control (placebo exercise) arm.
The dual isotope protocol was performed over 2 separate days. On the first day, the patients had a rest scan with thallium-201 followed by a Tc99m sestamibi adenosine-supine MPI; on a subsequent day, the patients underwent a Tc99m sestamibi study drug (i.e., regadenoson or placebo) low-level treadmill exercise MPI.
The multiday Tc99m sestamibi was performed over 3 days. On the first day, the patients had a Tc99m sestamibi adenosine supine MPI or Tc99m sestamibi rest scan. On the second day, the patients had either the rest or stress scan, whichever was not received on the first day, and on the third day, they had a Tc99m sestamibi study drug (i.e., regadenoson or placebo) low-level treadmill exercise MPI. The protocol allowed a window of 1 day to 7 days between the AdenoSup MPI and the RegEx or PlcEx MPI.
The stress MPI scans were to be performed 60 ± 10 minutes after the start of adenosine or study drug. Regions of interest, defined as the entire left ventricle, a 25 square pixel area over the right upper lobe of the liver excluding the common bile duct, and a 5 × 5-pixel square area of the gut beginning 5 pixels inferior to the mid-inferior wall of the heart17 were identified from a 60-second planar view of the thorax and abdomen, prior to each SPECT imaging. A region of interest in the gut area below the heart was chosen because of the potential deleterious effect on interpretation of inferior wall perfusion. Specifically, either direct overlap of the gut or activity immediately below the inferior wall greater than the inferior wall itself can result in an artifactual subtraction of counts from the inferior wall intrinsic to commonly used edge-detection software.
Statistical Methods
Changes in blood pressure, heart rate, and ECG intervals were computed over time and compared (regadenoson vs placebo) using repeated measures, mixed-model ANOVA. The incidence of symptomatic hypotension, systolic blood pressure decreases of ≥20 mm Hg, ECG abnormalities, and severe or related adverse events was compared using Fisher’s exact test. The quality of nuclear MPI scans following regadenoson and low-level exercise was compared to adenosine–supine MPI scans of the same subject using the sign test. Radiotracer target-to-background ratios were computed and compared between the two imaging regimens using the Wilcoxon signed ranks test. Semi-quantitative scoring of perfusion defects was conducted using a 17-segment polar map.18 The number of segments with reversible perfusion defects was defined as the median number across the three readers.
Subject comfort and tolerability were assessed using a 4-point scale and the regimens compared using a Cochran–Mantel–Haenszel test of equality of mean scores. Data are expressed as mean (SD) unless otherwise specified. Statistical analyses were conducted using SAS version 9.1. Hypotheses were tested at the 5% significance level.
Results
A total of 62 patients were enrolled in the study and underwent adenosine MPI; 60 of these patients were subsequently randomized to either regadenoson MPI (n = 39) or placebo MPI (n = 21). Two patients were not randomized following adenosine MPI and were prematurely terminated from the study because of the initiation of a beta-blocker within 6 days prior to the adenosine MPI and elective withdrawal, respectively. Of the 39 patients randomized to regadenoson MPI, 20 were in the reversible perfusion defects stratum and 19 were in the no reversible perfusion defects stratum; of the 21 patients randomized to placebo MPI, 10 were in the reversible perfusion defects stratum and 11 were in the no reversible perfusion defects stratum. All 60 randomized patients completed the 6-minute adenosine treatment, were treated with study drug, completed the low-level exercise per protocol, and completed the study. Of those randomized to RegEx, 28 (72%) underwent imaging protocol 1 (dual isotope), and 11 (28%) underwent imaging protocol 2 (2-day Tc99m sestamibi). Of those randomized to placebo, 21 (100%) underwent imaging protocol 1 (dual isotope).
Baseline Characteristics
Patient demographics are shown in Table 1. Patients receiving regadenoson were more frequently male compared to those receiving placebo (52% vs 21%, P = .011). The frequency of use of cardiovascular drugs (Table 2) in the study population is consistent with the high frequency of pre-existing comorbidities including coronary artery disease, hypertension, congestive heart failure, and diabetes (Table 1). The percentage of subjects receiving one or more anti-anginal drugs (β-blockers, calcium channel blockers, or nitrates) was 30/39 (77%) in the RegEx group and 20/21 (95%) in the PlcEx group (P = .069, chi-squared)
Safety
Following AdenoSup, 95% of the 62 patients experienced at least one adverse event, defined as any abnormal sign or symptom, regardless of perceived causality. The corresponding percentages following RegEx (n = 39) and PlcEx (n = 21) were 77% and 33%, respectively (Table 3). Dyspnea was the only adverse event that occurred with a higher frequency (≥10% difference) during RegEx (54%) compared to AdenoSup (41%) (exact McNemar P = .23).
One patient developed protocol-defined symptomatic hypotension (defined as the development of a sufficient decline in blood pressure that was likely related to simultaneously occurring symptoms that may accompany hypotension) and this occurred following adenosine treatment. A pre-defined blood pressure was not used as part of the above definition because symptoms of hypotension can occur at different levels of blood pressure. Severe adverse events occurred in 4/60 (6.7%) patients following AdenoSup (abdominal pain, chest pain, ST-segment depression, neck pain, headache, and paresthesia) and in no patient following RegEx or PlcEx. No patient was withdrawn from the study due to an adverse event, and no patient had a serious adverse event.
Compared to the peak HR following PlcEx (+28.9 (SE 3.7) bpm) and AdenoSup (+21.0 (SE 2.5) bpm), peak heart rate following RegEx was greater by 13 and 21 bpm, respectively (P = .006 and <.001, respectively). This represented a 41.9 (SE 2.7) bpm increase from the resting baseline. The heart rate remained significantly higher during RegEx vs PlcEx through 24 minutes following start of exercise (Figure 2A), although by 24 minutes, the HR in the RegEx and PlcEx patients had diminished to +4.6 (SE 1.5) and −1.33 (SE 2.1) bpm, respectively, over the pre-exercise baseline.
A, Effect of AdenoSup (blue), RegEx (red), and PlcEx (green) on Heart Rate. Data points shown represent means + SEM. At 4, 6, 8, 10, 14, and 24 minutes following the start of exercise (time 0), P-values comparing mean heart rate during regadenoson administration during exercise (RegEx) vs placebo (PlcEx) administration during exercise were <0.05 (AdenoSup time points were slightly different than those for RegEx and PlcEx; therefore, comparisons at individual time points were not possible). B, Effect of AdenoSup (blue), RegEx (red), and PlcEx (green) on Systolic Blood Pressure. Data points shown represent means + SEM. P-values for all comparisons between RegEx and PlcEx were >0.05 at all time points (AdenoSup time points were slightly different than those for RegEx and PlcEx; therefore, comparisons at individual time points were not possible)
During exercise, there were similar and transient mean increases in systolic blood pressure in the RegEx and PlcEx groups (Figure 2B). Pre-specified analyses of blood pressure, which included change from baseline in mean SBP, change from baseline to nadir SBP, and percentage of patients with a decline in SBP by ≥20 mm Hg, showed no important differences between RegEx and PlcEx or between RegEx and AdenoSup.
Arrhythmias reported as adverse events or ECG findings occurred in 3 patients following AdenoSup only (atrial fibrillation, atrial tachycardia, and supraventricular arrhythmia) and in 1 patient following RegEx only (supraventricular tachycardia). In 2 patients, arrhythmias occurred following both AdenoSup and RegEx: ventricular tachycardia, ventricular extrasystoles, and “premature ventricular contraction-mediated tachycardia” following adenosine, and a ventricular couplet and pacemaker-mediated tachycardia with RegEx.
The effects of AdenoSup, RegEx, and PlcEx on ECG intervals were similar. No occurrences of 2nd degree or higher AV block were observed following RegEx or PlcEx; one patient developed 2nd degree AV block following AdenoSup.
Image Quality
Target (heart)-to-background ratios (heart-to-liver, heart-to-gut, and heart-to-liver + gut) were significantly higher on the RegEx scans compared to the AdenoSup scans (Figure 3 and Table 4). The mean (SD) heart-to-liver ratio of RegEx and AdenoSup amongst the 39 patients undergoing both of these scans was 0.85 (0.34) and 0.65 (0.26), respectively, P < .001. The comparable values for the mean heart-to-gut ratio were 1.1 (0.36) vs 0.97 (0.34), P < .001, respectively, and those for the heart-to-liver + gut ratio were 0.93 (0.26) and 0.72 (0.18), respectively, P < .001. In side-by-side comparisons of studies from the 39 patients who received AdenoSup and were subsequently randomized to RegEx, the latter had significantly better overall image quality (P = .002) and image quality with respect to subdiaphragmatic interference (P = .004) (Figure 4). A representative example of the difference in image quality and target-to background ratios is shown in Figure 5.
Heart-to-background ratios following AdenoSup (blue) and RegEx (red). Data are from the 39 patients who underwent adenosine while supine (AdenoSup) and regadenoson during lowlevel exercise (RegEx). Data presented are means + SD. P-values are for differences between AdenoSup and RegEx (Wilcoxon matched pairs signed ranks test)
Side-by-side comparison of the overall image quality and image quality with respect to subdiaphragmatic imaging. Data are from 39 patients who underwent supine adenosine testing (AdenoSup) and regadenoson with low-level exercise (RegEx). P-values are for differences between AdenoSup and RegEx (Sign test, ignoring the same category)
Perfusion Defects
Agreement analysis for detection of perfusion defects appeared to be at least as good with the combined low-level exercise–regadenoson protocol compared to the standard resting supine–adenosine approach both on a per patient basis and on a coronary territory basis (Table 5 and Figure 6). With respect to the number of coronary territories with reversible perfusion defects, the agreement rate between RegEx and AdenoSup was 20/39 (51%). A total of 13 patients were assessed as showing more reversible territories with RegEx, and 6 were assessed as showing fewer (exact sign test P = .17) (Table 5). The agreement rate for the number of reversible segments between the RegEx and AdenoSup scans was 13/39 (33%). Compared to AdenoSup, more reversible segments were assessed on RegEx in 17/39 (44%) patients and fewer reversible segments were assessed in 9/39 (23%) patients (Figure 6).
Bland-Altman plot depicting the difference in the number of reversible segments detected following RegEx and AdenoSup studies with increasing numbers of mean reversible segments. Segments were counted as showing reversible perfusion defects if the stress score (scale 0 to 4) was greater than the rest score and at least 2. Diamonds above the line of identity signify more reversible segments when RegEx was performed, below the line when AdenoSup was performed. The numbers next to the diamonds signify the number of subjects represented by the diamond
Patient Tolerability and Comfort
Both RegEx and PlcEx were well tolerated: 59% and 95% of patients, respectively, reported the tests as being “comfortable” and 41% and 5%, respectively, as being “a little uncomfortable” on a 4-point scale. No patients reported being very uncomfortable or extremely uncomfortable. Compared to those receiving AdenoSup, 70% of patients receiving RegEx and 96% of patients receiving PlcEx felt that the test with exercise was “much better” or “somewhat better” (Figure 7).
Patient preference for RegEx and PlcEx in comparison to AdenoSup. Following the exercise test, all 60 patients were asked. How did the exercise test compare to the test when you were lying down? The P-value is a comparison of the responses in the RegEx group and PlcEx group (Cochran–Mantel–Haenszel)
Discussion
We report the results of the first trial combining a selective adenosine A2A agonist (regadenoson) with exercise for the purpose of radionuclide myocardial perfusion imaging. In this randomized, double-blind, placebo- and active-controlled study including a large proportion of elderly patients with pre-existing coronary artery disease, administration of a regadenoson bolus of 400 mcg during low-level stress testing was both feasible and well tolerated. Compared to AdenoSup, image quality overall, heart-to-gut and heart-to-liver ratios, and side-by-side comparisons of image quality with respect to subdiaphragmatic interference were significantly better with RegEx. In addition, agreement analysis appeared to be at least as good with the combined low-level exercise–regadenoson protocol compared to the standard resting supine adenosine approach. Patients also appeared to tolerate RegEx better than AdenoSup, based on their questionnaire self-reports and the lower frequency and diminished severity of adverse events.
The goals of combining low-level exercise testing with an adenosine agonist pharmacologic MPI agent are threefold: (1) To increase the tolerability of the pharmacologic agent by inducing a sympathetic response with exercise that offsets the hypotensive and other adverse effects of the adenosine agonists; (2) To obtain the benefits of exercise on enhancing image quality due to a greater relative distribution of blood flow to the heart over the gut and liver; (3) To improve test sensitivity for detecting ischemia. Prior to this pilot trial, regadenoson had not been administered in conjunction with exercise. The objective of this study, therefore, was to assess the feasibility, tolerability, and safety of regadenoson with low-level exercise testing and, as exploratory endpoints, its effects on image quality, extent of detectable ischemia, and patient tolerability. The exercise regimen created for this trial was designed to be one that could be performed by most patients who would be referred for pharmacologic testing (i.e., those who would not be expected to achieve 85% or more of maximum predicted heart rate with exercise) but one that would still elicit the desired sympathetic response. The testing was performed at a modest speed (1.7 mph) and at 0% grade over 4 minutes. Indeed, all the subjects were able to complete the exercise protocol.
The hemodynamic effects of exercise testing were as expected: there was a transient modest (non-statistically significant) mean increase in systolic blood pressure and a significant increase in mean heart rate relative to supine pharmacologic-only testing with adenosine. The combination of regadenoson with low-level exercise testing increased the mean maximum heart rate by 16.5 beats per minute over low-level exercise testing with placebo (+40.2 (1.5) bpm on regadenoson vs +23.7 (2.1) bpm on placebo). The heart rate difference vs placebo declined over time such that HR following regadenoson was <5 bpm higher than the pre-exercise baseline by 24 minutes following the study drug bolus.
The primary limitation of this study is related to the exploratory endpoints. Specifically, the stress protocols with adenosine and regadenoson differed on more than one variable: the pharmacologic stress agent and the inclusion (or not) of exercise. Given this, it is not possible to discern whether the differences observed between AdenoSup and RegEx were due to the exercise, the drugs, or both. Because this study was primarily intended to assess safety and not designed to definitively determine the relative benefits of various dual stress regimens, this limitation does not detract from the primary assessment of safety in this trial (i.e., a comparison between the safety of RegEx with that of PlcEx). The rationale for the choice of the AdenoSup protocol were threefold: (1) Adenosine was administered without exercise to comply with its FDA labeling; (2) The primary intent of the study was to evaluate the safety of regadenoson with exercise compared to placebo (the safety comparison to adenosine was secondary); (3) Performing a blinded comparison of adenosine with exercise to regadenoson with exercise would be challenging given that adenosine requires a short infusion whereas regadenoson is administered as a bolus injection. Although a double-dummy design might have been used, the exercise protocols for the two drugs would have had to differ. Nevertheless, it does appear that the benefits of combining low-level exercise with regadenoson are generally similar to those previously reported for the combination of adenosine with low-level exercise.1 A second limitation is that we evaluated only stable patients (those without recent myocardial infarction or coronary intervention); therefore, the results from this study cannot necessarily be extrapolated to patients with recent acute coronary syndromes. Third, the trial is relatively small, consistent with its pilot nature. A final limitation was that the order of testing with respect to the supine and exercise procedure was always the same. Performing the AdenoSup study first was necessary to stratify patients so as to ensure balance between the RegEx and PlcEx arms with respect to the numbers of patients with reversible and no reversible defects.
In conclusion, this randomized, controlled pilot trial demonstrated for the first time the feasibility and tolerability of administering regadenoson with low-level exercise. The addition of low-level exercise to regadenoson appears to provide benefits on image quality, patient acceptance, and side effects similar to those previously reported for imaging protocols in which exercise is added to adenosine. The safety and utility of the concomitant use of regadenoson with low-level exercise requires confirmation in a larger study.
References
Thomas GS, Miyamoto MI. Should simultaneous exercise become the standard for adenosine myocardial perfusion imaging? Am J Cardiol 2004;94:3D-10D; discussion 10D-11D
Thomas GS, Miyamoto MI, Morello AP, 3rd, et al. Technetium 99m sestamibi myocardial perfusion imaging predicts clinical outcome in the community outpatient setting. The Nuclear Utility in the Community (NUC) Study. J Am Coll Cardiol 2004;43:213-23.
Division IMI. Nuclear medicine census market summary reports. Greenbelt, MD; 2006.
Henzlova M, Cerqueria M, Mahmarian J, Yao S. Stress protocols and tracers. In: DePeuy EG, editor. Imaging guidelines for nuclear cardiology procedures: a report from the nuclear cardiology quality assurance committee. American Society of Nuclear Cardiology; 2006. p. 171.
Bedford Laboratories. Full prescribing information for dipyradamole injection. Bedford, OH; 2006.
Astellas Pharma US. Adenoscan (Adenosine Injection) full prescribing information. Deerfield, IL; 2005.
Miller DD. Impact of selective adenosine A2A receptor agonists on cardiac imaging feeling the lightning, waiting on the thunder. J Am Coll Cardiol 2005;46:2076-8
Iskandrian AE, Bateman TM, Belardinelli L, et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: Results of the ADVANCE Phase 3 multi-center international trial. J Nucl Cardiol 2007;14:645-58
Fan M, Mustafa SJ. Adenosine-mediated bronchoconstriction and lung inflammation in an allergic mouse model. Pulm Pharmacol Ther 2002;15:147-55
Feoktistov I, Biaggioni I. Adenosine A2b receptors evoke interleukin-8 secretion in human mast cells. An enprofylline-sensitive mechanism with implications for asthma. J Clin Invest 1995;96:1979-86.
Feoktistov I, Biaggioni I. Pharmacological characterization of adenosine A2B receptors: studies in human mast cells co-expressing A2A and A2B adenosine receptor subtypes. Biochem Pharmacol 1998;55:627-33
Shryock JC, Belardinelli L. Adenosine and adenosine receptors in the cardiovascular system: biochemistry, physiology, and pharmacology. Am J Cardiol 1997;79:2-10
Data on File. CV Therapeutics, 2006.
Gao Z, Li Z, Baker SP, et al. Novel short-acting A2A adenosine receptor agonists for coronary vasodilation: inverse relationship between affinity and duration of action of A2A agonists. J Pharmacol Exp Ther 2001;298:209-18
Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539-42
Updated imaging guidelines for nuclear cardiology procedures, part 1. J Nucl Cardiol 2001;8:G5-58.
Thomas GS, Prill NV, Majmundar H, et al. Treadmill exercise during adenosine infusion is safe, results in fewer adverse reactions, and improves myocardial perfusion image quality. J Nucl Cardiol 2000;7:439-46
Klocke FJ, Baird MG, Lorell BH, et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging-executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). J Am Coll Cardiol 2003;42:1318–33.
Acknowledgments
The authors would like to acknowledge and thank Raymond Taillefer for his leadership of the nuclear core lab, Kim A. Williams, MD, Diwakar Jain, MD and Ronald G. Schwartz, MD, MS, independent interpreters of the nuclear images at the core lab and Mahesh P. Shah for his contribution of 7 subjects into the trial. We would also like to thank all the study research team and especially the subjects who graciously volunteered to participate in the study.
Drs Thomas, Thompson, Miyamoto, and Ip received research funding from CV Therapeutics. Dr Thomas additionally serves as a consultant to Astellas Pharma and is a past consultant to CV Therapeutics. He receives grant support from General Electric and Molecular Insights Pharmaceuticals and is on the speakers’ bureau for Astellas Pharma. Dr Mathur and Doug Milikien are current and former consultants to CV Therapeutics, respectively, and Dr Lieu is a former employee of CV Therapeutics.
Source of Financial Assistance: CV Therapeutics, Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented, in part, at American College of Cardiology 56th Annual Scientific Session, New Orleans LA, March 25, 2007.
Rights and permissions
About this article
Cite this article
Thomas, G.S., Thompson, R.C., Miyamoto, M.I. et al. The RegEx trial: a randomized, double-blind, placebo- and active-controlled pilot study combining regadenoson, a selective A2A adenosine agonist, with low-level exercise, in patients undergoing myocardial perfusion imaging. J. Nucl. Cardiol. 16, 63–72 (2009). https://doi.org/10.1007/s12350-008-9001-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-008-9001-9