Abstract
Introduction
We report a randomized, double-blind, placebo-controlled, 4-week study to investigate the effect of empagliflozin on free fatty acids and blood ketone bodies in Japanese patients with type 2 diabetes mellitus.
Methods
Patients (baseline mean [standard deviation] glycated hemoglobin 7.91% [0.80%]; body mass index 24.3 [3.2] kg/m2) were randomized to empagliflozin 10 mg (n = 20), empagliflozin 25 mg (n = 19), or placebo (n = 21) daily as monotherapy for 28 days. Meal tolerance tests (MTTs; breakfast, lunch, dinner) were performed on day − 1, day 1 (first day of treatment), and day 28. On day 1 and day 28, study drug was administered 1 h before breakfast. Free fatty acids and blood ketone bodies were measured before and 1, 2, and 3 h after each MTT, and the next morning (overnight fast).
Results
Empagliflozin significantly reduced plasma glucose and insulin and reduced body weight vs. placebo. Empagliflozin increased free fatty acids and total ketones bodies at day 1 and day 28. At day 28, the adjusted mean (95% confidence interval) difference vs. placebo in the time-corrected area under curve over 24 h for total ketone bodies was 67.1 (12.3, 121.8) µmol·h/L·h (P = 0.017) with empagliflozin 10 mg and 178.1 (123.9, 232.2) µmol·h/L·h (P < 0.001) with empagliflozin 25 mg. Increases in ketones with empagliflozin vs. placebo peaked just before and declined after meals, with the highest peak before breakfast. Changes in total ketone bodies appeared to be associated with changes in plasma glucose, insulin, and free fatty acids.
Conclusion
Empagliflozin modestly increased free fatty acids and blood ketone bodies after a single dose and 28 days’ treatment. Increases in ketones appeared to be related to the duration of fasting and were most pronounced before breakfast. Increases in ketones appeared to be associated with changes in well-known metabolic determinants of ketone production.
Trial Registration
ClinicalTrials.gov identifier, NCT01947855.
Funding
Boehringer Ingelheim & Eli Lilly and Company.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Empagliflozin is a highly selective sodium-glucose co-transporter-2 (SGLT2) inhibitor [1] approved as a treatment for patients with type 2 diabetes mellitus (T2DM). Empagliflozin reduces renal glucose reabsorption, increasing urinary glucose excretion in patients with T2DM [2]. Increased urinary glucose excretion results in calorie loss and reduced plasma glucose with a decreased insulin-to-glucagon ratio [3], leading to lipolysis, increased free fatty acid levels, and ketogenesis [4, 5].
In international phase III trials, empagliflozin as monotherapy or add-on therapy significantly reduced glycated hemoglobin (HbA1c) and fasting plasma glucose (FPG) with additional benefits of weight loss and reductions in systolic blood pressure [6,7,8,9,10,11]. In Japanese patients with T2DM, empagliflozin as monotherapy or add-on therapy for 52 weeks led to sustained reductions in HbA1c, FPG, body weight, and systolic blood pressure [12]. In a randomized, placebo-controlled, 4-week trial in Japanese patients with T2DM, empagliflozin 10 or 25 mg as monotherapy for 28 days significantly reduced fasting and postprandial glucose and postprandial insulin levels [13]. At day 29, mean changes from baseline in weight were – 1.7 kg with empagliflozin 10 mg, – 2.1 kg with empagliflozin 25 mg, and – 0.9 kg with placebo [13]. No adverse events of diabetic ketoacidosis or adverse events related to ketone body levels were reported, but increases in fasting free fatty acids and significant increases in fasting blood ketone bodies were observed with empagliflozin 10 mg and 25 mg vs. placebo at day 29 [13].
The objective of this analysis was to investigate the effect of empagliflozin on daily profiles of free fatty acids and blood ketone bodies in Japanese patients with T2DM.
Methods
This was an analysis of data from patients enrolled in a randomized, double-blind, placebo-controlled, parallel-group study conducted at two sites in Japan [13]. The trial was registered with ClinicalTrials.gov (NCT01947855). The clinical trial protocol was approved by the institutional review boards of the participating centers, and complied with the Declaration of Helsinki in accordance with the International Conference on Harmonisation Harmonised Tripartite Guideline for Good Clinical Practice. All patients provided written informed consent.
Patients
Japanese patients with T2DM aged ≥ 20 and ≤ 74 years, with a body mass index (BMI) ≤ 40 kg/m2, who were on a diet and exercise regimen and were drug naïve (no antidiabetes agents for ≥ 12 weeks prior to consent) or treated with one oral antidiabetes agent (except as listed in the exclusion criteria) at an unchanged dose for ≥ 12 weeks prior to consent were eligible for inclusion. At screening, drug-naïve patients were required to have HbA1c ≥ 7.0% and ≤ 10.0%, and patients treated with one oral antidiabetes agent were required to have HbA1c ≥ 7.0% and ≤ 9.5%. All patients were required to have HbA1c ≥ 7.0% and ≤ 10.0% at the start of the placebo run-in period.
Key exclusion criteria included uncontrolled hyperglycemia (glucose level > 240 mg/dL) after an overnight fast confirmed by a second measurement; acute coronary syndrome, stroke, or transient ischemic attack ≤ 20 weeks prior to randomization; indication of liver disease (alanine aminotransferase, aspartate aminotransferase, or alkaline phosphatase levels greater than three times the upper limit of normal during screening, washout, or run-in); impaired renal function (estimated glomerular filtration rate [eGFR] < 60 mL/min/1.73 m2 according to the Japanese estimation equation [14]) during screening, washout, or run-in; treatment with insulin, glucagon-like peptide-1 analogs, or sulfonylurea at more than half the daily maximum approved dose or thiazolidinedione within 12 weeks prior to consent; and treatment with anti-obesity drugs within 12 weeks prior to consent.
Randomization and Interventions
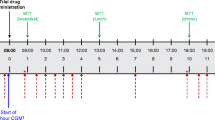
All patients underwent a 2-week, open-label, placebo run-in period. Patients pre-treated with an oral antidiabetes agent underwent a 2-week washout period prior to the placebo run-in. Following the run-in period, eligible patients were randomized (1:1:1) to receive empagliflozin 10 mg, empagliflozin 25 mg, or placebo for 28 days. Patients were monitored at the trial site from days – 2 to 2 and days 27 to 29. Meal tolerance tests (MTTs) were performed at day – 1, day 1 (first day of treatment), and day 28 (Fig. 1). Patients were assigned to test meals providing 1440, 1600, or 1840 kcal/day, based on standard weight, containing 50–60% carbohydrate, 15–21% protein, and 21–35% fat; details have been published [13]. On day 1 and day 28, study drug was administered 1 h before breakfast MTT (Fig. 1). At day – 1, day 1, and day 28, plasma glucose and serum insulin were measured at the times shown in Fig. 1, and blood ketone bodies and free fatty acids were measured before (after an overnight fast before breakfast MTT) and 1, 2, and 3 h after each MTT, and the next morning (after an overnight fast) (Fig. 1). Blood ketone bodies and free fatty acids were also measured at 08:00 h (after a 10-h overnight fast) on day 15. Insulin was measured at day – 1, day 1, and day 28 but not at 08:00 h on day 2 or 29, precluding an overnight fasting assessment on these days and a 24-h profile. Free fatty acids were measured using enzymatic determination.
Endpoints
Results for the primary endpoint (change from baseline in the area under the glucose concentration-time curve 3 h [AUC1–4h] after breakfast [for postprandial glucose] at day 28), additional measures of changes in postprandial glucose, changes in fasting free fatty acids and total ketone bodies at day 29, FPG, area under the curve (AUC) for postprandial insulin, and adverse events have been published [13]. In this paper, we present profiles of plasma glucose, serum insulin, free fatty acids, total ketone bodies, acetoacetic acid, and 3-hydroxybutyric acid over day – 1, day 1, and day 28 based on the sampling times shown in Fig. 1. We also present changes from baseline in fasting insulin at day 28; changes from baseline in time-corrected (normalized by observation time) AUC0–24h for postprandial free fatty acids, total ketone bodies, acetoacetic acid, and 3-hydroxybutyric acid on day 1 and day 28; changes from baseline in free fatty acids, total ketone bodies, acetoacetic acid, and 3-hydroxybutyric acid at 08:00 h (fasting) on day 2, day 15, and day 29; and changes from baseline in the 3-hydroxybutyric acid/acetoacetic acid ratio at 08:00 h (fasting) on day 2 and day 29. We used day 1 as the baseline for analyses of fasting values at day 2 and day 29 (except for the association analyses described below), and day – 1 as the baseline for analyses of fasting values at day 28, because of similarities in study procedures between these days. We used day 1 as the baseline for analyses of fasting values at day 15, and day – 1 as the baseline for analyses of AUC. We evaluated the association between changes in fasting total ketone bodies and changes in FPG, log-transformed fasting serum insulin, and fasting free fatty acids based on changes from 08:00 h on day – 1 to 08:00 h on day 2 (excluding insulin) and on day 28.
Changes from baseline in FPG on day 29 and in AUC1–4h for postprandial glucose, AUC1–4h for postprandial insulin, AUC0–24h for postprandial free fatty acids, and total ketone bodies on day 28 were also analyzed in subgroups by baseline age (< 65 and ≥ 65 years), BMI (< 25 and ≥ 25 kg/m2), time since diagnosis of T2DM (≤ 5, > 5 to 10, and > 10 years), HbA1c (< 8.0% and ≥ 8.0%), and AUC1–4h for postprandial insulin (below and above the median).
Statistical Analysis
Profiles of glucose, insulin, free fatty acids, and ketones over day – 1, day 1, and day 28 are based on descriptive statistics (i.e., visual inspection of diurnal profiles of each variable). Changes from baseline in fasting measurements and in AUC0–24h for free fatty acids, ketone bodies, and 3-hydroxybutyric acid/acetoacetic acid ratio were analyzed as safety variables using an analysis of covariance (ANCOVA) with baseline HbA1c and the baseline value of the endpoint in question as linear covariates and baseline eGFR (according to the Japanese estimation equation [14]) and treatment as fixed effects. Analyses of fasting measurements except 3-hydroxybutyric acid/acetoacetic acid ratio were pre-specified and analyses of time-corrected AUC0–24h for postprandial measurements were post hoc. Fasting insulin was analyzed post hoc as an efficacy variable using a model that included additional factors for baseline AUC1–4h for postprandial glucose and number of previous glucose-lowering medications. Missing data were not imputed. Subgroup analyses included a subgroup factor and a treatment-by-subgroup factor interaction as additional effects (post hoc). All endpoints were assessed in the treated set (patients treated with at least one dose of study drug) for individual empagliflozin dose groups vs. placebo, except for subgroup analyses that were performed for empagliflozin pooled vs. placebo. Slopes of the changes in fasting total ketone bodies were compared with changes in FPG, fasting serum insulin, or free fatty acids using linear regression, and treatment differences at day 2 and day 28 were analyzed post hoc using an ANCOVA with the change in the endpoint of interest (FPG, fasting serum insulin, or free fatty acids) and the change in the endpoint of interest by treatment interaction as linear covariates and treatment as a fixed effect (simple slope analysis). The slopes from the simple slope analysis were plotted in scatter plots. These slopes were also compared using a post hoc ANCOVA with changes in FPG, fasting serum insulin, and free fatty acids at day 28 and changes in each endpoint by treatment interactions as linear covariates and treatment as a fixed effect (multiple-factor slope analysis). The multiple-factor slope analysis was not plotted. The simple (one model for each endpoint of interest) and multiple-factor (one model including all endpoints of interest simultaneously) analyses tested slopes for each treatment, differences in slopes between treatments, and the interaction effect across all groups. A similar simple slope analysis including scatter plots was also made comparing changes in postprandial (AUC) with fasting levels for each of the endpoints of plasma glucose, insulin, free fatty acids, and ketones. Subsequently, as supportive information, we made a descriptive graphical assessment of the slopes using visual inspection of the scatter plots as the guidance for any differences for each slope compared to null or between slopes.
Plasma glucose, insulin, and free fatty acid values are presented as mg/dL, µIU/mL, and mg/dL, respectively. The following conversion factors can be used to convert plasma glucose to mmol/L, insulin to pmol/L, and free fatty acid to mmol/L: 0.0555, 6.945, and 0.0355, respectively.
Results
Patients
Of 78 patients screened, 60 were randomized and treated. Baseline characteristics are shown in Table S1 in the electronic supplementary material. One patient in the placebo group prematurely discontinued study medication.
Plasma Glucose and Serum Insulin
We previously reported analyses showing statistical significant reductions in fasting and most postprandial (including individual time points and AUC) measures of plasma glucose (P < 0.05), as well as in most postprandial (AUC) measures of serum insulin (P < 0.02) with both empagliflozin doses versus placebo [13]. Plasma glucose profiles over day – 1, day 1, and day 28, including fasting measurements at 0 h and 24 h, are presented in Fig. 2a. From visual inspection of the diurnal glucose profiles, plasma glucose was reduced with empagliflozin 10 mg and 25 mg vs. placebo on day 1 and day 28 (Fig. 2a). Plasma glucose peaked approximately 1.5 h after breakfast and dinner in every group (Fig. 2a). From visual inspection of scatter plots, changes in FPG generally correlated with changes in AUC1–4h for postprandial glucose. Likewise, the slopes were statistically significant (P < 0.009 for all), but there was no significant interaction across all groups (P = 0.252, Fig. S1a in the electronic supplementary material) or in pairwise comparisons between groups (data not shown), supporting a similar correlation between fasting and postprandial glucose levels for all treatment groups.
Serum insulin profiles over day – 1, day 1, and day 28, including the fasting measurement at 0 h, are presented in Fig. 2b. From visual inspection of the diurnal insulin profiles, serum insulin was reduced with empagliflozin 10 mg and 25 mg vs. placebo on day 1 and day 28 (Fig. 2b). Serum insulin peaked approximately 2 h after breakfast and dinner in every group (insulin was not measured after the lunch MTT; Fig. 2b). Fasting serum insulin was significantly reduced with both empagliflozin doses vs. placebo on day 28 (P < 0.05, Table S2 in the electronic supplementary material). From visual inspection of scatter plots, changes in fasting insulin generally correlated with changes in AUC1–4h for postprandial insulin, although this relationship appeared less pronounced for empagliflozin 25 mg than for placebo or empagliflozin 10 mg. The slopes were statistically significant only for empagliflozin 25 mg (P = 0.029), but there was no significant interaction across all groups (P = 0.459, Fig. S1b in the electronic supplementary material) or in pairwise comparisons between groups (data not shown), supporting a similar correlation between fasting and postprandial insulin levels for all treatment groups.
Free Fatty Acids
Fasting free fatty acids significantly increased with empagliflozin 10 mg and 25 mg vs. placebo at day 2 (P < 0.001, Fig. 3a) and significantly increased with empagliflozin 25 mg vs. placebo at day 29 (MTTs were given on day 1 and day 28) (P < 0.001, Fig. 3a). Increases with empagliflozin vs. placebo at day 2 did not appear to be dose dependent (as evaluated descriptively). Increases in fasting free fatty acids with empagliflozin vs. placebo did not reach significance at day 15 (P > 0.05; MTTs were not given on day 14) (Fig. S2a in the electronic supplementary material).
a Fasting free fatty acids (at 08:00 h) on day 2 and day 29 based on ANCOVA, b free fatty acids over time elapsed from 08:00 h on day – 1, day 1, and day 28 based on descriptive statistics, and c time-corrected AUC0–24h for free fatty acids at day 1 and day 28 based on ANCOVA. Data are from the treated set. ANCOVA analysis of covariance, AUC0–24h area under the curve from time 0 to 24 h, CI confidence interval, SD standard deviation, SE standard error
Free fatty acids over day – 1, day 1, and day 28 are presented in Fig. 3b. From visual inspection of the diurnal free fatty acids profiles, free fatty acids dose-dependently increased with empagliflozin 10 mg and 25 mg vs. placebo on day 1 and day 28. There was a peak in free fatty acids in all groups just before each meal; the highest peak was before breakfast (Fig. 3b). From visual inspection of scatter plots, changes in fasting free fatty acids were generally correlated with changes in AUC1–4h for postprandial free fatty acids. The slopes were statistically significant for empagliflozin 10 mg and 25 mg (P = 0.046 and P = 0.004, respectively), but there was no significant interaction across all groups (P = 0.565, Fig. S1c in the electronic supplementary material) or in pairwise comparisons between groups (data not shown), supporting a similar correlation between fasting and postprandial free fatty acid levels for all treatment groups. Compared with placebo, AUC0–24h for postprandial free fatty acids dose-dependently increased from baseline with empagliflozin 10 mg and 25 mg at day 1 and day 28 (P < 0.001; dose-dependency was evaluated descriptively, Fig. 3c).
Ketone Bodies
There were significant, dose-dependent increases in fasting levels of total ketone bodies, acetoacetic acid, and 3-hydroxybutyric acid with empagliflozin 10 mg and 25 mg vs. placebo at day 2 and day 29 (P < 0.03; dose-dependency was evaluated descriptively, Fig. 4a; Figs. S3a and S4a in the electronic supplementary material). On day 15, fasting levels increased with empagliflozin 25 mg vs. placebo (P < 0.001); increases with empagliflozin 10 mg did not reach statistical significance vs. placebo (P > 0.05, Fig. S2b–d in the electronic supplementary material).
a Fasting total ketone bodies (at 08:00 h) on day 2 and day 29 based on ANCOVA, b total ketone bodies over time elapsed from 08:00 h on day – 1, day 1, and day 28 based on descriptive statistics, and c time-corrected AUC0–24h for postprandial total ketone bodies at day 1 and day 28 based on ANCOVA. Data are from the treated set. ANCOVA analysis of covariance, AUC0–24h area under the curve from time 0 to 24 h, CI confidence interval, SD standard deviation, SE standard error
Total ketone bodies over day – 1, day 1, and day 28 are presented in Fig. 4b. From visual inspection of the diurnal ketone profiles, compared with placebo, total ketone bodies dose-dependently increased with empagliflozin 10 mg and 25 mg on day 1 and on day 28. There was a peak in total ketone bodies with empagliflozin 10 mg and 25 mg just before, and with a decline after, each meal on day 1 and day 28; the highest peak was before breakfast (Fig. 4b). Pre-meal peaks were smaller in the placebo group than in the empagliflozin groups (Fig. 4b). From visual inspection of scatter plots, changes in fasting ketone bodies were generally correlated with changes in AUC1–4h for postprandial ketone bodies. The slopes were statistically significant only for empagliflozin 10 mg and empagliflozin 25 mg (P < 0.001), but there was no significant interaction across all groups (P = 0.161, Fig. S1d in the electronic supplementary material) or in pairwise comparisons between groups (data not shown), supporting a similar correlation between fasting and postprandial ketone levels for all treatment groups. Compared with placebo, AUC0–24h for postprandial total ketone bodies dose-dependently increased with empagliflozin 10 mg and 25 mg at day 1 and day 28 (P < 0.02; dose-dependency was evaluated descriptively, Fig. 4c). The highest level of total ketone bodies observed in an individual patient was 1449 μmol/L, observed at 11:00 h on day 28 in a patient receiving empagliflozin 25 mg. The highest levels in the placebo and empagliflozin 10 mg groups were 389 and 890 μmol/L, respectively, both observed at 08:00 h on day 2.
Patterns in the daily profiles and AUC0–24h for postprandial acetoacetic acid and 3-hydroxybutyric acid were similar to those of total ketone bodies on day – 1, day 1, and day 28 (Figs. S3b, c and S4b, c in the electronic supplementary material). The 3-hydroxybutyric acid/acetoacetic acid ratio was increased with empagliflozin 10 mg and 25 mg vs. placebo on day 2 and day 29 (P < 0.02, Fig. S5 in the electronic supplementary material).
Changes in fasting ketone bodies compared with changes in FPG, fasting serum insulin, and fasting free fatty acids are shown in Fig. 5 and S6 in the electronic supplementary material. Visual inspection of the scatter plots suggests that increases in fasting ketone bodies with empagliflozin may be associated with reductions in FPG and fasting serum insulin and increases in fasting free fatty acids at day 2 and day 28 (although the opposite trend was observed for fasting free fatty acids with empagliflozin 10 mg at day 2). Slopes for the change in fasting ketone bodies vs. changes in other fasting variables at day 2 and day 28 reached statistical significance for empagliflozin 25 mg (P < 0.003 for all, Fig. 5 and S6), except for the change vs. fasting free fatty acids at day 2 (P = 0.062, Fig. S6 in the electronic supplementary material). The slopes for placebo and empagliflozin 10 mg did not reach statistical significance (P > 0.05 for all, Fig. 5 and S6 in the electronic supplementary material). In the simple slope analysis, a significant interaction was observed across all groups between slopes of changes in fasting ketone bodies and changes in fasting serum insulin at day 28 (P = 0.008, Fig. 5b) and the slopes of change were significantly different between empagliflozin 25 mg and placebo (P = 0.002) and between empagliflozin 25 mg and empagliflozin 10 mg (P = 0.040). A significant interaction was observed across all groups between slopes of changes in fasting ketone bodies and changes in FPG at day 2 (P = 0.004, Fig. S6 in the electronic supplementary material) but not at day 28 (P = 0.109, Fig. 5a); slopes of change were significantly different between empagliflozin 25 mg and placebo at day 2 (P = 0.002) and day 28 (P = 0.039), and between empagliflozin 25 mg and empagliflozin 10 mg at day 2 (P = 0.031). Other interactions, either across all groups or in pairwise comparisons between groups, did not reach statistical significance (P > 0.05), although a dose-dependent difference was suggested from visual inspection of the scatter plots. Results from multiple-factor slope analyses at day 28 were consistent with the simple slope analyses. In these analyses, P values for interaction across all groups were 0.017 for changes in fasting ketone bodies vs. changes in FPG, 0.003 for changes in fasting ketone bodies vs. changes in fasting free fatty acids, and 0.228 for changes in fasting ketone bodies vs. changes in fasting insulin. Pairwise tests of differences in slopes between groups also suggested nominal or significant interactions (data not shown). Thus, in both the simple and multiple-factor slope analysis, a significant interaction between treatment groups was observed with greater changes (steeper slopes) in ketone levels for the changes in FPG, insulin, or free fatty acids with empagliflozin than placebo, as also suggested from the visual inspection of the scatter plots.
Association between change in fasting ketone bodies from day – 1 to day 28 and a change in FPG from day – 1 to day 28 (P = 0.109 for change in FPG by treatment interaction across all groups), b change in log-transformed fasting serum insulin from day – 1 to day 28 (P = 0.008 for change in fasting insulin by treatment interaction across all groups), and c change in fasting free fatty acids from day – 1 to day 28 (P = 0.197 for change in fasting free fatty acids by treatment interaction across all groups) (c). Data are from the treated set. Figures include regression equations and P values for slopes. FPG fasting plasma glucose
In subgroup analyses, reductions in AUC1–4h for postprandial glucose with empagliflozin vs. placebo at day 28 were greater in patients with baseline HbA1c ≥ 8.0% than baseline HbA1c < 8.0% (P = 0.0037 for treatment by subgroup interaction); changes were consistent across the other subgroups assessed (age, BMI, time since diagnosis of T2DM, and AUC1–4h for postprandial insulin). Increases in AUC0–24h for postprandial free fatty acids with empagliflozin vs. placebo at day 28 were greater in patients with baseline AUC1–4h for postprandial insulin below or equal to the median than in those with AUC1–4h for postprandial insulin above the median (P = 0.0284 for treatment by subgroup interaction); changes were consistent across the other subgroups assessed. Changes in FPG, AUC1–4h for postprandial insulin, or total ketone bodies with empagliflozin vs. placebo were consistent across all subgroups assessed. Except for the P values for interaction included above, data from the subgroup analyses are not shown.
Discussion
The objective of these analyses was to investigate the effect of empagliflozin on free fatty acids and blood ketone bodies in Japanese patients with T2DM from a randomized, placebo-controlled, 4-week trial. Free fatty acids and total ketone bodies over 24 h increased with empagliflozin 10 mg and empagliflozin 25 mg vs. placebo at day 1 and day 28. Increases in ketones with empagliflozin vs. placebo peaked just before and declined after meals, with the highest peak before breakfast. Patterns in the daily profiles of acetoacetic acid and 3-hydroxybutyric acid matched those of total ketone bodies. Fasting ketones dose-dependently increased with empagliflozin 10 mg and 25 mg vs. placebo at day 2 and day 29. Changes in total ketone bodies appeared to be associated with changes in plasma glucose, insulin, and free fatty acids. In general, changes in fasting glucose, insulin, free fatty acids, and ketones appeared to correlate with changes in AUC for postprandial measurements.
Consistent with the mechanism of action of empagliflozin, which inhibits renal glucose reabsorption leading to increased urinary glucose excretion, reduced plasma glucose [2], and reduced plasma insulin [3], we observed reductions in plasma glucose and insulin profiles over 24 h with empagliflozin vs. placebo at day 1 and day 28. Peaks in glucose and insulin were observed approximately 1.5 h after each meal. The majority of the reduction in glucose and insulin was apparent at day 1. The findings from these analyses are in line with the significant reductions in AUC1–4h and AUC10–13h for postprandial glucose observed in previous analyses of data from this study, in which 80% to 90% of the reduction was achieved at day 1, accompanied by a reduction in AUC1–4h and AUC10–13h for postprandial insulin and a reduction in FPG [13]. In our subgroup analyses, the reduction in AUC1–4h for postprandial glucose with empagliflozin was greater in patients with higher baseline HbA1c, consistent with known effects of glucose-lowering agents [15].
Glucose-lowering agents with other mechanisms of action, such as metformin, sulfonylurea, and incretin-based therapies, have been reported to reduce free fatty acids as they increase insulin secretion or have no effect on insulin [16, 17]. In contrast, mild-to-moderate increases in free fatty acids and ketone bodies have been reported with the use of SGLT2 inhibitors in patients with type 1 diabetes and T2DM [3, 18,19,20,21,22]; however, the underlying mechanism for these increases has not been fully established. Ketogenesis is a physiologic adaptation to glucose depletion and/or fasting, and is mainly controlled by insulin (which is decreased during fasting and inhibits ketogenesis) and glucagon (which is increased during fasting and promotes ketogenesis). During fasting, reduced nutrient intake and decrease in insulin-to-glucagon ratio promote a shift from carbohydrate to lipid utilization and, in turn, toward ketogenesis. Therefore, the mode of action of SGLT2 inhibitors may mimic starvation by promoting urinary glucose excretion, which lowers plasma glucose and, consequently, insulin.
Our simple slope analyses indicated that increases in fasting ketone bodies with empagliflozin may be associated with reductions in FPG and fasting serum insulin, and with increases in free fatty acids. These associations would be expected after a shift toward lipid utilization and ketogenesis. Changes in these metabolic determinants of ketone production were associated with greater changes (steeper slopes) in ketone levels with empagliflozin than placebo, and in a dose-dependent manner. The multiple-factor slope analysis corroborated the results of the simple analysis. The attenuation of the P value for interaction across all groups for fasting insulin in the multiple-factor analyses compared with the simple analyses, with significant interactions for FPG and fasting free fatty acids in the multiple-factor analyses but not the simple analyses, suggests that FPG and fasting free fatty acids may provide additional information to fasting insulin for the change in fasting ketones. The reason not all associations reached significance may be the small sample size and/or the wide variability in the endpoints measured. The mechanisms underlying these results, and the potential clinical impact, remain to be established. In subgroup analyses, the effect of empagliflozin vs. placebo on AUC0–24h for ketone bodies showed no interactions across the subgroups assessed, whereas greater increases in free fatty acids with empagliflozin vs. placebo were observed in patients with lower baseline insulin levels, consistent with the anti-lipolytic effect of insulin [23]. Correlations between changes in ketones and changes in plasma glucose, insulin, and free fatty acids after 2 weeks’ treatment with empagliflozin have been reported in a recent single-arm study [24].
The diurnal patterns of glucose, insulin, free fatty acids, and ketone bodies appeared to be preserved during empagliflozin treatment. There were peaks in ketone levels just before each meal, with the highest peaks observed before breakfast. Despite the overall increase in ketone levels, postprandial decreases in ketones were not blunted with empagliflozin; rather, the differences vs. placebo appeared to be increased. The data are consistent with a study in non-Japanese patients treated with empagliflozin, although a placebo group was not included in that study [25]. The diurnal pattern of serum ketone levels was also maintained with the SGLT2 inhibitor luseogliflozin in Japanese patients with T2DM (N = 18) on normal or low-carbohydrate diets, although serum ketone levels were higher in patients on a low-carbohydrate diet [26]. These data suggest the effect of SGLT2 inhibitors on ketones may vary by time of day, the degree of fasting, and/or the carbohydrate content of a patient’s diet.
In our study, the highest level of ketone bodies observed in an individual patient was 1449 μmol/L in a patient receiving empagliflozin. This is comparable with levels of up to approximately 1300 μmol/L reported for subjects without T2DM after an overnight fast [27]. An increase in the 3-hydroxybutyric acid/acetoacetic acid ratio is observed when ketones are increased, such as under fasting conditions [28]. At day 29 in our analyses, this ratio was increased by 0.5 with empagliflozin 10 mg (from a baseline of 1.6) and by 0.8 with empagliflozin 25 mg (from a baseline of 1.9). These increases were considerably smaller than those observed in patients with acute diabetic ketoacidosis, in whom the ratio increased from 1:1 (normal) to as high as 10:1 [28].
No adverse events of diabetic ketoacidosis or those related to abnormal ketone body levels were reported during this study [13]. The incidence of ketoacidosis was low and comparable between empagliflozin and placebo in the EMPA-REG OUTCOME trial in 7020 patients with T2DM and established cardiovascular disease over a median observation time of 3.1 years [29] and in analysis of pooled safety data from 15 placebo-controlled trials of empagliflozin based on more than 15,000 patient-years’ exposure [30]. In a post-marketing surveillance study of 8505 elderly Japanese patients treated with the SGLT2 inhibitor ipragliflozin for up to 1 year (mean treatment duration 9 months), adverse drug reactions related to ketone bodies were reported in two patients (0.02%) [31]. The mechanism of action of empagliflozin can lead to an increase in ketone body levels and, on the basis of global post-marketing data, ketoacidosis is listed as a possible side effect in the prescribing information [32]. At-risk patients, such as those with infections, trauma patients, or post-surgery, may need to be monitored closely for signs and symptoms of ketoacidosis [32,33,34]. Of note, patients taking SGLT2 inhibitors may experience ketoacidosis in the absence of significant hyperglycemia [33]. The relationship between the metabolic variables assessed in this study and other clinical outcomes, such as cardiovascular or renal events, requires further investigation.
The strengths of this study include that it was a randomized study, that patients were to follow a controlled diet, and that plasma glucose, blood ketone bodies, and free fatty acids were measured throughout each day patients were at the trial site. Limitations include the relatively small sample size and short duration, and the facts that no samples were taken at night, that plasma insulin was only measured up to 13 h after drug administration, and that glucagon was not measured. It should also be noted that MTTs were not performed on day 14, which is a possible reason why not all differences with empagliflozin vs. placebo in changes in fasting ketones and free fatty acids reached significance at day 15, in contrast to other study days.
Conclusions
Compared with placebo, Japanese patients with T2DM treated with empagliflozin showed improvements in glycemia, insulinemia, and body weight, with increases in free fatty acids and blood ketone bodies, after a single dose and after 28 days’ treatment. Levels of total ketones were comparable with those observed during fasting conditions in individuals without diabetes as reported by others. The diurnal pattern of changes in ketones appeared to be preserved during empagliflozin treatment. Increases in ketones appeared to be related to the duration of fasting, were most pronounced before and declined after meals, and with the highest peak before breakfast. The changes in ketones with empagliflozin appeared to be associated with changes in well-known metabolic determinants of ketone production.
References
Grempler R, Thomas L, Eckhardt M, et al. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab. 2012;14:83–90.
Heise T, Seewaldt-Becker E, Macha S, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15:613–21.
Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124:499–508.
Carlson MG, Snead WL, Campbell PJ. Fuel and energy metabolism in fasting humans. Am J Clin Nutr. 1994;60:29–36.
Ferrannini E, Baldi S, Frascerra S, et al. Renal handling of ketones in response to sodium-glucose co-transporter-2 inhibition in patients with type 2 diabetes. Diabetes Care. 2017;40:771–6.
Häring H-U, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36:3396–404.
Häring H-U, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37:1650–9.
Kovacs CS, Seshiah V, Swallow R, et al. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2014;16:147–58.
Roden M, Weng J, Eilbracht J, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1:208–19.
Rosenstock J, Jelaska A, Frappin G, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37:1815–23.
Rosenstock J, Jelaska A, Zeller C, et al. Impact of empagliflozin added-on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: a 78-week randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2015;17:936–48.
Araki E, Tanizawa Y, Tanaka Y, et al. Long-term treatment with empagliflozin as add-on to oral antidiabetes therapy in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2015;17:665–74.
Nishimura R, Tanaka Y, Koiwai K, et al. Effect of empagliflozin monotherapy on post-prandial glucose and 24-h glucose variability in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, 4-week study. Cardiovasc Diabetol. 2015;14:11.
Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Sherifali D, Nerenberg K, Pullenayegum E, Cheng JE, Gerstein HC. The effect of oral antidiabetic agents on A1C levels: a systematic review and meta-analysis. Diabetes Care. 2010;33:1859–64.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–9.
Perriello G, Misericordia P, Volpi E, et al. Acute antihyperglycemic mechanisms of metformin in NIDDM. Evidence for suppression of lipid oxidation and hepatic glucose production. Diabetes. 1994;43:920–8.
Inagaki N, Kondo K, Yoshinari T, Maruyama N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, 12-week study. Diabetes Obes Metab. 2013;15:1136–45.
Kadokura T, Akiyama N, Kashiwagi A, et al. Pharmacokinetic and pharmacodynamic study of ipragliflozin in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study. Diabetes Res Clin Pract. 2014;106:50–6.
Kaku K, Watada H, Iwamoto Y, et al. Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter-2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined phase 2 and 3 randomized, placebo-controlled, double-blind, parallel-group comparative study. Cardiovasc Diabetol. 2014;13:65.
Nishimura R, Osonoi T, Kanada S, et al. Effects of luseogliflozin, a sodium-glucose co-transporter 2 inhibitor, on 24-h glucose variability assessed by continuous glucose monitoring in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, crossover study. Diabetes Obes Metab. 2015;17:800–4.
Pieber TR, Famulla S, Eilbracht J, et al. Empagliflozin as adjunct to insulin in patients with type 1 diabetes: a 4-week, randomized, placebo-controlled trial (EASE-1). Diabetes Obes Metab. 2015;17:928–35.
Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis—a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res. 2011;50:14–27.
Al JH, Daniele G, Adams J, et al. Determinants of the increase in ketone concentration during SGLT2 inhibition in NGT, IFG and T2DM patients. Diabetes Obes Metab. 2017;19:809–13.
Ferrannini E, Baldi S, Frascerra S, et al. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65:1190–5.
Nishimura R, Omiya H, Sugio K, Ubukata M, Sakai S, Samukawa Y. Sodium-glucose cotransporter 2 inhibitor luseogliflozin improves glycaemic control, assessed by continuous glucose monitoring, even on a low-carbohydrate diet. Diabetes Obes Metab. 2016;18:702–6.
Foster KJ, Alberti KG, Hinks L, et al. Blood intermediary metabolite and insulin concentrations after an overnight fast: reference ranges for adults, and interrelations. Clin Chem. 1978;24:1568–72.
Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev. 1999;15:412–26.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Kohler S, Zeller C, Iliev H, Kaspers S. Safety and tolerability of empagliflozin in patients with type 2 diabetes: pooled analysis of phase I-III clinical trials. Adv Ther. 2017;34:1707–26.
Yokote K, Terauchi Y, Nakamura I, Sugamori H. Real-world evidence for the safety of ipragliflozin in elderly Japanese patients with type 2 diabetes mellitus (STELLA-ELDER): final results of a post-marketing surveillance study. Expert Opin Pharmacother. 2016;17:1995–2003.
Boehringer Ingelheim. Prescribing information: JARDIANCE® (empagliflozin) tablets, for oral use. 2018. https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Jardiance/jardiance.pdf. Accessed 12 Feb 2019.
Umpierrez GE, Kitabchi AE. Diabetic ketoacidosis: risk factors and management strategies. Treat Endocrinol. 2003;2:95–108.
Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38:1687–93.
Acknowledgements
The authors would like to thank the patients who participated in the study. The authors thank Rabia Ahmad of inVentiv Health, New Jersey, USA; Kohei Inoue; and Lee Ganghyuck of Nippon Boehringer Ingelheim Co. Ltd for providing support with the statistical analyses. The authors thank Atsutaka Yasui of Nippon Boehringer Ingelheim Co. Ltd for support with this manuscript.
Funding
This study was funded by Boehringer Ingelheim & Eli Lilly and Company, who also provided financial support for the article processing charges. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and Editorial Assistance
Medical writing and editing assistance, supported financially by Boehringer Ingelheim, was provided by Elizabeth Ng of FleishmanHillard Fishburn, London, UK, and Radhika Bhatia, PhD, CMPP, of Elevate Scientific Solutions, Horsham, UK, during the preparation of this manuscript. The authors sent the manuscript for accuracy review to the sponsor (Boehringer Ingelheim) on 19 February 2018. The sponsor reviewed and eventually released the manuscript on 24 January 2019, without changes. The authors were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development, and have approved the final version.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Rimei Nishimura has received research support from Japan Diabetes Foundation, Boehringer Ingelheim, Daiichi-Sankyo, and Astellas; has participated in speaker’s bureau/advisory panels for Novo Nordisk, Eli Lilly, Sanofi, Kissei, Astellas, Boehringer Ingelheim, Daiichi-Sankyo, Tanabe-Mitsubishi, Astra Zeneca, Kowa, Ono, Johnson & Johnson, Medtronic, Takeda, and Astellas; and served as a consultant for Boehringer Ingelheim and Eli Lilly. Yuko Tanaka is an employee of Boehringer Ingelheim. Afshin Salsali is an employee of Boehringer Ingelheim. Stefan Kaspers is an employee of Boehringer Ingelheim. Sven Kohler is an employee of Boehringer Ingelheim. Søren S. Lund is an employee of Boehringer Ingelheim and owns shares in Novo Nordisk A/S and shares in dynamically traded investment funds, which may own stocks from pharmaceutical companies. Kosuke Ishida is an employee of Rakuten Medical Japan, K.K. and was an employee of Boehringer Ingelheim at the time the analyses were conducted. Kazuki Koiwai is an employee of Amgen Astellas BioPharma K.K. and was an employee of Boehringer Ingelheim at the time the analyses were conducted.
Compliance with Ethics Guidelines
The clinical trial protocol was approved by the Institutional Review Board of Heishinkai Medical Group Incorporated OPHAC Hospital (Osaka, Japan), and complied with the Declaration of Helsinki in accordance with the International Conference on Harmonisation Harmonised Tripartite Guideline for Good Clinical Practice. All patients provided written informed consent.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.9072128.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nishimura, R., Tanaka, Y., Koiwai, K. et al. Effect of Empagliflozin on Free Fatty Acids and Ketone Bodies in Japanese Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Adv Ther 36, 2769–2782 (2019). https://doi.org/10.1007/s12325-019-01045-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-01045-x