Abstract
Introduction
Long-standing acanthamoeba keratitis (AK) may result in corneal neovascularization, extension of the infiltrate to the limbus or sclera, broad peripheral synechiae, mature cataract or ischemic posterior segment inflammation. We investigated the impact of early emergency penetrating keratoplasty (PKP) in therapy-resistant cases among the patients of a highly specialized tertiary care center.
Methods
In this retrospective, observational cohort within a single institution, we collected data on best-corrected visual acuity (BCVA), epithelial wound healing, graft survival and secondary complications of AK patients who underwent PKP. A total of 23 eyes of 23 patients diagnosed with acute, therapy-resistant AK between 2006 and 2015 were enrolled. Postoperative combined topical treatment was tapered for 6–9 months.
Results
Eyes were grouped based on preoperative disease duration as shorter (group 1) or longer (group 2) than the median. The median was 5.3 (0.66–36) months. The BCVA in group 1 (20/44 ± 20/18; 0.32 ± 0.18 logMAR) was significantly better than in group 2 (20/1200 ± 20/1133; 1.28 ± 0.89; logMAR); p = 0.015. Persisting epithelial defects occurred in 5 patients (50%) of group 1 and in 10 patients (77%) of group 2. In 5 eyes (group 2), no epithelial healing could be achieved. After 36 months, graft survival (Kaplan–Meier) was 78% (18 grafts) for all patients (90% in group 1 and 44% in group 2).
Conclusion
PKP à chaud within 5.3 months after first symptoms of therapy-resistant AK seems to result in better final BCVA than delayed graft surgery if the disease is resistant to a classical topical triple therapy. In addition, early PKP may have a favorable impact on epithelial healing and graft survival.
Funding
We thank the Alexander von Humboldt Foundation for supporting the work of Prof. N. Szentmáry at the Department of Ophthalmology of Saarland University Medical Center in Homburg/Saar, Germany. We thank the University of Saarland for funding the medical writing assistance and the Rapid Service Fees. The funding organisation had no role in the design or conduct of this research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first cases of acanthamoeba keratitis (AK) were reported in the 1970s [1]. Since then, the occurrence and prevalence of AK has dramatically increased all over the world. Approximately 5% of contact lens related keratitis is caused by acanthamoeba [2].
In the early stages, AK usually manifests with severe corneal pain, photophobia, epithelial and stromal alterations. During the first 2–4 weeks, about 50% of the patients exhibit the characteristic “pseudodendritiform epitheliopathy”, 20% exhibit a ring infiltrate and 2.5–63% develop perineuritis followed by decreased corneal sensitivity [3]. Besides often uncharacteristic symptoms in the early stages, the wide spectrum of possible clinical manifestations impedes differentiation between bacterial, mycotic or herpetic keratitis. The lack of characteristic symptoms and the common mixed bacterial or mycotic infections lead to initial misdiagnoses in up to 90% of the cases [4]. According to the German Acanthamoeba keratitis registry, more than two-thirds of the cases are primarily misdiagnosed as herpetic (47.6%), bacterial (25.2%) or mycotic (3.9%) keratitis with an average diagnostic delay of more than 3 months (3.1 ± 5.2 months) from the onset of the symptoms [5].
Due to the various clinical manifestations and the extremely resistant cystic form of acanthamoebae, several different chemotherapeutic agents are applied in individually modified combinations depending on the manifestation and length of the disease, as well as the empirical experience of the clinicians. Experts worldwide have reached a consensus concerning the immediate application of the following topical antimicrobial agents in high concentration but in various combinations: biguanides, aromatic diamidines and neomycin [6].
The diagnostic delay and the partial responsiveness to the topical combination therapy expedite the development of sight-threatening complications, such as persisting epithelial defects, severe corneal neovascularization, limbal stem cell deficiency, expansion of the infiltrate to the limbus or sclera, broad peripheral synechiae, iris atrophy, mature cataract and reactive posterior segment inflammation [3, 7].
Prior to the aforementioned topical antimicrobial options, penetrating keratoplasty (PKP) was the only reasonable causative treatment for AK [8]. Currently, a vast majority of corneal experts advise against performing keratoplasty in an inflamed eye [9,10,11]. Some suggest that the surgical treatment should be postponed to approximately 3 months after the active interval of the disease [12]. In our opinion, penetrating keratoplasty is a better option to treat severe AK before severe neovascularization and anterior chamber or peripheral corneal involvement could threaten the sclera, when conservative treatment failed to control the infection. We would also like to explicitly point out the impact and importance of the length of the treatment period before penetrating keratoplasty, performed in our highly specialized corneal center.
We hypothesized that early emergency PKP in therapy-resistant cases performed on the patient population of a tertiary care corneal subspecialty center might have a favorable impact on best-corrected visual acuity (BCVA), epithelial wound healing and graft survival in therapy-resistant, acute AK.
Methods
A total of 29 eyes of 27 patients (bilateral infection in 2 patients) were diagnosed with AK between January 2006 and November 2015 at the Department of Ophthalmology, Saarland University Medical Center. Of the 29 eyes, 6 in 5 patients (one patient with bilateral involvement) showed favorable therapeutic response and were successfully treated with a combination of topical antimicrobial agents within 3 weeks from symptom onset. In our retrospective review of a defined cohort, we enrolled and further investigated the remaining 23 eyes of our 23 patients, which were “therapy-resistant” cases that underwent PKP. We defined “therapy-resistant” AK as worsening of the clinical symptoms (decrease in BCVA, persistent corneal ulcer, expansion of stromal infiltrates toward the limbus and increased anterior chamber inflammation) over at least 1 week despite the aforementioned intensive topical triple-antimicrobial therapy (and cross-linking) at our Department. However, most patients have already been treated for several weeks or months with different antimicrobial agents by other ophthalmologists.
The study was performed in adherence to the guidelines of the Declaration of Helsinki and the Ethical Committee of the Medical Association of Saarland. Informed consents for participation and publication of patient data were collected from all participants.
For diagnostic purposes, all patients underwent corneal scraping or diagnostic keratectomy for PCR and microbiological culture analysis. In case a keratectomy or a keratoplasty was performed postoperative histological evaluation was also carried out. Confocal microscopy was carried out at the Department of Ophthalmology, Saarland University Medical Center, by one experienced examiner (Loay Daas). AK was diagnosed by PCR in 17 (59%), by microbiological culture analysis in 9 (31%), by histological analysis of the corneal tissue in 14 (48%) and by in vivo confocal microscopy in 4 eyes (14%). In some cases, more than one method confirmed the diagnosis. No further evaluation was carried out to distinguish between different acanthamoeba subspecies.
Following the first examination at our Department and a clinical diagnosis of AK, topical combination therapy of polyhexamethylene biguanide (Lavasept®; B. Braun, Melsungen, Germany), propamidine isethionate (Brolene®; Sanofi, Guildford, Surrey, UK) and neomycin sulfate/gramicidin/polymixin B sulfate (Polyspectran®; Alcon Pharma, Freiburg, Germany) was initiated immediately for each patient every half-hour for 48 h before having the results of the above diagnostics, then five–eight times a day (Table 1). Our clinical diagnosis was justified later by at least one of the aforementioned diagnostic methods in 100% of the patients.
We performed a riboflavin–UVA cross-linking in 13 eyes (6 eyes of group 1, 7 eyes of group 2) using the CCL 365 System (Peschke, Waldshut-Tiengen, Germany) 11.5 ± 13.6 days prior to PKP. In 14 therapy-resistant cases (60.9%), the emergency PKP was performed within 2 weeks after the first examination at our Department. In all but one of the patients, we applied the 193-nm excimer laser for trephination of the donor and host (MEL 80; Carl Zeiss, Jena, Germany, or SCHWIND Eye-Tech-Solutions, Kleinostheim, Germany). In 1 eye, Hessburg-Barrone vacuum trephine (Jedmed Instrument, St. Louis, MO, USA) was used. We performed simultaneous cryotherapy before trephination [13] in all eyes and simultaneous cataract surgery (triple procedure) in 6 eyes. The keratoplasties were performed using round (21 eyes, excimer laser or vacuum trephination) or elliptical (2 eyes, excimer laser trephination) trephination with a double running cross-stitch suture according to Hoffmann in 19 eyes and with 24 single interrupted sutures in 4 eyes. The trephination edge was marked with the respective metal mask, the size and shape of which was chosen according to the size and shape of the AK infiltrate. Then, cryotherapy (“freezing–thawing–freezing”) was applied at the graft–host junction, while the eye was still closed. A donor diameter with 0.1 mm oversize for excimer laser and 0.25 mm oversize for Hessburg-Barrone trephination was used [14]. The recipient diameters are displayed in Table 2. No intraoperative complications occurred.
Postoperatively, we applied the above-mentioned topical triple combination antimicrobial therapy (five times daily each) supplemented with sodium hyaluronate eye-drops (Hylogel; Ursapharm Arzneimittel, Saarbrücken, Germany) 5 times a day. Additionally, prednisolone acetate (Inflanefran forte; Allergan Pharm., Westport, Co. Mayo, Ireland) eye-drops were applied two times daily until epithelial closure, and thereafter five times daily for 6 weeks. From the day of the surgery, a 1 mg/kg systemic corticosteroid therapy was introduced and gradually reduced for 14 days. The triple antimicrobial combination therapy was gradually reduced over 6 months together with the corticosteroid eye-drops. Meanwhile, sodium hyaluronate eye-drops were applied continuously five times a day. The postoperative therapeutic regimen of our Department is displayed in Table 3.
Our primary main outcome measures were BCVA, persistent epithelial defects (PEDs) and graft survival time. Secondary main outcome measures were the subsequent operations and ocular diseases. BCVA was tested with spectacle glasses; hand motion or less was counted as 2.1 (log MAR). PED was defined as at least 4 weeks of incomplete epithelial closure. Graft failure was defined as primary or secondary endothelial decompensation or necrotic corneal graft melting. Graft survival was defined as elapsed time between first postoperative day and the day of diagnosed graft failure in patients’ data record.
Statistical evaluation was carried out for comparison of BCVA by Mann–Whitney U test. Graft survival was evaluated by Kaplan–Meier analysis.
Results
In our 23 patients (average age 39.6 ± 13.3 years), the preoperative duration of the disease ranged from 1 to 36 months. Our patients were categorized based on the time interval between the symptom onset and the first PKP. The threshold value was set to 5.3 months, which refers to the median preoperative disease duration. Patients were assigned to group 1 in cases of preoperative disease duration shorter than 5.3 months and to group 2 in cases of preoperative disease duration longer than or equal to 5.3 months (0.66–5.25, 5.33–36 months, respectively). The interval between symptom onset and application of the triple antimicrobial therapy ranged between 1 week and 1 year. As a mean value, we established 3.6 ± 3.4 months for all 23 patients: 3.3 ± 1.6 months in group 1 and 3.7 ± 4.2 months in group 2. Seven patients had preoperative cataract and 4 limbal insufficiency; all of them were patients of group 2. We performed triple procedure in 6 eyes (1 in group 1 and 5 in group 2) and sequential cataract surgery in 5 eyes (3 in group 1 and 2 in group 2) during the mean follow-up of 27 months in cases where a perceptible visual loss could be certified. Prior to PKP, the mean BCVA was finger counting (20/1100; 1.73 ± 0.48 logMAR). There was no significant difference between the groups in preoperative BCVA (20/1000 and 20/1250; 1.67 and 1.78 in group 1 and 2, respectively; p = 0.418). None of our 23 patients exhibited aphakia or luxation of the intraocular lens preoperatively or after surgeries conducted in our Department.
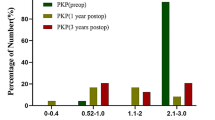
The postoperative BCVA varied from no light perception (20/2500; 2.1 logMAR) to 20/20 (0.0 logMAR) with a median of 20/50 (0.4 logMAR). The BCVA at the last follow-up ranged from 20/80 (0.6 logMAR) to 20/20 (0.0 logMAR) in group 1 (n = 10). Five patients had a BCVA of 20/80 (0.2 logMAR) or better and no patients had a BCVA of 20/200 (1.0 logMAR) or worse. In group 2 (n = 13), BCVA ranged from no light perception to 20/20; 2 patients had a BCVA of 20/80 or better, and 7 patients had a BCVA of 20/200 or worse (Fig. 1). The median BCVA was 20/36 (0.25 logMAR) in group 1 and hand motion (1.9 logMAR) in group 2 (p = 0.015) (Figs. 2, 3, 4, 5).
A 53-year-old female patient (patient no. 23) (preoperative disease duration 36.0 months) at first presentation (top) in our Department, 2 weeks after penetrating keratoplasty as triple-procedure (middle) and at last follow-up (bottom), 28 months after penetrating keratoplasty à chaud with graft failure and hypotony; BCVA: no light perception
For each eye, the postoperative time period during which ambulatory vision was continuously maintained was recorded, following a method described by Dohlman and Terada (Table 4).
PEDs following PKP had to be treated in 17 patients (74%): 5 patients (50%) in group 1 and 12 patients (92%) in group 2. Preoperative duration of the disease, duration of autologous serum (AS) therapy, the number of amniotic membrane transplantations (AMT) as patch and the number of repeat keratoplasties (repeat PKP) in our patients are summarized in Table 5.
In order to treat PEDs following PKP, 100% autologous serum (AS) eye drops were applied in 14 eyes (61%) (5 patients in group 1, 9 patients in group 2). A total of 40 AMTs as patch [15] were applied in 14 patients (61%) postoperatively to treat PEDs: 11 (28%) in group 1 (4 patients) and 29 (72%) in group 2 (10 patients).
In 18 patients (78%), we carried out a single keratoplasty (8 in group 1 and 10 in group 2), in 5 patients (22%) we carried out a second (2 in group 1 and 3 in group 2) and in 2 patients (9%) a third (1 in each group). Repeat keratoplasties were performed due to PEDs (2 patients in each group), endothelial graft decompensation (2 patients in group 2) and necrotic graft melting without any proven superinfection in an eye that suffered previous ocular trauma (1 patient in group 1).
Six patients (26%) reached complete epithelial healing without autologous serum application, AMT or repeat PKP (5 patients in group 1, 1 patient in group 2). During follow-up, epithelial healing was achieved in all patients of group 1, although 2 patients underwent multiple keratoplasties. In 5 cases of group 2 (22%), no permanent epithelial healing could be achieved.
The mean follow-up was 27 ± 19.4 months overall, 25 ± 16 months for group 1, 19 ± 22 months for group 2 and varied between 3 and 76 months. Seven patients developed immunological endothelial graft rejection (2 patients in group 1, 5 patients in group 2), which could be resolved in 3 cases by intensifying of the topical and systemic corticosteroid therapy (2 patients in group 1, and 1 patient in group 2) (Table 6). In cases of a graft failure or persisting epithelial defects, a microbiological evaluation, histopathology and confocal microscopy were repeated to rule out a possible relapse of AK.
At 36 months, the cumulative probability of graft survival for the first 23 transplants was 78% (18 grafts) for all patients, 90% in group 1 and 44% in group 2 (Fig. 6). There was no significant difference between the survival curves according to the log rank test (p = 0.167).
Secondary ocular diseases and their distribution in both groups are presented in Table 6. Two patients (one in each group) were treated for primary open angle glaucoma prior to AK. Both were treated 5–10 years with a combination of beta-blocker and acetazolamide. During the follow-up, 4 patients of group 1 and 8 patients of group 2 exhibited elevated eye pressure (> 20 mmHg), among them, 6 patients (1 in group 1 and 5 in group 2) underwent a single cyclophotocoagualtion and needed antiglaucomatic eye-drops for more than 4 weeks. Multiple keratoplasties were necessary in 3 patients with and 2 patients without glaucoma. All patients with ocular hypertension received round grafts with a diameter of 7.5–8.0 mm diameter.
One blind eye (group 2) showed signs of central retinal vein occlusion by a histologically diagnosed severe chorioretinitis and was enucleated 5 months after the second keratoplasty due to persistent epithelial defects and major psychological problems of the patient.
Discussion
Penetrating keratoplasty within 5.3 months after the first symptoms of therapy-resistant AK resulted in our patients having significantly better final BCVA than after delayed graft surgery. In addition, early keratoplasty had a favorable impact on epithelial healing and overall graft survival.
Some authors recommended that PKP in AK should only be performed in cases of frank or definitely pending corneal perforation, intumescent cataract, fulminant corneal abscess formation or in cases of the involvement of paralimbal corneal tissue despite intensive anti-amoebic therapy. PKP was claimed to have poor prognosis and should not be used for the treatment of acute AK [16, 17]. Most of the authors suggest that lamellar or penetrating keratoplasty is best performed after stabilizing the disease to treat corneal scars and irregular astigmatism, and should be postponed at least 1–3 months after the cessation of “active” inflammation [11, 12, 18].
In accordance with our own findings, other authors have reported favorable results for penetrating or lamellar keratoplasty in the acute stage of AK. Illingworth [19] published a series of 9 cases with a homogenously long graft survival of more than 15 months and a BCVA of 0.8–1.0 (0.1–0.0 logMAR) in each patient after performing PKP in the inflamed eye, as in our patients. In these patients, surgery took place on average 45 days after applying anti-amoebic therapy, and the postoperative treatment consisted of polyhexamethylene biguanide, propamidine isethionate and topical corticosteroids.
In another study [20], 15 patients underwent penetrating keratoplasty more than 2 months after the onset of AK symptoms, 64% of them reached a visual acuity of 0.3 (0.5 logMAR) or better, the graft survival was approximately 84% after 1 year, and penetrating keratoplasty had an 87% success rate in eradicating the infection in AK.
Sarnicola [21] reported on a case series of 11 patients who underwent early deep anterior lamellar keratoplasty within 30–60 days from symptom onset. The BCVA varied between 0.6 and 1.0 (0.2–0.0 logMAR) after an average follow-up of approximately 2 years. All grafts remained clear and no ocular surface defects were reported. The postoperative therapy consisted of a combination of antimicrobial agents with neomycin, propamidine isethionate and chlorhexidine supplemented with topical steroids, lubricant preparations and 500 mg/day systemic levofloxacin. No systemic steroids were applied. All patients exhibited a postoperative delay of epithelialization which could in each case be resolved after 15 days of eye patching.
Despite the much disputed role of keratoplasty in AK, all these studies suggest that the most important prognostic factors in AK are the severity of the disease at first manifestation and the interval from symptom onset to application of the antimicrobial treatment, as over time the trophozoites invade deeper layers of the cornea [9]. Patients, who are adequately treated within 3 weeks from symptom onset seem to have significantly better chances than the ones with delayed application of the triple anti-amoebic therapy [4]. Patients who have not obtained adequate antimicrobial therapy within this short period are quite likely to respond only partially or with significant and irreversible corneal damage. In these therapy-resistant cases with typically persistent (para)central corneal ulcus, our early surgical approach could limit the involvement of the peripheral cornea, major circular corneal neovascularization and the inflammation of the anterior chamber, thus minimizing the likelihood of severe, sight-threatening, potentially irreversible complications.
Since acanthamoeba cysts can be seen within 30–50 µm of Descemet’s membrane, we prefer penetrating keratoplasty over lamellar techniques in order to avoid residual cyst reproduction in the deep host cornea [13].
The reason for prolonged and more frequently occurring ocular surface defects and the often minor BCVA increase in our patients in group 2 could have been the insufficient eradication of the infection, as well as the epithelial toxicity of the antimicrobial and anti-inflammatory drugs over many months or even years, resulting in partial stem cell deficiency as some authors already described [22, 23]. The latter typically results in severe circular stromal vascularisation, which in turn is a very unfavorable predisposition for future optical corneal transplantation (Fig. 7).
The main limitation of this study is the nature of our sample, which makes it difficult to generalize results to the average patient with AK. There may be a considerable bias in the patient population referred to a single tertiary, highly specialized corneal center. Cases that heal well after correct treatment application within the first 3 weeks after symptom onset are rarely introduced to our Department. The results of the present study are also limited due to the fact that a complete control of confounding parameters was not reached. However, the main purpose of our analysis was not to control all the confounders and present the penetrating keratoplasty as the only key to success. Our purpose was to dispute and reemphasize the role of this surgical treatment option, mainly because we wanted to examine and explicitly point out the impact and importance of the shorter treatment period before penetrating keratoplasty, as a valid method for timely infection control.
Future longitudinal studies could be of interest to investigate whether, after an early PKP à chaud with excision of the entire pathological tissue, the postoperative antimicrobial therapy could be shortened or even avoided, thus preventing epithelial defects on the graft. In this case, however, in vivo confocal microscopy would be crucial for the recurrence control [24].
Penetrating keratoplasty à chaud in the acute stage of AK may, in contrast to the current contention, result in better final BCVA than delayed graft surgery, in cases of a severe infection and poor therapeutic response to a classical topical triple therapy. In addition, early PKP may have a favorable impact on epithelial healing and graft survival.
Conclusions
Our results from a tertiary, highly specialized corneal center suggest that PKP, performed at the early, acute stage in therapy-resistant AK, can significantly increase the chances of a better visual outcome by maintaining ocular surface integrity and graft clarity. Therefore, PKP à chaud could possibly be considered as an effective method to eradicate the infection timely and prevent sight-threatening complications. Moreover, it can shorten the expensive conservative treatment and the long hospitalisation period that seems unbearable for the typically young and active AK patients.
References
Naginton J, Watson PG, Playfair TJ, McGill J, Jones BR, Steele AD. Amoebic infection of the eye. Lancet. 1974;2:1537–40.
Meltendorf C, Duncker G. Akanthamöben-Keratitis. Klin Monbl Augenheilkd. 2011;228:R29–43.
Szentmary N, Daas L, Matoula P, Goebels S, Seitz B. Akanthamöbenkeratitis. Ophthalmologe. 2013;110:1203–10.
Claerhout I, Goegebuer A, Van Den Broecke C, Kestelyn P. Delay in diagnosis and outcome of Acanthamoeba keratitis. Graef Arch Clin Exp Ophthalmol. 2004;242:648–53.
Daas L, Szentmary N, Eppig T, et al. Das Deutsche Akanthamöbenkeratitis-Register: Erste Ergebnisse einer multizentrischen Erhebung. Ophthalmologe. 2015;112:752–63.
Lorenzo-Morales J, Khan NA, Walochnik J. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite. 2015;22:10.
Awwad ST, Heilman M, Hogan RN, et al. Severe reactive ischemic posterior segment inflammation in acanthamoeba keratitis: a new potentially blindign syndrome. Ophthalmology. 2007;114:313–20.
Blackman HJ, Rao NA, Lemp MA, Visvesvara GS. Acanthamoeba keratitis successfully treated with penetrating keratoplasty: suggested immunogenic mechanisms of action. Cornea. 1984;3:125–30.
Dart JK, Saw VP, Kilvington S. Acanthamoeba keratitis: diagnosis and treatment update 2009. Am J Ophthalmol. 2009;148:487–99.
Robaei D, Carnt N, Minassian DC, Dart JK. Therapeutic and optical keratoplasty in the management of Acanthamoeba keratitis: risk factors, outcomes, and summary of the literature. Ophthalmology. 2015;122:17–24.
Illingworth CD, Cook SD. Acanthamoeba keratitis. Surv Ophthalmol. 1998;42:493–508.
Awwad ST, Parmar DN, Heilman M, Bowman RW, McCulley JP, Cavanagh HD. Results of penetrating keratoplasty for visual rehabilitation after Acanthamoeba keratitis. Am J Ophthalmol. 2005;140:1080–4.
Hager T, Hasenfus A, Stachon T, Seitz B, Szentmary N. Crosslinking and corneal cryotherapy in acanthamoeba keratitis—a histological study. Graefes Arch Clin Exp Ophthalmol. 2015;254:149–53.
Küchle M, Seitz B, Langenbucher A, Naumann GO. Nonmechanical excimer laser penetrating keratoplasty for perforated or predescemetal corneal ulcers. Ophthalmology. 1999;106:2203–9.
Seitz B, Resch M, Schlötzer-Schrehardt U, Hofmann-Rummelt C, Sauer R, Kruse FE. Histopathology and ultrastructure of human corneas after amniotic membrane transplantation. Arch Ophthalmol. 2006;124:1487–90.
Robaei D, Carnt N, Minassian DC, Dart JK. The impact of topical corticosteroid use before diagnosis on the outcome of Acanthamoeba keratitis. Ophthalmology. 2014;121:1383–8.
Kashiwabuchi RT, de Freitas D, Alvarenga LS, et al. Corneal graft survival after therapeutic keratoplasty for Acanthamoeba keratitis. Acta Ophthalmol. 2008;86:666–9.
Ficker LA, Kirkness C, Wright P. Prognosis for keratoplasty in Acanthamoeba keratitis. Ophthalmology. 1993;100:105–10.
Illingworth CD, Cook SD, Karabatsas CH, Easty DL. Acanthamoeba keratitis: risk factors and outcome. Br J Ophthalmol. 1995;79:1078–82.
Chen WL, Wu CY, Hu FR, Wang IJ. Therapeutic penetrating keratoplasty for microbial keratitis in Taiwan from 1987 to 2001. Am J Ophthalmol. 2004;137:736–43.
Sarnicola E, Sarnicola C, Sabatino F, Tosi GM, Perri P, Sarnicola V. Early Deep Anterior Lamellar Keratoplasty (DALK) for Acanthamoeba keratitis poorly responsive to medical treatment. Cornea. 2016;35:1–5.
Ehlers N, Hjortdal J. Are cataract and iris atrophy toxic complications of medical treatment of acanthamoeba keratitis? Acta Ophthalmol Scand. 2004;82:228–31.
Herz NL, Matoba AY, Wilhelmus KR. Rapidly progressive cataract and iris atrophy during treatment of Acanthamoeba keratitis. Ophthalmology. 2008;115:866–9.
Daas L, Viestenz A, Schnabel PA, et al. Confocal microscopy as an early relapse marker for Acanthamoeba keratitis. Clin Anat. 2018;31:60–3.
Dohlman CH, Terada H. Keratoprosthesis in pemphigoid and Stevens-Johnson syndrome. Adv Exp Med Biol. 1998;438:1021–5.
Bach M, Kommerell G. Sehschärfebestimmung nach Europäischer Norm: wissenschaftliche Grundlagen und Möglichkeiten der automatischen Messung. Klin Monbl Augenheilkund. 1998;212(4):190–5.
Acknowledgements
Funding
We thank the Alexander von Humboldt Foundation for supporting the work of Prof. N. Szentmáry at the Department of Ophthalmology of Saarland University Medical Center in Homburg/Saar, Germany. We thank the University of Saarland for funding the medical writing assistance and the Rapid Service Fees. The funding organisation had no role in the design or conduct of this research.
Medical Writing Assistance
Language editing and assistance for this article was provided by Chrisitna Turner of the ACT-Fachübersetzunge and was funded by the University of Saarland.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Kornélia L. Laurik, Nóra Szentmáry, Loay Daas, Achim Langenbucher and Berthold Seitz have nothing to disclose.
Compliance with Ethics Guidelines
All procedures performed in our study involving human participants were in accordance with the ethical standards of the Ethical Committee of the Medical Association of Saarland and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent for participation and publication of patient data was obtained from all individual participants included in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
We hereby thank all the participants of the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.8546129.
Rights and permissions
About this article
Cite this article
Laurik, K.L., Szentmáry, N., Daas, L. et al. Early Penetrating Keratoplasty À Chaud May Improve Outcome in Therapy-Resistant Acanthamoeba Keratitis. Adv Ther 36, 2528–2540 (2019). https://doi.org/10.1007/s12325-019-01031-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-01031-3