Abstract
Oxaliplatin in combination with 5-fluorouuacil and leucovorin (FOLFOX) is one of the most commonly used first-line chemotherapies for patients with advanced or metastatic colorectal cancer. Pulmonary toxicity, including interstitial pneumonia (IP)/peumonitis, is a very rare complication. We report a case of fatal IP associated with FOLFOX therapy in a patient with metastatic rectal cancer. A 74-year-old man with rectal adenocarcinoma and associated liver metastases underwent palliative surgery and 21 cycles of modified FOLFOX6 therapy. After starting the 22nd therapy cycle, the patient developed a high fever with non-productive cough. Chest X-ray demonstrated diffuse ground-glass opacities in both lungs, and computed tomography showed severe disorder of the bilateral lung architecture. On the basis of a lymphocyte stimulation test (DLST), oxaliplatin-induced IP was diagnosed. Intravenous administration of high-dose methylprednisolone was started, but the symptoms and radiological findings were not improved. The patient died of respiratory failure 16 days after the last administration of oxaliplatin. Although IP is a rare but potentially fatal complication of oxaliplatin-based treatment in colorectal cancer patients, clinicians should pay careful attention to the clinical respiratory symptoms and radiographic findings in colorectal cancer patients receiving FOLFOX therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The management and treatment of patients with metastatic colorectal cancer have changed dramatically over the last decade. Since it was first reported in the late 1990s, the FOLFOX regimen [1], which comprises bolus/infusional 5-fluorouracil (5-FU) with leucovorin (LV) and oxaliplatin, has become one of the most commonly used first-line chemotherapies for patients with advanced or metastatic colorectal cancer. In Japan, oxaliplatin (Elplat®, Yakult Co. Ltd., Tokyo, Japan) was approved in March 2005, and several types of FOLFOX regimens are frequently used for patients with advanced or metastatic colorectal cancer [2]. Although FOLFOX therapy has helped to improve the clinical outcome of these patients [1, 3], several therapeutic issues, including the adverse effects of this chemotherapy, still remain. Hematologic, gastrointestinal and neurosensory toxicities have frequently been observed in colorectal cancer patients who receive FOLFOX therapy [4]. Pulmonary toxicity, including interstitial pneumonia (IP)/peumonitis, is a very rare complication. Herein, we report a case of fatal IP associated with FOLFOX therapy in a patient with metastatic rectal cancer.
Case report
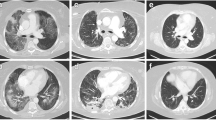
The patient was a 74-year-old man with rectal adenocarcinoma and liver metastases. The tumor was located in the middle rectum and had invaded the serosa with regional node metastases. The patient underwent palliative surgery and modified FOLFOX6 therapy consisting of 200 mg/m2 l-LV as a 2-h infusion and 85 mg/m2 oxaliplatin on day 1, followed by bolus injection of 400 mg/m2 5-FU and 46-h infusion of 2400 mg/m2 5-FU. Modified FOLFOX6 therapy was repeated every 2 weeks, and 21 cycles of the treatment were administered. For premedication, antiemetic prophylaxis with a 5-hydroxytryptamine receptor type-3 antagonist and dexamethasone was administered with each cycle. The patient had no history of food or medication allergy, and had no preoperative or postoperative complications, including respiratory dysfunction. Furthermore, the patient had never smoked. Before starting modified FOLFOX6 therapy, we confirmed the absence of any lung metastasis or respiratory disorders by chest computed tomography (CT) (Fig. 1a). By 16 cycles, he developed neutropenia (grade 3), peripheral neuropathy (grade 2), thrombocytopenia (grade 1), and stomatitis (grade 1) according to the Common Terminology Criteria for Adverse Events (CTCAE ver.3.0). Oxaliplatin and 5-FU were reduced to doses of 63 and 1,800 mg/m2, respectively. Bolus injection of 5-FU was stopped from 17 cycles of therapy.
Chest computed tomography. a Before starting chemotherapy, there is neither metastasis nor respiratory disorder. b After 18 cycles of FOLFOX, there are minimal consolidative changes and linear subpleural changes in the right lower lung. c At the onset of interstitial pneumonia, there is severe disorder of the bilateral lung architecture, including honeycombing, traction bronchiectasis and bronchiolectasis
After 18 cycles of modified FOLFOX6 therapy, progression of the liver metastases was observed. CT showed minimal consolidative changes and linear subpleural changes in the right lower lung (Fig. 1b). At the time, the patient had no respiratory symptom, and his performance status was good. Lung auscultation revealed no inspiratory crackles. For the above reason, we continued reduced dose chemotherapy. There was no abnormal finding from 18 cycles to 22 cycles of therapy in laboratory data such as WBC, C-reactive protein (CRP), and lactate dehydrogenase (LDH) levels. Krebs von den Lungen-6 (KL-6) was not measured.
One day after starting the 22 cycles of modified FOLFOX6 therapy (Fig. 2), the patient developed a high fever (38.8°C) with a non-productive cough. Peripheral oxygen saturation (SpO2) level measured by a pulse oximeter was under 90% on room air. Chemotherapy was stopped promptly, and supplemental oxygen (2 l/min) was started. The patient was treated empirically with cefdinir and carbocisteine. Despite transient clinical improvement, hypoxemia persisted, and the oxygen requirement increased.
Three days later, the non-productive cough had worsened, and signs of severe respiratory distress appeared. Fine crackles were heard in the bilateral lungs. Arterial blood gas analysis showed pH 7.467, PaCO2 34.6 mmHg, and PaO2 71.3 mmHg under nasal oxygen supplementation (3 l/min). Laboratory values were: WBC count 5700/μl (neutrophils 73.5%, eosinophils 2.6%), CRP 14.26 mg/dl (normally under 0.10 mg/ml), erythrocyte sedimentation rate 95.0 mm/h (normally 2.0–10.0 mm/h), LDH 462 IU/ml (normally 114–220 IU/ml), alkaline phosphatase 853 IU/ml (normally 124–367 IU/ml), and KL-6 4341 U/ml (normally 105–401 U/ml). All cultures and stains for infectious etiologies, including common bacteria, fungi, Pneumocystis and Legionella, were negative in the sputum. Chest X-ray demonstrated diffuse ground-glass opacities in both lungs. Chest CT showed severe disorder of the bilateral lung architecture, including honeycombing, traction bronchiectasis and bronchiolectasis (Fig. 1c). In addition, ground-glass attenuation was observed in the peripheral region of both lungs. Because of severe dyspnea, bronchoscopy and bronchoalveolar lavage were not performed. A peripheral blood lymphocyte stimulation test (DLST) with oxaliplatin, 5-FU and l-LV showed that the value for oxaliplatin was higher than that of the control (1106 vs. 529 c.p.m.). Therefore, oxaliplatin-induced IP was suspected. Intravenous administration of high-dose methylprednisolone (1,000 mg/day) with ciprofloxacin (600 mg/day) was started, but the symptoms and radiological findings were not improved, and so administration of high-dose steroid was repeated. Despite intensive treatments with mechanical ventilation and further administration of azathioprine (50 mg/day), the patient died of respiratory failure 16 days after the last administration of oxaliplatin (Fig. 2). Permission for autopsy was not granted by the patient’s family.
Discussion
Chemotherapy with oxaliplatin in combination with 5-FU and l-LV has been shown to have synergistic activity for the treatment of patients with colorectal cancer. On the other hand, FOLFOX therapy has frequent adverse effects, including toxicities of the bone marrow, gastrointestinal tract and neurosensory system. Initial safety studies of FOLFOX therapy for colorectal cancer patients did not find any significant pulmonary complications with the exception of dyspnea related to infusional or hypersensitivity reactions [4]. In addition, pulmonary toxicity, including pulmonary fibrosis, has been noted in less than 1% of colorectal cancer patients treated with the FOLFOX regimen during clinical trials (see manufacturer’s comments at http://www.eloxatin.com). Several types of pulmonary complications associated with oxaliplatin-based treatment have been reported in colorectal cancer patients, including acute lung injury [5], eosinophilic lung disease [6], cryptogenic organizing pneumonia [7], and IP or pulmonary fibrosis [8–16].
IP is induced via various pathogenic pathways, such as associations with collagen vascular disease, asbestosis, hypersensitivity reactions for several antigens, infections, sarcoidosis and several drugs. Drug-induced IP has been recorded for several chemotherapeutic agents used for malignancies, and oxaliplatin is known to be one of them. There are several causes of oxaliplatin-induced IP, but the underlying mechanism and the individual contribution of oxaliplatin to pulmonary toxicity are not sufficiently clear. Wilcox et al. [12] have suggested that oxaliplatin may predispose patients to glutathione depletion, and this situation may be part of the pathogenesis of oxaliplatin-related liver injury leading to hepatic sinusoidal obstruction associated with endothelial injury and perivenular fibrosis. Glutathione is a major small molecular antioxidant in the human lung that may be protective against oxidant-mediated lung disease; its depletion secondary to oxaliplatin treatment may provide the key to explain the progression of IP. In the present case, a positive reaction for oxaliplatin was observed upon DLST, and the result suggested a type-3 or type-4 allergic reaction. To our knowledge, this is the first documented case showing a positive DLST for oxaliplatin. Allergy to oxaliplatin has already been reported in the largest clinical trial of oxaliplatin-MOSAIC involving 2,246 patients, 10.6% of whom experienced an allergic reaction, but only 2.9% had grade 3–4 [17].
Although in the present case steroid therapy was started 2 days after the onset of slight respiratory symptoms, the patient died. When patients being treated with oxaliplatin develop IP, drug-induced IP should be considered as a differential diagnosis.
Over the last decade (January 2000 and December 2009) in Japan and the West, 17 cases of oxaliplatin-induced IP or acute lung injury have been reported, as revealed in a search of PubMed and Japana Centro Revuo Medicina. A review of the 18 colorectal cancer patients with oxaliplatin-induced IP, including the present one, is summarized in Table 1. The patients ranged in age from 30 to 82 years (mean value: 67.8), and comprised 14 men and 4 women. Colon and rectal cancer was observed in 14 and 4, respectively. Unresectable or metastatic colorectal cancer was observed in 10, and curative surgery was performed in 8. The chemotherapeutic regimens were FOFLOX and others in 16 and 2 patients, respectively. IP developed after between 4 and 22 cycles of FOLFOX therapy or oxaliplatin-based treatment (mean number of cycles, 8.7). Pulmonary complications before chemotherapy were observed in 4 cases: chronic obstructive pulmonary disease in 1, lung metastasis in 2 and pulmonary infiltrates in 1. Comparison of the clinicopathologic features between patients with IP who died and survived showed that those with pulmonary complications tended to have a high mortality rate. There was no difference with regard to the frequency or number of cycles of FOLFOX therapy or the total dose of oxaliplatin. The mean interval between diagnosis of IP and death in the patients who died was 19.7 days (range 8–33 days). Many of the early symptoms were dry cough, dyspnea and shortness of breath. Although there was no difference in the time until introduction of steroid treatment after the appearance of respiratory symptoms, rapid aggravation of the condition was seen in the patients who subsequently died.
In the present case, after 18 cycles of FOLFOX therapy, CT showed slight consolidative changes. At that time, if treatment was stopped or changed to the other therapy, it seemed to be possible to save the patient. But the patient had no respiratory symptoms or inspiratory crackles, and it was difficult to stop the chemotherapy. Thus, we should have used CT examinations more frequently and carefully.
In conclusion, IP is a rare, but potentially fatal complication of oxaliplatin-based treatment in patients with colorectal cancer. Pulmonary complications can become life-threatening in patients receiving anticancer chemotherapy, and careful attention should be paid to respiratory symptoms and radiographic findings in colorectal cancer patients receiving FOLFOX therapy, especially more than four cycles. When IP is suspected, chemotherapy should be promptly withdrawn, diagnosis should be made by chest X-ray or CT, and administration of steroids should be considered. DLST testing may be useful for confirming any allergy to the FOLFOX regimen.
References
de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–47.
Shimizu T, Satoh T, Tamura K, Ozaki T, Okamoto I, Fukuoka M, et al. Oxaliplatin/fluorouracil/leucovorin (FOLFOX4 and modified FOLFOX6) in patients with refractory or advanced colorectal cancer. Post-approval Japanese population experience. Int J Clin Oncol. 2007;12:218–23.
Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, et al. A randomized control trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combination in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30.
Ramanathan RK, Clark JW, Kemeny NE, Lenz HJ, Gococo KO, Haller DG, et al. Safety and toxicity analysis of oxaliplatin combined with fluorouracil or as a single agent in patients with previously treated advanced colorectal cancer. J Clin Oncol. 2003;15:2904–11.
Trisolini R, Lazzari Agli L, Tassinari D, Rondelli D, Cancellieri A, Patelli M, et al. Acute lung injury associated with 5-fluorouracil and oxaliplatinum combined chemotherapy. Eur Respir J. 2001;18:243–5.
Gagnadoux F, Roiron C, Carrie E, Monnier-Cholley L, Lebeau B. Eosinophilic lung disease under chemotherapy with oxaliplatin for colorectal cancer. Am J Clin Oncol. 2002;25:388–90.
Garrido M, O’Brien A, González S, Clavero JM, Orellana E. Cryptogenic organizing pneumonitis during oxaliplatin chemotherapy for colorectal cancer: case report. Chest. 2007;132:1997–9.
Yagüe XH, Soy E, Merino BQ, Puig J, Fabregat MB, Colomer R. Interstitial pneumonitis after oxaliplatin treatment in colorectal cancer. Clin Transl Oncol. 2005;11:515–7.
Pasetto LM, Monfardini S. Is acute dyspnea related to oxaliplatin administration? World J Gastroenterol. 2006;12:5907–8.
Mundt P, Mochmann HC, Ebhardt H, Zeitz M, Duchmann R, Pauschinger M. Pulmonary fibrosis after chemotherapy with oxaliplatin and 5-fluorouracil for colorectal cancer. Oncology. 2007;73:270–2.
Fuse N, Doi T, Ohtsu A, Takeuchi S, Kojima T, Taku K, et al. Feasibility of oxaliplatin and infusional fluorouracil/leucovorin (FOLFOX4) for Japanese patients with unresectable metastatic colorectal cancer. Jpn J Clin Oncol. 2007;37:434–9.
Wilcox BE, Ryu JH, Kalra S. Exacerbation of pre-existing interstitial lung disease after oxaliplatin therapy: a report of three cases. Respir Med. 2008;102:273–9.
Pena Alvarez C, Suh Oh HJ, Saenz de Miera Rodriguez A, Garcia Arroyo FR, Covela Rua M, Boquwtw LS, et al. Interstitial lung disease associated with oxaliplatin: description of two cases. Clin Transl Oncol 2009; 11: 332–3.
Muneoka K, Shirai Y, Sasaki Y, Wakai T, Sakata J, Hatakeyama K. Interstitial pneumonia arising in a patient treated with oxaliplatin, 5-fluorouracil, and leucovorin (FOLFOX). Int J Clin Oncol. 2009;14:457–9.
Ohori H, Takahashi M, Ogasawara N, Suzuki M, Miyate Y, Kato S. Two cases of interstitial lung disease in patients treated with oxaliplatin, 5-fluorouracil and leucovorin (FOLFOX). Jpn J Cancer Chemother. 2009;36:295–8. (Japanese).
Arevalo LS, Sagastibeltza MN, Elejoste EI, Mele MO, Egana LO, Basterretxea LB, et al. Fatal pneumonitis induced by oxaliplatin. Clin Transl Oncol. 2008;10:764–7.
Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hicksh T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Eng J Med. 2004;350:2343–51.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ishizone, S., Koide, N., Akita, N. et al. Fatal interstitial pneumonia associated with oxaliplatin-based therapy in a patient with metastatic rectal cancer. Clin J Gastroenterol 4, 157–161 (2011). https://doi.org/10.1007/s12328-011-0217-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-011-0217-x