Abstract
Introduction
Cytomegalovirus (CMV) infections continue to cause significant morbidity and mortality in hematopoietic stem cell transplant (HSCT) recipients. Successful pre-emptive therapy in transplant patients depends on the availability of reliable diagnostic tests for CMV infections. The purpose of this retrospective study was to evaluate CMV DNA viral load, incidence of CMV disease and CMV seropositivity, risk factors and correlation between CMV DNA positivity and clinical course in HSCT patients.
Methods
Two hundred and twenty-five patients who underwent peripheral blood stem cell or bone marrow transplantation between June 2003 and April 2010 were included. A real-time polymerase chain reaction (RT-PCR) assay was used for CMV monitoring.
Results
Recipient median age was 42.5 years. CMV seropositivity was 95.6%. CMV DNA positivity determined by RT-PCR was 24.9% among the entire patient group. CMV DNA positivity with RT-PCR was found to be significantly higher in allogeneic transplant recipients than autologous transplant recipients (46.7% vs 14.0%; P < 0.0001). Gender, age, conditioning regimen, stem cell source, underlying disease and recipient and donor seropositivity (alone or paired) were not significant risk factors for CMV DNAemia. We did not observe any CMV end-organ disease.
Conclusion
CMV DNAemia was significantly higher in allogeneic transplant recipients than in autologous transplant patients. End-organ disease could be prevented with appropriate pre-emptive therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cytomegalovirus (CMV) infection is an important cause of morbidity and mortality in patients undergoing hematopoietic stem cell transplantation (HSCT) [1]. Since CMV disease occurs in up to 35% of allogeneic HSCT recipients and carries a high mortality rate despite treatment [2], prevention and treatment of active CMV infection are essential. Pre-emptive therapy administered on the basis of early detection of CMV reactivation has become a common strategy in the treatment of HSCT recipients [3]. It has been shown that with an optimized pre-emptive therapy, the incidence of CMV disease is reduced and overall survival is improved significantly after allogeneic HSCT [3, 4]. Therefore, diagnostic assays that are sensitive, rapid and quantitative and accurately reflect active CMV infection are essential for the success of pre-emptive therapy. Quantification of CMV viral load by real-time polymerase chain reaction (RT-PCR) is one of the most widely used methods for monitoring active CMV infection and guiding pre-emptive therapy in a variety of clinical settings [5–9].
The purpose of this single-center study was to retrospectively determine the incidence of CMV disease among patients who underwent HSCT. We also aimed to analyze risk factors, CMV DNA viral load, incidence of CMV seropositivity and the effectiveness of pre-emptive therapy in our population.
Subjects and Methods
Patients
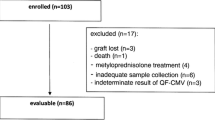
Two hundred and twenty-five patients who underwent autologous peripheral blood stem cell (PBSC) transplantation or allogeneic bone marrow transplantation at the Bone Marrow Transplantation Unit of the Hematology Department at the Eskisehir Osmangazi University Medical School between June 2003 and April 2010 were included in the study. All patients were enrolled prior to transplantation and subsequently received HSCT from human leukocyte antigen (HLA)-matched related donors. Age, sex, underlying disease, CMV serostatus, conditioning regimen, stem cell source, and transplant type were recorded. Patients were selected for enrollment if either the recipient (R) or the donor (D) had immunoglobulin G (IgG) antibody to CMV, as tested by enzyme immunoassay. Quantitative RT-PCR assay was used for CMV monitoring post-engraftment. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients prior to inclusion in the study.
Quantitative RT-PCR for CMV DNA
CMV DNA was extracted from whole blood using the BioRobot M48 automated system (QIAGEN, Valencia, CA, USA) and purified using MagAttract Virus Mini M48 kits (QIAGEN, Valencia, CA, USA). The sample input volume was 200 μL, and the elution volume was 75 μL. For extraction with the BioRobot M48 system, the ready-to-use reagents were placed in the instrument. The artus ® CMV RG PCR Kit (QIAGEN Inc., Hamburg, Germany) was used in the Rotor-Gene™ 6000 (Corbett Research, Sydney, Australia) instrument for the detection of CMV DNA. Primer information is proprietary information and cannot be disclosed; however, the CMV assay targeted a conserved part of the CMV major immediate-early gene.
For RT-PCR, 30 μL of the master mix and 20 μL of the eluted sample DNA were mixed in a PCR tube and loaded onto the Rotor-Gene™ 6000 instrument. Equal volumes (20 μL each) of the four quantitation standards and water were added to 30 μL of the master mix as a positive and negative control, respectively. The four standard samples included in each of the kits were amplified within the same run as the test samples to quantify the DNA of the samples. Briefly, the RT-PCR conditions were as follows: an initial 10 min at 95 °C, followed by 45 cycles of 15 s for denaturing at 95 °C, 30 s of annealing at 65 °C, and a 20-s extension at 72 °C.
Pre-Emptive Therapy and CMV Disease
The diagnosis of CMV disease was made according to the presence of symptoms and signs compatible with end-organ damage, together with the detection of CMV using a validated method in an appropriate clinical specimen [10]. Surveillance for CMV infection was routinely performed with the RT-PCR assay. Patients were monitored at 1- to 2-week intervals during the first 100 days after transplantation. After the first 100 days, patients were followed for the duration that they took immunosuppressive drugs for late CMV disease. Pre-emptive therapy with ganciclovir at 5 mg/kg twice daily was started when a positive result was detected and it was continued for 1 week at the same dose, then the dose was tapered to 5 mg/kg once daily and continued for two additional weeks. All patients were administered prophylactic acyclovir (400 mg orally every 8 h) and all patients who developed graft-versus-host disease (GVHD) were treated with steroids. Valgancyclovir was not used in any of our study patients.
Statistical Analysis
Statistical analysis was performed using commercially available software (SAS version 9.1; SAS Institute, Inc., Cary, USA). Data with non-normal distribution were expressed as median (range) for comparison using the Mann–Whitney test. The Chi-square or Fisher exact tests were used to compare differences between groups of categorical data. Stepwise logistic regression analyses were used to evaluate risk factors. P < 0.05 was considered to be significant.
Results
Peripheral blood samples from 225 HSCT recipients were analyzed. Table 1 summarizes the clinical characteristics of the autologous and allogeneic transplant patients. Recipient median age was 42.5 (14–65) years, and 42.6% of the patients were female. Almost all patients had a malignant hematologic disorder and, of these, 60 (26.7%) had multiple myeloma. Most of the patients (66.7%) underwent autologous transplantation. Peripheral blood cells were the primary source of stem cells. The choice of conditioning regimen was myeloablative in 93.8% of the patients. The donors of all allogeneic HSCT recipients were HLA-identical siblings or parents.
In our population, recipient CMV seropositivity, which is a risk factor for CMV disease, was 95.6%. Seventy-one patients were D+/R+, 3 were D–/R+ and 1 was D−/R−. The CMV DNA positivity rate, determined using RT-PCR, was 24.8%. The cutoff value of CMV DNA for initiating pre-emptive therapy was ≥10,000 copies/mL recorded on a single occasion, ≥5,000 copies/mL recorded on consecutive occasions, or if a progressive increase in CMV DNAemia was recorded. The viral loads for patients at the initiation of therapy were between 832 and 557,491 copy numbers. None of the patients had developed CMV end-organ disease and there were no associated deaths. The median time from HSCT to the start of pre-emptive therapy was 46 (26–203) days. Pre-emptive therapy with ganciclovir was generally well tolerated and the median treatment duration was 21 days.
We also divided the patients into two groups based on autologous or allogeneic transplantation (Table 2). We found that CMV DNA positivity using RT-PCR was significantly higher in allogeneic transplant recipients than in the autologous group (46.7% vs 14.0%; P < 0.0001). In the allogeneic group, donor CMV seropositivity was 94.7%. Similarly, recipient CMV seropositivity was 98.6% and 94.0% in the allogeneic and autologous groups, respectively (P < 0.05).
We used stepwise logistic regression analysis to evaluate risk factors for CMV DNAemia. Gender, conditioning regimen, stem cell source, and recipient and donor seropositivity (alone or paired), underlying disease and GVHD were not found to be significant risk factors for CMV DNAemia.
Discussion
Despite recent advances in surveillance strategies, antivirals and diagnostic techniques, CMV infection continues to be a major opportunistic infectious agent in HSCT recipients. In our study, we found that CMV DNAemia was significantly higher in allogeneic transplant recipients than in autologous transplant patients. However, there was no active CMV disease or related deaths.
Although 24.9% of our patients were positive for CMV DNA, we did not observe any CMV end-organ disease. Recipient CMV seropositivity has been shown to be a risk factor for CMV disease [11–13]. In our study, most of the patients (95.6%) were seropositive for CMV before transplantation. This was similar to the previously reported rate of 93.6% by Ataman et al. [14], which is reflective of the high CMV seropositivity rate in Mediterranean populations [14, 15]. Also, in a recent study by Peres et al. [16], it was shown that the highest incidence of active CMV infections occurred during the second post-transplant month (31–60 days after transplantation). Given the fact that we initiated pre-emptive therapy at a median day-46, this may be one of the reasons that we did not observe any active CMV disease in our patients. Infection is termed primary CMV infection when it occurs in a CMV IgG-negative patient, and CMV reactivation when the patient or donor is known to be CMV antibody positive. Prevention of CMV reactivation appears to be mostly dependent on immune response [17]. In this respect, when secondary infection occurs, immune memory may limit the disease; therefore the disease may appear asymptomatic or mild. In our study population, CMV seropositivity was high. We believe this may be another reason for the lack of post-transplant CMV disease or reactivation of disease in our study. Several studies have shown that transplantation with alternative donors (unrelated and/or mismatched) is a risk factor for post-transplant CMV disease [18–21]. In a recent study, Yoon et al. [22] showed that patients who were recipients of HSCT from alternative donors were at highest risk of CMV disease when compared with those from matched sibling donors. In our study, all our patients had HLA-matched sibling or related alternative donors. We assume that this may be another reason for the lack of observed CMV infection in our population. On the other hand, some studies have revealed no difference in the incidence of CMV disease by graft sources [23, 24]. Further studies are needed to determine the exact role of graft sources in post-transplant CMV disease.
All CMV-seropositive HSCT recipients are at risk for CMV disease. This may be related to the function of individual post-immune reconstitution after transplantation. Therefore, there is a lower risk for CMV disease in patients who have undergone autologous transplantation as opposed to allogeneic transplantation [25, 26]. Similarly, we found that the incidence of CMV DNA positivity was significantly higher in allogeneic transplant recipients than in autologous transplant recipients. We believe this could be explained by more efficient reconstitution of immunity after autologous transplantation. In addition, recipient seropositivity was found to be higher in the allogeneic transplant group than the autologous group in our study. Considering that recipient seropositivity is a risk factor for CMV disease, this may support our finding that CMV DNA positivity was higher in the allogeneic transplant group.
In the present study, we analyzed some risk factors, such as gender, stem cell source, conditioning regimen and underlying disease in relation to CMV disease. We were not able to show any relation between these factors and CMV DNAemia.
There are some limitations in our study. We failed to analyze more CMV disease risk factors, which have been identified in previous studies, such as T cell depletion, anti-thymocyte globulin-containing regimen and initial viral load [10, 27]. CMV antigenemia also could have been studied in parallel to CMV DNAemia. In the literature, many studies have analyzed and compared both techniques simultaneously [8, 28, 29]. As a consequence of these limitations, it is difficult to compare our results with these previous findings.
Conclusion
We conclude that CMV DNAemia was significantly higher in allogeneic transplant recipients than autologous transplant patients. The rate of CMV seropositivity was similar to that reported in the literature. End-organ disease is preventable with appropriate pre-emptive therapy. Further prospective studies are needed to evaluate both the risk factors for CMV disease and the regular use of RT-PCR for determining CMV infection status.
References
Boeckh M, Nichols WG, Papanicolaou G, et al. Cytomegalovirus in hematopoietic stem cell transplant recipients: current status, known challenges, and future strategies. Biol Blood Marrow Transplant. 2003;9:543–58.
Leruez-Ville M, Ouachee M, Delarue R, et al. Monitoring cytomegalovirus infection in adult and pediatric bone marrow transplant recipients by a real-time PCR assay performed with blood plasma. J Clin Microbiol. 2003;41:2040–6.
Goodrich JM, Mori M, Gleaves CA, et al. Early treatment with ganciclovir to prevent cytomegalovirus disease after allogeneic bone marrow transplantation. N Engl J Med. 1991;325:1601–7.
Goodrich JM, Bowden RA, Fisher L, et al. Ganciclovir prophylaxis to prevent cytomegalovirus disease after allogeneic marrow transplant. Ann Intern Med. 1993;118:173–8.
de la Cruz-Vicente F, Perez-Romero P, Aguilar-Guisado M, et al. Differences in cytomegalovirus replication quantified using quantitative polymerase chain reaction and antigenemia after allogeneic stem cell transplantation. Transplant Proc. 2010;42:3230–1.
Farfan UM, Torres TJ, Vergara AA, et al. Comparison of real-time polymerase chain reaction and antigenemia assay to detect cytomegalovirus in pediatric transplants. Rev Chilena Infectol. 2011;28:113–7.
Zhai WJ, Wei JL, Zhao MF, et al. Comparison between CMV quantitative PCR and CMV-pp 65 antigen test for detection of CMV infection in allogeneic hematopoietic stem cell transplantation. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2009;17:1522–6.
Choi SM, Lee DG, Lim J, et al. Comparison of quantitative cytomegalovirus real-time PCR in whole blood and pp 65 antigenemia assay: clinical utility of CMV real-time PCR in hematopoietic stem cell transplant recipients. J Korean Med Sci. 2009;24:571–8.
Gimeno C, Solano C, Latorre JC, et al. Quantification of DNA in plasma by an automated real-time PCR assay (cytomegalovirus PCR kit) for surveillance of active cytomegalovirus infection and guidance of preemptive therapy for allogeneic hematopoietic stem cell transplant recipients. J Clin Microbiol. 2008;46:3311–8.
Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Hematol Oncol Clin North Am. 2011;25:151–69.
Meyers JD, Flournoy N, Thomas ED. Risk factors for cytomegalovirus infection after human marrow transplantation. J Infect Dis. 1986;153:478–88.
Patel SR, Ridwan RU, Ortin M. Cytomegalovirus reactivation in pediatric hemopoietic progenitors transplant: a retrospective study on the risk factors and the efficacy of treatment. J Pediatr Hematol Oncol. 2005;27:411–5.
Zaucha-Prazmo A, Wojcik B, Drabko K, Choma M, Kowalczyk JR. Cytomegalovirus (CMV) infections in children undergoing hematopoietic stem cell transplantation. Pediatr Hematol Oncol. 2005;22:271–6.
Ataman S, Colak D, Gunseren F, et al. Investigation of cytomegalovirus seroepidemiology in Antalya with a population-based cross-sectional study and review of related data in Turkey. Mikrobiyol Bul. 2007;41:545–55.
Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20:202–13.
Peres RM, Costa CR, Andrade PD, et al. Surveillance of active human cytomegalovirus infection in hematopoietic stem cell transplantation (HLA sibling identical donor): search for optimal cutoff value by real-time PCR. BMC Infect Dis. 2010;10:147.
Emery V, Zuckerman M, Jackson G, et al. Management of cytomegalovirus infection in haemopoietic stem cell transplantation. Br J Haematol. 2013;162:25–39.
Nichols WG, Boeckh M. Recent advances in the therapy and prevention of CMV infections. J Clin Virol. 2000;16:25–40.
de la Camara R, Fernandez-Ranada JM. Increased incidence of CMV infection after allogeneic bone marrow transplantation from unrelated donors but doubts about an increase in CMV-associated disease. Bone Marrow Transplant. 1997;20:181.
Takami A, Mochizuki K, Asakura H, et al. High incidence of cytomegalovirus reactivation in adult recipients of an unrelated cord blood transplant. Haematologica. 2005;90:1290–2.
Takenaka K, Gondo H, Tanimoto K, et al. Increased incidence of cytomegalovirus (CMV) infection and CMV-associated disease after allogeneic bone marrow transplantation from unrelated donors. The Fukuoka Bone Marrow Transplantation Group. Bone Marrow Transplant. 1997;19:241–8.
Yoon HS, Lee JH, Choi ES, et al. Cytomegalovirus infection in children who underwent hematopoietic stem cell transplantation at a single center: a retrospective study of the risk factors. Pediatr Transplant. 2009;13:898–905.
Walker CM, van Burik JA, De For TE, Weisdorf DJ. Cytomegalovirus infection after allogeneic transplantation: comparison of cord blood with peripheral blood and marrow graft sources. Biol Blood Marrow Transplant. 2007;13:1106–15.
Asano-Mori Y, Oshima K, Sakata-Yanagimoto M, et al. High-grade cytomegalovirus antigenemia after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;36:813–9.
Zaia JA. Prevention of cytomegalovirus disease in hematopoietic stem cell transplantation. Clin Infect Dis. 2002;35:999–1004.
Reusser P, Attenhofer R, Hebart H, et al. Cytomegalovirus-specific T-cell immunity in recipients of autologous peripheral blood stem cell or bone marrow transplants. Blood. 1997;89:3873–9.
Emery VC, Sabin CA, Cope AV, et al. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet. 2000;355:2032–6.
Gentile G, Picardi A, Capobianchi A, et al. A prospective study comparing quantitative cytomegalovirus (CMV) polymerase chain reaction in plasma and pp 65 antigenemia assay in monitoring patients after allogeneic stem cell transplantation. BMC Infect Dis. 2006;6:167.
Yakushiji K, Gondo H, Kamezaki K, et al. Monitoring of cytomegalovirus reactivation after allogeneic stem cell transplantation: comparison of an antigenemia assay and quantitative real-time polymerase chain reaction. Bone Marrow Transplant. 2002;29:599–606.
Acknowledgments
No funding or sponsorship was received for this study or publication of this article. Dr. Deniz Goren Sahin is the guarantor for this article and takes responsibility for the integrity of the work as a whole.
Conflict of interest
Deniz Goren Sahin, Eren Gunduz, Nilgun Kasifoglu, Olga Akay, Tercan Us and Zafer Gulbas declare that they have no conflicts of interest.
Compliance with ethics guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients prior to inclusion in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sahin, D.G., Gunduz, E., Kasifoglu, N. et al. Cytomegalovirus DNAemia Detected with Real-Time Polymerase Chain Reaction in Hematopoietic Stem Cell Transplant Patients. Adv Ther 30, 784–791 (2013). https://doi.org/10.1007/s12325-013-0049-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-013-0049-9