Abstract
Von Hippel–Lindau disease (VHLD) is an autosomal dominant familial syndrome associated with multiple neoplasms. Medical management of pancreatic lesions is still controversial, especially for pancreatic neuroendocrine tumors (NET). We report an experience of total pancreatectomy for multiple pancreatic neuroendocrine tumors in a VHLD patient, and discuss the indication of surgical treatment. The patient was a 33-year-old Japanese female with a medical history of VHLD-associated tumors. At 27 years of age, abdominal computed tomography revealed a number of strongly enhanced round tumors throughout the pancreas. She underwent total pancreatectomy with portal vein resection because of back pain and an increase of tumor size. Pathological examination reconfirmed the diagnosis of multiple pancreatic NET invading the portal vein. She has been well with intensive insulin therapy and has shown no recurrence of NET for more than one year. This is a rare case of total pancreatectomy with portal vein resection for treatment of pancreatic NET in a VHLD patient. Total pancreatectomy is a viable option for treatment of multi-centric or extensive pancreatic NET because of a favorable prognosis of NET after radical surgical treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Von Hippel–Lindau disease (VHLD) is an autosomal dominant familial disorder caused by a germline mutation of the VHL tumor suppressor gene, and is characterized by benign and malignant tumors affecting multiple organs, including the central nervous system, kidney, adrenal grand, pancreas, and reproductive system [1]. The pancreatic lesions occur in 40–60% of VHLD patients and they are mostly cystic with a notable incidence of serous cystadenoma [1–3], a benign tumor without need for specific treatment [1].
By contrast, pancreatic neuroendocrine tumors (NET) occur in 8–18% of VHLD patients [1, 2]. In VHLD disease patients, NET are exclusively hormone-inactive [1–3], and surgical treatment is sometimes required to prevent local invasion and distant metastasis. However, when surgery should be recommended and what type of surgery should be chosen are still problems.
Case study
A 33-year-old Japanese woman had suffered back pain for seven years. She was referred to our institute because multiple solid tumors in the pancreas had gradually increased in size, which was pointed out at 27 years of age. She had a medical history of treatment for a variety of VHLD-associated tumors, including a pancreatic NET surgically resected at 14 years of age, hemangiomas of the fourth ventricle and cervical spinal cord operated at 19 years of age, and a renal cell carcinoma of the right kidney treated by nephrectomy at 30 years of age.
Mutational analysis of the VHL gene, performed using DNA extracted from her peripheral blood cells according to a previously reported method [4], revealed a G-to-A point mutation at nucleotide 500 (exon 1) resulting in an Arg167Glu amino acid substitution. Her father and elder brother were also diagnosed with VHLD, with her father having a medical history of two surgical resections for pancreatic NET and associated metastatic recurrence in the liver.
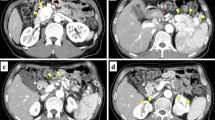
Blood tests showed increased serum levels of amylase (146 U/l, normal range; 40–115 U/l) and lipase (121 U/l, normal range; 13–60 U/l). There was no abnormality in serum levels for gastrointestinal and pancreatic hormones and tumor markers for NET, such as neuron-specific enolase. Abdominal computed tomography (CT) revealed multiple solid mass lesions throughout the pancreas (Fig. 1). These lesions were round with irregular alignment, strongly enhanced at the marginal region, and heterogeneously enhanced within the tumors. The tumor in the pancreatic head had a maximum diameter of 4.1 cm and appeared to invade the portal vein (Fig. 1a). Positron emission tomography with [18F]fluorodeoxyglucose (FDG-PET) and FDG-PET-CT revealed positive signals for FDG accumulation in the pancreas, but no significant signal in other organs (Fig. 2). The maximum standardized uptake value (SUVmax) is high and differs among tumors, ranging from 9.0 to 16.3. Endoscopic retrograde cholangiopancreatogram failed to show the main pancreatic duct, but demonstrated narrowing of the common bile duct because of compression by the tumor in the pancreatic head. Fine needle aspiration biopsy was not performed because of the risk of hemorrhage from the tumor with abundant blood flow shown by the abdominal CT.
She underwent total pancreatectomy and the preoperative diagnosis was multiple malignant NET. Laparotomy revealed that the mass of the pancreatic head had partly invaded the portal vein but was totally removed by portal vein resection. Pathological investigation of the resected specimen showed that the mass lesion was composed of round tumor cells with oval nuclei and slightly eosinophilic cytoplasm growing in a nested pattern and surrounded by an abundance of blood vessels (Fig. 3a). The tumor cells had invaded the wall of the portal vein, but had not eroded the inner surface (Fig. 3b). No metastasis in peripancreatic lymph nodes was found. Immunohistochemical studies revealed that the tumor cells were positive for synaptophysin and chromogranin A. Mitosis was rarely observed, but positive staining for Ki-67 was observed in 1–10% of tumor cells. Taken together, these findings provide evidence for diagnosis of well-differentiated neuroendocrine carcinoma according to the WHO classification system.
Following the operation, watery diarrhea appeared because of pancreatic exocrine insufficiency, but subsided with oral administration of a large amount of digestive enzymes. She was discharged on the 30th postoperative day. She has been well with intensive insulin therapy and has shown no recurrence of NET for one year.
Discussion
To our knowledge, this is the third reported case of a VHLD patient with pancreatic NET that was treated with total pancreatectomy. Total pancreatectomy is a rarely chosen treatment option for pancreatic NET in VHLD patients. A large study of 633 VHLD patients from a single institute reported only two total pancreatectomies among 39 patients who underwent definitive pancreatic surgery [2]. Total pancreatectomy combined with portal vein resection, as performed in our case, for pancreatic NET in a VHLD patient is extremely rare, and provides an invaluable instance of successful radical surgical treatment.
Generally, VHLD patients are subjected to many surgical operations during their lifetime, because of the occurrence and recurrence of a variety of tumors in various organs. Therefore surgical treatment may be avoided and careful observation may be chosen. However, operation is necessary when the tumor has high malignancy potential, because it improves the prognosis of patients with malignant pancreatic NET [5, 6].
In this case, the radiological images were suggestive of malignant nature of pancreatic NET. First, the size of the tumor was large with the maximum diameter of 4.1 cm. When the tumor size is greater than 3 cm, the tumor has an increased risk of liver metastasis and a shorter doubling time [2, 3]. Second, the pancreatic NETs were enhanced heterogeneously on CT. Typically, contrast-enhanced CT demonstrates pancreatic NET as a homogeneously hypervascular mass. In the present case, however, the tumor is heterogeneously enhanced, which possibly reflects the irregularity of the vascular structure, perfusion, and permeability, and also necrosis within the tumor, indicating the malignant potential of the pancreatic NET [3]. Third, the significant accumulation of FDG in pancreatic NET was demonstrated on FDG-PET-CT. A positive signal suggests that a tumor is likely malignant with high proliferative activity, while indolent pancreatic NET generally fails to show a positive signal [7].
A mutation in exon 3 of the VHL gene is reported to be associated with the malignant potential of pancreatic NET [2]. Our case showed a mis-sense mutation in exon 1, but not in exon 3 of the VHL gene. Further investigation of genotype–phenotype correlations with a large number of patients may provide the information necessary to determine the appropriate treatment option for pancreatic NET in VHLD patients.
In cases when surgical treatment is required, less-invasive surgery should be chosen to maximize preservation of pancreatic and neighboring organ function [8]. In the present case, however, the pancreatic tumors in the body and tail displayed heterogeneous enhancement, which is similar to the tumor in the pancreatic head. For this reason, the decision was made to resect all tumors by total pancreatectomy.
In conclusion, total pancreatectomy with portal vein resection was successfully achieved in a VHLD patient with pancreatic NET. The postoperative course was uneventful and the patient has been well more than one year after the surgery. Radical and curative resection should be considered for treatment of malignant pancreatic NET to improve the prognosis of VHLD patients with pancreatic NET.
References
Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, et al. von Hippel–Lindau disease. Lancet. 2003;361:2059–67.
Blansfield JA, Choyke L, Morita SY, Choyke PL, Pingpank JF, Alexander HR, et al. Clinical, genetic and radiographic analysis of 108 patients with von Hippel–Lindau disease (VHL) manifested by pancreatic neuroendocrine neoplasms (PNETs). Surgery. 2007;142:814–8.
Marcos HB, Libutti SK, Alexander HR, Lubensky IA, Bartlett DL, Walther MM, et al. Neuroendocrine tumors of the pancreas in von Hippel–Lindau disease: spectrum of appearances at CT and MR imaging with histopathologic comparison. Radiology. 2002;225:751–8.
Yoshida M, Ashida S, Kondo K, Kobayashi K, Kanno H, Shinohara N, et al. Germ-line mutation analysis in patients with von Hippel–Lindau disease in Japan: an extended study of 77 families. Jpn J Cancer Res. 2000;91:204–12.
Norton JA, Kivlen M, Li M, Schneider D, Chuter T, Jensen RT. Morbidity and mortality of aggressive resection in patients with advanced neuroendocrine tumors. Arch Surg. 2003;138:859–66.
Heidt DG, Burant C, Simeone DM. Total pancreatectomy: indications, operative technique, and postoperative sequelae. J Gastrointest Surg. 2007;11:209–16.
Bombardieri E, Maccauro M, De Deckere E, Savelli G, Chiti A. Nuclear medicine imaging of neuroendocrine tumours. Ann Oncol. 2001;12:S51–61.
Maeda H, Okabayashi T, Nishimori I, Kobayashi M, Sugimoto T, Kohsaki T, et al. Duodenum-preserving pancreatic head resection for pancreatic metastasis from renal cell carcinoma: a case report. Langenbecks Arch Surg. 2007;392:649–52.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maeda, H., Nishimori, I., Okabayashi, T. et al. Total pancreatectomy for multiple neuroendocrine tumors of the pancreas in a patient with von Hippel–Lindau disease. Clin J Gastroenterol 2, 222–225 (2009). https://doi.org/10.1007/s12328-009-0071-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-009-0071-2