Abstract
Cholangiolocellular carcinoma (CoCC) is a rare malignant primary liver tumor that is considered to originate from the canals of Hering, where hepatic progenitor cells are located. CoCC has various clinicopathological findings, therefore it is difficult to describe a clear diagnostic criteria for CoCC. Reported is a case of a large CoCC in a 45-year-old Japanese woman, which could not be preoperatively diagnosed as CoCC. The final diagnosis of CoCC was determined by pathological observation. Since both the biological behavior and diagnostic criteria of CoCC remain unclear, it is necessary to accumulate more information on CoCCs in order to elucidate these characteristic findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cholangiolocellular carcinoma (CoCC), a rare malignant primary liver tumor that accounts for 0.56% of all primary liver cancers [1, 2], is considered to originate from the ductules/canals of Hering, where hepatic progenitor cells (HPCs) are located [3]. The most characteristic histopathological feature of CoCC is a tendency for the neoplastic cells to be composed of a mixture of small monotonous glands, with antler-like anastomosing patterns and an abundant hyalinized and/or edematous fibrous stroma with lymphocytic infiltration [4]. HPCs are liver-specific adult stem cells that are activated when mature hepatocytes and/or cholangiocytes are damaged and become capable of differentiating into both cell types. Therefore, components of cholangiocellular carcinoma (CCC) and/or hepatocellular carcinoma (HCC) often coexist with CoCC. The varied clinicopathological findings make it difficult to describe clear diagnostic criteria, so CoCC cannot be diagnosed preoperatively and only a few cases have been reported in the English literature [5]. Here, we present a case of a large CoCC with imaging and clinicopathological findings and a review of the literature, with a particular focus on the hepatic stem cell origin of CoCC.
Case report
A 45-year-old Japanese woman was referred and admitted to our hospital for evaluation of a liver mass that was detected by abdominal ultrasound during a close inspection for epigastric discomfort. She had a history of appendicitis and asthma but no history of blood transfusion or high alcohol intake. Physical examination upon admission was normal. No ascites, peripheral edema or flapping tremor was observed. She was 161.9 cm in height and weighed 52.3 kg. Her blood pressure was 116/75 mmHg, and her pulse was 6 L/min.
Laboratory examinations showed normal values: white blood cell count, 7900/mL; red blood cell count, 411 × 104/mL; platelet count, 21.1 × 104/mL; prothrombin time, >100%; albumin, 4.1 g/dL; total bilirubin, 0.7 mg/dL; aspartate aminotransferase (AST), 16 IU/L; alanine aminotransferase (ALT), 13 IU/L; alkaline phosphatase, 136 IU/L; and γ-glutamyl transpeptidase, 20 IU/L. Serum biomarkers indicated normal liver function. Her Child class was A and her indocyanine green retention rate at 15 min (ICGR15) was 3.0%. Hepatitis B virus (HBV) surface antigen and hepatitis C virus (HCV) antibodies were negative. Levels of serum tumor markers were within normal limits, including α-fetoprotein, carbohydrate antigen 19-9, carcinoembryonic antigen and prothrombin induced by vitamin K absence or antagonist-II (Table 1).
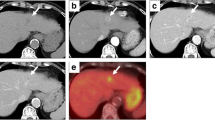
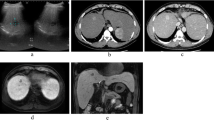
Abdominal computed tomography (CT) depicted an isoattenuating to hypoattenuating pear-shaped tumor measuring 45 × 75 mm in the medial segment (S4) of the liver (Fig. 1a). Predominant peripheral enhancement was seen with lower central density in the early phase, and persistent enhancement was seen in the delayed phase of dynamic contrast imaging (Fig. 1b, c). The margin of the tumor was clear. Magnetic resonance imaging (MRI) showed low- and high-intensity nodules in T1- and T2-weighted images, respectively. Peripheral enhancement was seen in the early phase during contrast enhancement in a gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA) dynamic study. In that imaging study, persistent enhancement was seen in the delayed phase, and hypoattenuation was seen relative to the surrounding liver tissue in the hepatic cell contrast phase (Fig. 2a–e). The tumor showed high intensity on diffusion-weighted images (Fig. 2f) and low signal intensity was seen on an apparent diffusion coefficient (ADC) map (Fig. 2g). 18F-fluorodeoxy glucose positron emission tomography (FDG-PET)/CT also revealed a focal region of increased uptake in S4 of the liver that correlated with the lesion observed by CT and MRI, with a standardized uptake value (SUV) maximum of 5.0, and with no other sites of abnormal FDG uptake identified on a whole-body PET (Fig. 3).
Abdominal computed tomography (CT) findings. a Plain CT shows a hypoattenuating pear-shaped tumor in the median segment (S4) of the liver. b Marked enhancement is observed in the periphery of the tumor in the arterial phase of dynamic contrast CT. c Persistent enhancement is observed in the delayed phase of dynamic contrast CT
Magnetic resonance imaging (MRI) findings. a The tumor shows low intensity on T1-weighted imaging. b The tumor shows a slightly high intensity on T2-weighted imaging. c A gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA) dynamic study shows peripheral enhancement of the tumor in the early contrast enhancement. d, e Persistent enhancement is observed in the delayed phase. f High intensity is observed on a diffusion-weighted image. g An apparent diffusion coefficient (ADC) map shows the tumor with low signal intensity
Based on these preoperative imaging findings, the hepatic tumor was diagnosed as a hepatic carcinoma, and the patient safely underwent a partial hepatectomy. The patient’s postoperative course was uneventful, and she was discharged on postoperative day 5.
The resected tumor measured 7.5 × 7.1 × 4.4 cm, and was a whitish, solid, non-encapsulated double nodule with a relatively regular margin. It was pear-shaped and contained a central scar comprising coarse fibrosis with a respective nodule (Fig. 4). Microscopic examination revealed that small ductules composed of cells with large ovoid nuclei and mild atypia had partially proliferated in an anastomosing pattern resembling Hering canal-like small glands. However, the ductules of the tumor were larger than the Hering canals in diameter and were not contiguous with the hepatic cords (Fig. 5a, b). Moreover, since mucin production was not observed in the tumor cells, the tumor was thought to be a CoCC. The tumor cells were immunohistologically positive for the biliary/HPC markers cytokeratin (CK) 7 and CK19, and negative for the hepatocytic markers hepatocyte paraffin-1 and α-fetoprotein. A strong intraluminal staining pattern for epithelial membrane antigens (EMAs) was seen in the gland (Fig. 5c). On the other hand, immunostaining for the carcinoid markers chromogranin A, synaptophysin and neuron-specific enolase (NSE) was negative. CoCC was confirmed as the final pathological diagnosis. The patient was alive without recurrence when this report was written at 12 months after the operation.
Pathological features of the tumor. a A low-power view of the tumor demonstrates that tumor cells have proliferated with an irregular, lobulated border. H&E staining, ×100. b A high-power view shows that the tumor cells have proliferated in an anastomosing pattern of small ductules resembling the canals of Hering with abundant fibrosis. H&E staining, ×400. c Immunohistochemical staining of epithelial membrane antigens reveals a strong intraluminal staining pattern in the gland. Magnification, ×400. d The luminal structure in the tumor was positive for cytokeratin 7 antibody. Magnification, ×400
Discussion
CoCC is an extremely rare primary malignant tumor of the liver, and its frequency is as low as 0.56% in Japan [1]. CoCC may originate from HPCs and may have the pluripotency to proliferate into CCC and/or HCC. In fact, in a previous study, all cases of CoCCs exhibited an HCC-like trabecular growth pattern located at the tumor/non-tumor boundary, while 63.3% of cases showed small tumor areas of papillary and/or clear glandular formation with mucin production and abundant fibrous stroma, which are considered to be typical features of CCC [5]. Furthermore, it has been postulated that HPCs are candidate cells for carcinogenesis, and they also give rise to CoCC in chronic liver diseases. Although it has been reported that tumors showing HPC features have a poorer prognosis than tumors without these features [4], it is very difficult to make a preoperative diagnosis of CoCC. Therefore, it is necessary to acquire more information on CoCCs in order to clarify these issues.
The diagnostic criteria for CoCC using imaging studies have not been clearly established, and as a consequence, CoCC is difficult to diagnose preoperatively. In the present case, the imaging findings were recognized as being similar to those of most reported cases of CoCC, but this cannot be considered a differential diagnosis. As reported in the literature, in the hepatic arterial phase of dynamic CT, CoCC is depicted with peripheral enhancement or a mosaic pattern [6]. In the portal venous and delayed phases, dynamic CT depicts CoCC with continuous enhancement or homogenous hyperattenuation. MRI of a typical CoCC reveals a low-signal intensity liver tumor in T1-weighted images and a high-signal intensity tumor in T2-weighted images. Marked contrast enhancement of the tumor has also been observed at the periphery of the tumor on MRI obtained during the arterial and portal venous phases [7]. These findings are very similar to those of CCC. Although a differential diagnosis of HCC and CCC may be possible with conventional imaging, it is very difficult to distinguish between CCC and CoCC with conventional imaging at the present time.
Characteristics of CoCC that differ from those of CCC include significantly higher rates of detection during follow-up of chronic hepatitis, ALT and/or AST abnormalities, hepatitis virus infection and fibrosis. It has been reported that 56% of CoCC patients were infected with HCV, 11% were infected with HBV and only 33% were negative for both HCV antibodies and HBs antigen [8]. The surrounding liver tissue showed liver cirrhosis in 26.7% of patients and chronic hepatitis with varying degrees of fibrosis in 73.3% of patients [1]. However, similar to the present case, a history of liver damage is not necessarily seen in all cases of CoCC. Therefore, CoCC should be considered when the imaging findings are not typical of HCC or CCC without elevation of serum tumor markers.
Pathological features of CoCC must be confirmed in order to make a definitive diagnosis, but because CoCC is a rare tumor and presents some conceptual difficulties, it is rarely diagnosed by pathologists. Nevertheless, the histopathological morphology of CoCC has distinct and characteristic features, as described by the World Health Organization criteria [9]. As mentioned above, the tumor cells have small ovoid nuclei and an eosinophilic cytoplasm with mild atypia, and proliferate in an anastomosing pattern of small ductules resembling the canals of Hering with fibrous stroma and without mucin production. However, it is difficult to diagnose CoCC with a needle biopsy because CoCC may have HCC-like trabecular areas and CCC-like areas in addition to the CoCC areas. These 3 different areas can coexist to various degrees. In the present case, areas of papillary and/or clear glandular formation with mucin production and abundant fibrous stroma were confirmed, findings which are considered to be typical features of CCC [5]. In the literature, only 3 CoCC cases have been precisely diagnosed preoperatively, 1 case by recurrence and 2 cases by needle biopsy. Moreover, hematoxylin and eosin (H&E) staining alone cannot determine a CoCC diagnosis because the pathological findings are varied and depend both on which component of the tumor is predominant and on the degree of CoCC differentiation. Consequently, immunohistochemical findings should be taken together with histological findings for the diagnosis of CoCC.
In the present case, tumor cells in the CoCC areas showed strong cytoplasmic positivity for CK7, CK19 and neural cell adhesion molecule as biliary/HPC markers, which was very similar to nonmalignant ductular reactions. In addition, specific adenosine triphosphate-binding cassette transporters, such as multidrug resistance protein 1 (MDR1), multidrug resistance-associated protein 1 (MPR1), MPR3 and breast cancer resistance protein (BCRP) generally show stronger staining intensity in CoCC compared with nonneoplastic HPC/ductular reactions. Other indications of CoCC include tumor cell expression of hematopoietic markers such as receptors for stem cell factor c-kit and prominin-1, embryonic stem cell markers such as POU5F1, and the leukemia inhibitory factor. In contrast, hepatocytic markers such as hepatocyte paraffin-1, canalicular polyclonal carcinoembryonic antigen and CD10 are negative in CoCC. In this present case, it is important to note that there was a strong intraluminal EMA staining pattern in the gland.
The question invariably arises as to whether CoCC differentiates into CCC or HCC during the process of growth. In the present case, from the pathological findings it was thought that the tumor differentiated into CCC. Indeed, it has been reported that the maximum tumor diameter of CoCCs ranges from 0.8 to 11 cm (mean ± SD, 4.9 ± 3.2 cm). In a total of 33.3% of cases, the CoCCs were less than 3 cm in diameter. One reason for this could be that most patients with CoCCs have a history of chronic hepatitis, and it is possible that such patients may have a greater frequency of periodic medical checkups that lead to detection of CoCC at an early stage. However, recent studies have described that activated HPCs are a target population for carcinogenesis, and that activated HPCs are present not only in hepatic malignant tumors such as HCC, combined HCC-CCC and CCC, but also in premalignant precursor lesions and hepatocellular adenomas [10].
One example of this carcinogenic potential is the expression of CK19, which is normally found in HPCs and cholangiocytes but not in hepatocytes. It has been reported that 12% of HCCs express CK19, and compared to CK19-negative HCCs, CK19-positive HCCs are associated with increased malignancy. CK19-positive HCCs do not express hepatocyte paraffin-1 like CoCCs. This suggests that these HCCs originate from HPCs, despite the histological evidence of a hepatocyte origin, poor histological differentiation, and intrahepatic or extrahepatic metastases. Also, referring to the gene expression profiles, CoCC and CK19-positive HCC have high homology. This evidence suggests the possibility that while keeping the characteristics of HPC, CoCC may differentiate into HCC or CCC during the process of growth.
Conclusions
The biological behavior of CoCC remains unknown, although some reports have shown that CoCCs exhibiting HPC-like features have a poorer prognosis than tumors without these features. Since a preoperative diagnosis of CoCC is generally difficult, CoCC should be considered as a differential diagnosis when a tumor with imaging findings similar to CCC is observed.
Abbreviations
- PT:
-
Prothrombin time
- ICG:
-
Indocyanine green
References
Shiota K, Taguchi J, Nakashima O, Nakashima M, Kojiro M. Clinicopathologic study on cholangiolocellular carcinoma. Oncol Rep. 2001;8:263–8.
Steiner PE, Higginson J. Cholangiolocellular carcinoma of the liver. Cancer. 1959;12:753–9.
Theise ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L, et al. The canals of Hering and hepatic stem cells in humans. Hepatology. 1999;30:1425–33.
Durnez A, Verslype C, Nevens F, Fevery J, Aerts R, Pirenne J, et al. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology. 2006;49:138–51.
Komuta M, Spee B, Vander Borght S, De Vos R, Verslype C, Aerts R, et al. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology. 2008;47:1544–56.
Asayama Y, Tajima T, Okamoto D, Nishie A, Ishigami K, Ushijima Y, et al. Imaging of cholangiolocellular carcinoma of the liver. Eur J Radiol. 2010;75:e120–5.
Fukukura Y, Hamanoue M, Fujiyoshi F, Sasaki M, Haruta K, Inoue H, et al. Cholangiolocellular carcinoma of the liver: CT and MR findings. J Comput Assist Tomogr. 2000;24:809–12.
Kanamoto M, Yoshizumi T, Ikegami T, Imura S, Morine Y, Ikemoto T, et al. Cholangiolocellular carcinoma containing hepatocellular carcinoma and cholangiocellular carcinoma, extremely rare tumor of the liver: a case report. J Med Invest. 2008;55:161–5.
Motosugi U, Ichikawa T, Nakajima H, Sou H, Sano M, Sano K, et al. Imaging of small hepatic metastases of colorectal carcinoma: how to use superparamagnetic iron oxide-enhanced magnetic resonance imaging in the multidetector-row computed tomography age? J Comput Assist Tomogr. 2009;33:266–72.
Libbrecht L, Desmet V, Van Damme B, Roskams T. The immunohistochemical phenotype of dysplastic foci in human liver: correlation with putative progenitor cells. J Hepatol. 2000;33:76–84.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kadono, M., Kimura, K., Imamura, J. et al. A case of a large cholangiolocellular carcinoma. Clin J Gastroenterol 4, 340–346 (2011). https://doi.org/10.1007/s12328-011-0250-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-011-0250-9