Abstract
Introduction
The incidence of inflammatory bowel disease (IBD) in Denmark is among the highest in the world, with Crohn’s disease and ulcerative colitis occurring at rates of 9.1 and 18.6 per 100,000 person-years respectively in 2010–2013. Anemia is the most prevalent extraintestinal complication of IBD, most commonly caused by iron deficiency. In treating IBD-associated iron deficiency anemia (IDA), intravenous iron is more effective and better tolerated and shows a faster response than oral iron. The present study evaluated resource use and costs associated with using iron isomaltoside (Monofer; IIM) versus ferric carboxymaltose (Ferinject; FCM) in patients with IDA and IBD in Denmark.
Methods
A budget impact model was developed to evaluate the cost of IIM compared with FCM from a Danish healthcare payer perspective. Iron deficits were modeled using dosing tables and a joint distribution of bodyweight [mean 75.4 kg, standard deviation (SD) 17.4 kg] and hemoglobin (mean 10.8 g/dL, SD 1.4 g/dl) based on observational data from patients with IBD. Retreatment frequency was modeled using a pooled retrospective analysis of randomized trial data, and costs were modeled using diagnosis-related groups with an outpatient infusion cost of DKK 2855.
Results
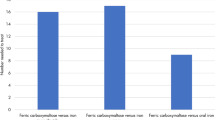
Using IIM required 1.2 infusions (per treatment) to correct the mean iron deficit compared with 1.6 with FCM. Treating 2.54 patients with IIM would therefore avoid one infusion compared with FCM. Patients using IIM required multiple infusions in 25.0% of cases compared with 64.3% with FCM. Over 5 years, total estimated costs were DKK 21,406 per patient with IIM compared with DKK 28,137 with FCM, corresponding to savings of DKK 6731 with IIM.
Conclusion
Using IIM in place of FCM markedly reduced the number of iron infusions required in patients with IBD and IDA in Denmark. The reduction in infusions was accompanied by reductions in cost compared with FCM.
Funding
Pharmacosmos A/S.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory bowel disease (IBD) is a collection of disorders characterized by chronic relapsing intestinal inflammation [1]. Crohn’s disease (CD) and ulcerative colitis (UC) are the most prevalent subtypes of IBD [1]. In Denmark, the incidence of IBD has markedly increased over the past few decades. Between 1980 and 2012, the incidence of CD increased from 5.2 per 100,000 to 9.1 per 100,000, while the incidence of UC increased from 10.7 per 100,000 to 18.6 per 100,000 [2]. Although the incidence of IBD has also increased globally, Denmark has among the highest incidence rates of IBD in the world [3].
Data on the overall economic burden of IBD in Denmark are scarce, but the EC-IBD study, published in 2006, reported that the overall costs associated with IBD in Denmark were the highest of the nine countries (eight in Europe and Israel) included in the study [4]. The mean annual cost of total health care and hospitalization in Denmark was € 3705 per patient, although this varied substantially between patients with a standard deviation of € 8720 and costs at the 10th and 90th percentiles of € 128 and € 11,421, respectively[4].
Iron deficiency anemia (IDA) is a common extraintestinal complication of IBD, arising from a combination of ongoing blood loss from the intestinal mucosa and impaired absorption of micronutrients such as iron and B12 [5]. IDA is diagnosed using a combination of hemoglobin (Hb) and ferritin levels; the World Health Organization defines anemia as Hb concentrations < 13 g/dl (7.7 mmol/l) in men or < 12 g/dl (7.4 mmol/l) in non-pregnant women [6]. Serum ferritin concentrations < 30 μg/l then confirm a diagnosis of ID in patients without clinical, endoscopic or biochemical evidence of active disease, while concentrations up to 100 μg/l may be indicative of ID depending on other factors such as chronic inflammation [7].
Due to the potential for exacerbation of gastrointestinal side effects and the malabsorption of oral iron, intravenous (IV) iron is the first-line treatment in patients with clinically active IBD, intolerance to oral iron or hemoglobin levels < 10 g/dl. IV iron is more effective, shows a faster response and is better tolerated than oral iron in these patients [7]. Numerous IV iron formulations are available, including iron isomaltoside (Monofer, Pharmacosmos A/S, Holbaek, Denmark; IIM) and ferric carboxymaltose (Ferinject, Vifor France SA, Victor, France; FCM), which differ in their posologic characteristics. For instance, IIM can be dosed up to 20 mg/kg bodyweight in a single infusion, while FCM can be dosed up to a maximum of 1000 mg in a single infusion. The objective of the present analysis was to evaluate how these differences in posology affect the cost and resource use implications of using IIM compared with FCM in the treatment of IDA in patients with IBD from the perspective of a Danish healthcare payer.
Methods
A cohort-level budget impact model (BIM) of iron deficiency and iron delivery was developed in Microsoft Excel (Microsoft Corp., Redmond, WA, USA) to characterize the resource use and costs associated with using IIM compared with FCM in patients with IBD and IDA from the perspective of a Danish healthcare payer. No head-to-head trials have yet been completed that directly compare the efficacy of IIM and FCM, but a recent network meta-analysis based on randomized trials of IV iron in patients with IBD concluded that there was no statistically significant difference in hematologic response with FCM, IIM and iron sucrose [8]. In line with this meta-analysis and a previous health economic analysis of IIM and FCM, the present analysis was conducted based on the assumption that the iron formulations were equivalent in terms of safety and efficacy [8, 9]. The present study did not include any studies of human participants or animals performed by any of the authors.
Model and Scenarios Analyzed
The BIM was structured in two parts: an iron deficiency model designed to model the iron deficiency in a given population of patients with IBD and IDA and an iron supply model designed to model the number of infusions of each IV iron formulation required to supply enough iron to correct the modeled deficit (Fig. 1).
The iron deficiency model included three different approaches to calculating the iron deficiency: a simplified dosing table (Table 1), a modified version of the Ganzoni formula (Eq. 1) or a normal distribution of the average iron deficiency in milligrams. The European Crohn’s and Colitis Organisation (ECCO) recommends using simplified dosing tables to establish the appropriate iron dose in patients with IDA associated with IBD [7]. Treating patients in line with the Ganzoni formula has been shown to result in low serum ferritin levels (< 100 μg/l) in patients with IBD and is considered to be impractical to use in routine clinical practice; the modified Ganzoni equation was therefore used exclusively for the purposes of sensitivity analysis [10, 11]. The simplified table dose calculation approach was therefore selected for use in the base case analysis.
Equation 1 Modified Ganzoni Formula
When using the simplified table approach or modified Ganzoni formula, the iron deficiency model distributed the cohort over bivariate lognormal distributions of bodyweight and hemoglobin in line with techniques employed by dosing models in other disease areas [12]. The bodyweight and hemoglobin distributions were assumed to be independent based on the mixed evidence identified in a 2011 systematic literature review and meta-analysis in which four out of ten cohorts showed a significant association between obesity and hemoglobin concentrations (compared with non-obese control subjects), while no significant association was identified in the remaining six cohorts [13]. When using an average iron deficiency distribution, the same bodyweight distribution was used to inform dosing limits, without the need for a corresponding hemoglobin distribution to inform the dosing models.
The iron supply model consisted of simple models of the ability of each comparator to address the iron deficit. IIM was modeled based on a maximum dose of 20 mg/kg bodyweight, while FCM was able to dose up to a maximum of 1000 mg in a single dose, in line with the summaries of product characteristics [14].
Budget impact analyses consisted of pairwise head-to-head comparisons of IV iron in which the “with IIM” scenario modeled IIM at 100% market share, while the “without IIM” scenario assumed 100% market share for FCM. Model outputs included the projected mean number of infusions required per patient, the mean number of patients requiring more than one infusion, and the absolute and incremental costs in the “with IIM” and “without IIM” scenarios. The model also calculated a “number needed to treat” (NNT), which represented the projected number of patients with IBD who would need to be treated with IIM to avoid one infusion with FCM.
Population and Cohort Characteristics
The bodyweight and hemoglobin distributions were based on a subgroup of patients with anemia and IBD in the Non-Interventional Monofer® (NIMO) study, a prospective, observational, multicenter study in patients with IDA and IBD [15]. Patients in the anemic subgroup had a mean bodyweight of 75.4 kg (SD 17.4 kg) and hemoglobin levels of 10.8 g/dl (SD 1.4 g/dl). The hemoglobin and bodyweight distributions were not covaried based on the assumption of independence. A minimum bodyweight of 50 kg was assumed in line with the lowest bodyweight threshold listed in the IIM summary of product characteristics, and the analysis was therefore restricted to patients with IBD weighing 50 kg or more. The lognormal bodyweight distribution was truncated by mirroring the distribution around the minimum, as previously reported [14, 16].
The continuous and/or recurrent blood loss in patients with IBD combined with reduced iron absorption typically necessitates multiple iron treatments in those patients who experience IDA. In the model, all patients were assumed to require retreatment, and the frequency of repeat treatments was captured based on a retrospective analysis of data pooled from observational follow-up data from three randomized clinical trials. In the pooled analysis, the median time to recurrence of anemia was 10 months [95% confidence interval (CI) 8–12 months], which was used as the interval between iron treatments in the base case [17]. Sensitivity analysis was conducted in which the median time to retreatment of 16 months (95% CI 7–24 months) [17]. Probabilistic sensitivity analysis (PSA) was conducted to capture uncertainty around the retreatment frequency by sampling from a normal distribution around the average 10-month interval with a standard error of 1.02 months (the width of the confidence interval divided by 3.92). One thousand model iterations were conducted in the PSA, which reported the median, interquartile range, minimum and maximum cost differences between IIM and FCM.
The base case analysis focused on the budgetary implications of using IIM in place of FCM in a single patient. In the interests of evaluating the budget impact across the whole population, a top-down approach was used to estimate the size of the Danish IBD population with IDA who would be eligible to receive IV iron treatment (Fig. 2). The Danish population size of 5,785,864 was taken from the 2018 Q2 Danmarks Statistik estimate for all of Denmark [18]. Estimates of the Danish prevalence of CD and UC (151 and 294 per 100,000 population, respectively) were then taken from a 2006 study by Jacobsen et al. [19], which was the most recent prevalence estimate identified in recent epidemiologic reviews of IBD by Molodecky et al. and Burisch et al. [20, 21]. Finally, estimates of the proportion of patients with IBD and anemia (24%) and the proportion of anemic IBD patients with iron deficiency (57%) were taken from a systematic review by Filmann et al. [22]. The resulting population size estimate was then treated as a closed cohort over the 5-year time horizon in an analysis assuming all patients would be treated with IV iron in line with ECCO guidelines [7].
Costs, Time Horizon and Discounting
The budget impact of IIM versus FCM was evaluated over a 5-year time horizon. A diagnosis-related group (DRG)-based approach was used to capture the costs of each infusion, specifically utilizing an outpatient DRG (formerly the “Dansk ambulant grupperingssystem” or Danish outpatient grouping system) tariff for iron infusion of DKK 2855. No other costs of IBD treatment were captured in the analysis based on the assumption that other ongoing treatment costs would be independent of the iron formulation in use, and the iron formulation costs would be covered by the DRG cost. Future costs were not discounted in the base case analysis in line with budget impact modeling guidelines from the International Society for Pharmacoeconomic and Outcomes Research (ISPOR) [23].
Sensitivity Analyses
A series of one-way sensitivity and scenario analyses was conducted to establish the extent to which changes in key input parameters changed the modeled outcomes. The analysis time horizon was changed from 5 years in the base case to 1 and 3 years in two separate sensitivity analyses, both assuming the same number of IV iron treatment courses per year as the base case. The mean cohort bodyweight of 75.40 kg in the base case was changed to lognormal distributions with expected values of 65, 70, 75, 80 and 85 kg, each with an assumed standard deviation of 25% of the mean. A mean bodyweight of 82.36 kg (SD 22.47 kg) was also analyzed based on the average bodyweight across six RCTs included in a review of RCTs in patients of various IDA etiologies published by Koch et al. [10]. The modified Ganzoni formula (Eq. 1) and the mean iron deficit modeling approaches were used in place of the simplified dose table approach employed in the base case. In the mean iron deficit approach, the iron deficit distribution was based on a pooled mean and SD from the studies included in the Koch et al. review [10].
Results
Using IIM required 1.2 infusions per patient to correct the mean iron deficit compared with 1.6 with FCM (Fig. 3a). Patients using IIM required multiple infusions in 25.0% of cases compared with 64.3% of patients with FCM (Fig. 3b). Based on a DRG-based costing methodology, total costs per patient over 5 years were estimated to be DKK 21,406 per patient with IIM compared with DKK 28,137 with FCM, corresponding to a saving of 23.9% compared with FCM (Table 2). The NNT to avoid a single infusion with IIM was 2.54 compared with FCM.
Sampling around the retreatment frequency showed changes in the magnitude of the cost savings (Fig. 4). Savings with IIM compared with FCM ranged from 7.6 to 38.3%, and mean percentage cost savings were consistent with the deterministic analysis at 23.8% with IIM compared with FCM (SD 5.0%).
One-way sensitivity analysis showed that cohort bodyweight assumptions did not affect the directionality of the outcomes, but did have a notable effect on the magnitude of the cost savings (Table 3). Specifically, cost savings with IIM increased with increasing mean cohort weight. Switching from the base case iron deficit calculation approach based on a simplified dosing table to a population mean approach and the Ganzoni formula increased cost savings with IIM compared with FCM (Table 3). Using the Ganzoni formula increased the cost savings with IIM compared with FCM to DKK 10,313 or 33.9% (from savings of DKK 6731 or 23.9% in the base case). Similarly, the average iron deficit-based approach increased cost savings compared with FCM to DKK 8085 (24.5%) from DKK 6731 (23.9%) in the base case. Both alternative dosing approaches resulted in increases in the absolute costs associated with iron replacement (Table 3).
The top-down approach to estimating the eligible IBD population size with anemia and iron deficiency in Denmark yielded an estimate of 3522 patients across the whole of Denmark (Fig. 2). Over a 5-year period, assuming all patients were treated with FCM and would switch to IIM, total cost savings would be DKK 23.7 m, with costs decreasing from DKK 99.1 m with FCM to DKK 75.4 m with IIM.
Discussion
In patients with IBD and IDA, using IIM in place of FCM reduced the mean number of infusions (and the associated costs) required to correct the iron deficit by 23.9%. The modeling analysis showed that the number of infusions and cost difference increased with increasing average bodyweight, and no sensitivity analyses fundamentally altered the conclusions of the base case.
The analysis may have been conservative from the perspective of routine clinical practice; the modeled cohort was based on the anemic, IBD subgroup in the NIMO Study, and yet the modeled outcome of a 25% repeat visit rate with IIM differed substantially from the repeat treatment rate of 5% observed in the NIMO study [15]. This was likely driven by the low mean iron dose administered in the NIMO study (1010 mg). Indeed, the authors noted that this fell substantially below the average of 1363 mg that would have been administered according to the simplified dosing table as modeled in the present analysis. Furthermore, 27% of patients still had anemia after treatment in the NIMO study, suggesting that dosing in routine clinical practice was insufficient to fully address the iron deficiency. The discrepancy in the repeat visit estimates could therefore be explained by the assumption in the present model that all patients would receive exactly the dose recommended by the simplified dosing tables. Other recent data from NIMO show that patients receiving doses > 1000 mg having 65% lower odds of needing iron retreatment than patients receiving 1000 mg (p = 0.001), which would support the explanation of the discrepancy between the modeled outcomes and the NIMO study outcomes [15].
One potential limitation of the model was the lack of “clinical discretion modeling” in determining the need for subsequent iron infusions. For instance, in a patient with a bodyweight of 71 kg and hemoglobin level of 10 g/dl, the simplified dosing tables for IIM or FCM both specify an iron requirement of 1500 mg. At an IIM dose of 20 mg/kg, a maximum of 1420 mg of iron could be administered to the patient and the model would therefore report a requirement of two infusions, one of 1420 mg and one of 80 mg. In practice, the clinician may decide that a dose of 1420 mg (95% of the calculated requirement) would be sufficient and that no subsequent infusion would be required. This approach may therefore somewhat mitigate the effect of the conservatism introduced by modeling exactly in line with the treatment guidelines; however, this effect was applied across both iron formulations and hence would be unlikely to drive any substantive incremental differences between comparators.
While the present study represents the second published budget impact analysis of IIM in patients with IDA and IBD from a national payer perspective, a previous study has reported findings of a cost-minimization analysis of IIM in the UK hospital setting [9, 24], and previous analyses of the budget impact of parenteral iron have broadly agreed with the findings of the present analysis with regard to FCM. When interpreting the findings of a budget impact analysis, the payer perspective is an important consideration; here, the use of a DRG-based analysis ultimately gives cost estimates from the perspective of the Danish Ministry of Health (Sundheds-og Ældreministeriet). The hospital at which the outpatient infusion takes place charges the DRG tariff to one of Denmark’s five regional health authorities, which are in turn funded (predominantly) by the Ministry of Health [25]. The actual costs borne by the hospital, including IV iron drug costs, nurse time, infusion center running costs, giving sets, cannulas and dressings, may vary; from the hospital perspective, the DRG tariff represents the income for each infusion rather than the expenditure.
The reductions in infusions modeled in the present analysis with IIM compared with FCM show that improvements in patient throughput could also be achieved by the use of different IV iron formulations. Specifically, treating 2.54 patients with IBD with IIM rather than FCM would result in one avoided infusion. Furthermore, the improvements with IIM would be accompanied by substantial reductions in the direct costs of treatment, saving DKK 6731 per patient compared with FCM over 5 years.
The healthcare payer perspective combined with the exclusive use of DRG-based cost estimates yielded an analysis that is likely to be particularly conservative compared with other perspectives. Given that reduction in infusion center visits was the primary driver of the analysis, indirect costs borne by other payers, such as patient transportation costs, infusion center running costs and costs of lost workplace productivity, would probably substantially amplify the savings from any given broader perspective. These costs may be considered in future analyses.
Conclusions
IIM is the only fast-infusion IV iron formulation that can be administered in doses exceeding 1000 mg iron (20 mg/kg bodyweight). As a direct corollary of this potential for fast infusion, using IIM in place of FCM was projected to result in reductions in the number of infusions required to correct iron deficits in patients with IBD and IDA. Just 23.9% of patients required multiple infusions with IIM compared with 64.3% with FCM. The reduction in infusions resulted in corresponding reductions in cost with IIM compared with FCM over 5 years. Based on a DRG costing of the IV iron therapies, IIM should represent the iron replacement therapy of choice in patients with IBD and IDA in the Danish setting.
References
Loddo I, Romano C. Inflammatory bowel disease: genetics, epigenetics, and pathogenesis. Front Immunol. 2015;6:551.
Lophaven SN, Lynge E, Burisch J. The incidence of inflammatory bowel disease in Denmark 1980–2013: a nationwide cohort study. Aliment Pharmacol Ther. 2017;45(7):961–72.
Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(46–54):e42.
Odes S, Vardi H, Friger M, Wolters F, Russel MG, Riis L, Munkholm P, Politi P, Tsianos E, Clofent J, Vermeire S, Monteiro E, Mouzas I, Fornaciari G, Sijbrandij J, Limonard C, Van Zeijl G, O’Morain C, Moum B, Vatn M, Stockbrugger R. Cost analysis and cost determinants in a European inflammatory bowel disease inception cohort with 10 years of follow-up evaluation. Gastroenterology. 2006;131(3):719–28.
Kaitha S, Bashir M, Ali T. Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol. 2015;6(3):62–72.
WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and mineral nutrition information system. World Health Organization, Geneva, Switzerland. 2011. Available form: http://www.who.int/vmnis/indicators/haemoglobin.pdf. Accessed 26 Jun 2018.
Dignass AU, Gasche C, Bettenworth D, et al. European Crohn’s and Colitis Organisation [ECCO]. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis. 2015;9(3):211–22.
Aksan A, Işık H, Radeke HH, Dignass A, Stein J. Systematic review with network meta-analysis: comparative efficacy and tolerability of different intravenous iron formulations for the treatment of iron deficiency anaemia in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2017;45(10):1303–18.
Bhandari S. Update of a comparative analysis of cost minimization following the introduction of newly available intravenous iron therapies in hospital practice. Ther Clin Risk Manag. 2011;7:501–9.
Koch TA, Myers J, Goodnough LT. Intravenous iron therapy in patients with iron deficiency anemia: dosing considerations. Anemia. 2015;2015:763576.
Kulnigg S, Stoinov S, Simanenkov V, et al. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT) randomized controlled trial. Am J Gastroenterol. 2008;103(5):1182–92.
Pollock RF, Kappelgaard AM, Seitz L. An analysis of product wastage arising from dosing increment granularity in four modern growth hormone administration devices. Expert Opin Drug Deliv. 2015;12(3):353–60.
Cheng HL, Bryant C, Cook R, O’Connor H, Rooney K, Steinbeck K. The relationship between obesity and hypoferraemia in adults: a systematic review. Obes Rev. 2012;13(2):150–61.
Electronic Medicines Compendium (eMC). Ferinject (ferric carboxymaltose) summary of product characteristics. Available from: https://www.medicines.org.uk/emc/medicine/24167. Accessed 26 Jun 2018.
Frigstad SO, Haaber A, Bajor A, Fallingborg J, Hammarlund P, Bonderup OK, Blom H, Rannem T, Hellström PM. The NIMO Scandinavian Study: a prospective observational study of iron isomaltoside treatment in patients with iron deficiency. Gastroenterol Res Pract. 2017;2017:4585164.
Pollock RF, Muduma G. Intravenous iron treatments for iron deficiency anemia in inflammatory bowel disease: a budget impact analysis of iron isomaltoside 1000 (Monofer) in the UK. Expert Opin Drug Deliv. 2017;14(12):1439–46.
Kulnigg S, Teischinger L, Dejaco C, Waldhör T, Gasche C. Rapid recurrence of IBD-associated anemia and iron deficiency after intravenous iron sucrose and erythropoietin treatment. Am J Gastroenterol. 2009;104(6):1460–7.
Statistics Danmark. Folketal den 1. i kvartalet. 2018K2. Available from: https://www.dst.dk/da/Statistik/emner/befolkning-og-valg/befolkning-og-befolkningsfremskrivning/folketal. Accessed 22 Jul 2018.
Jacobsen BA, Fallingborg J, Rasmussen HH, Nielsen KR, Drewes AM, Puho E, Nielsen GL, Sørensen HT. Increase in incidence and prevalence of inflammatory bowel disease in northern Denmark: a population-based study, 1978–2002. Eur J Gastroenterol Hepatol. 2006;18(6):601–6.
Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54.
Burisch J, Jess T, Martinato M, Lakatos PL, ECCO-EpiCom. The burden of inflammatory bowel disease in Europe. J Crohns Colitis. 2013;7(4):322–37.
Filmann N, Rey J, Schneeweiss S, Ardizzone S, Bager P, Bergamaschi G, Koutroubakis I, Lindgren S, Morena Fde L, Moum B, Vavricka SR, Schröder O, Herrmann E, Blumenstein I. Prevalence of anemia in inflammatory bowel diseases in european countries: a systematic review and individual patient data meta-analysis. Inflamm Bowel Dis. 2014;20(5):936–45.
Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Health. 2014;17(1):5–14.
Bhandari S. A hospital-based cost minimization study of the potential financial impact on the UK health care system of introduction of iron isomaltoside 1000. Ther Clin Risk Manag. 2011;7:103–13.
Olejaz M, Nielsen AJ, Rudkjøbing A, Birk HO, Krasnik A, Hernández-Quevedo C. Denmark: health system review. Health systems in transition. 2012;14(2):1–192. Available from: http://www.euro.who.int/__data/assets/pdf_file/0004/160519/e96442.pdf. Accessed 18 Oct 2018.
Acknowledgements
Funding
Sponsorship for this study and the article publication charges were provided by Pharmacosmos A/S.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Richard F. Pollock is a full-time employee of Ossian Health Economics and Communications GmbH, which received consultancy fees to develop the budget impact model and conduct the Danish analysis. Gorden Muduma is a full-time employee of Pharmacosmos A/S, which is the EU marketing authorization holder for iron isomaltoside.
Compliance with Ethics Guidelines
This article does not include any studies of human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.7235048.
Rights and permissions
About this article
Cite this article
Pollock, R.F., Muduma, G. An Economic Evaluation of Iron Isomaltoside 1000 Versus Ferric Carboxymaltose in Patients with Inflammatory Bowel Disease and Iron Deficiency Anemia in Denmark. Adv Ther 35, 2128–2137 (2018). https://doi.org/10.1007/s12325-018-0827-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-018-0827-5