Abstract
Spinocerebellar ataxia type 31 (SCA31) is known as a late-onset, relatively pure cerebellar form of ataxia, but a longitudinal prospective study on the natural history of SCA31 has not been done yet. In this prospective cohort study, we enrolled 44 patients (mean ± standard deviation 73.6 ± 8.5 years) with genetically confirmed SCA31 from 10 ataxia referral centers in the Nagano area, Japan. Patients were evaluated every year for 4 years using the Scale for the Assessment and Rating of Ataxia (SARA) and the Barthel Index (BI). Of the 176 follow-up visits (91.5%), 161 were completed in this study. Five patients (11.4%) died during the follow-up period, and two patients (4.5%) were lost to follow-up. The annual progression of the SARA score was 0.8 ± 0.1 points/year and that of the BI was −2.3 ± 0.4 points/year (mean ± standard error). Shorter disease duration at baseline was associated with faster progression of the SARA score. Our study indicated the averaged clinical course of SCA31 as follows: the patients develop ataxic symptoms at 58.5 ± 10.3 years, become wheelchair bound at 79.4 ± 1.7 years, and died at 88.5 ± 0.7 years. Our prospective dataset provides important information for clinical trials of forthcoming disease-modifying therapies for cerebellar ataxia. It also represents a useful resource for SCA31 patients and their family members in genetic counseling sessions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinocerebellar ataxia type 31 (SCA31) (MIM 117210) accounts for 8–42% of autosomal dominant cerebellar ataxia (ADCA) families in Japan, with a considerable regional difference in frequency [1–7]. It is caused by a 2.5–3.8-kb insertion containing pentanucleotide repeats (TGGAA)n, which is located in overlapping introns of the BEAN and TK2 genes on chromosome 16q21–q22 [8, 9]. Recent progress in understanding the pathogenic mechanisms of ADCAs has raised hopes for the development of new disease-modifying therapies for cerebellar ataxia. Thus, knowledge about the natural history of ADCAs is important for well-designed clinical trials. To address these issues, some prospective cohort studies of patients with SCA1, SCA2, SCA3, or SCA6 have been conducted in Europe, the USA, Cuba, Taiwan, and Japan [10–15]. SCA31 may be a desirable ADCA subtype for clinical trials of forthcoming disease-modifying therapies for cerebellar ataxia because cerebellar Purkinje cells are preferentially affected in SCA31 [16–19]. Cross-sectional studies on the estimated clinical course of SCA31 have been reported [1–7]; however, prospective longitudinal studies on the rate of disease progression in SCA31 have not been performed. One possible reason for this is that SCA31 is almost exclusively seen in the Japanese population [20–22].
The main purpose of this study was to quantify the clinical progression rate of cerebellar ataxia and activities of daily living (ADLs) in SCA31 patients.
Methods
Organization and Patients of the Study
In this 4-year prospective cohort study, we enrolled 44 patients with genetically confirmed SCA31. We excluded the patients with any comorbid diseases that apparently prevented us from evaluating the severity of ataxia or ADLs. All patients were residents of Nagano prefecture, one of the most prevalent areas for SCA31 in Japan [6, 7], where the latest incidence of SCA31 is approximately 44% (103/235) of ADCA families. We calculated the size of Hae III fragment containing the insertion and substituted it for the TGGAA repeat length, as previously described [7, 8].
Patients were seen at a baseline visit (visit 1), followed by an annual evaluation (for a total of five visits) over a period of 4 years between 2011 and 2015. The present study was performed at 10 hospitals located in the Nagano area, Japan. A written informed consent form, which was approved by the institutional review board of all hospitals and by the Ethics Committee of the Shinshu University School of Medicine, was completed by all study participants.
Clinical Evaluation
Age at onset was determined on the basis of information provided by the patients themselves and their close relatives. We used the Scale for the Assessment and Rating of Ataxia (SARA) and Barthel Index (BI) to evaluate the severity of ataxia [23] and ADLs of the patients [24], respectively. Each patient was followed by the same neurologist, who was experienced in neurology practice and trained for SARA.
Statistical Analysis
Statistical analyses were performed with IBM SPSS Statistics software (version 22; IBM, Armonk, NY, USA). To investigate whether the baseline characteristics of male patients differed from those of female patients, we used t tests for quantitative variables and χ 2 tests for categorical variables. Correlations between clinical scores and covariates were tested by the Pearson’s correlation test. For analysis of disease progression, we applied a linear mixed model with random effects on the intercept and slope. The rate at which patients became wheelchair dependent and their survival were calculated by the Kaplan-Meier method. The test results were considered significant at the 0.05 level. To identify factors that affected the SARA score at baseline and delta SARA (∆SARA), multiple regression analysis was used to validate the relationships between the SARA score and sex, age at onset, disease duration, and TGGAA repeat length of the expanded allele as independent variables. Independent factors that were significant in the univariate analysis were included in a multivariable model, including interactions with backward selection. ∆SARA was defined as the change of the SARA score between baseline and visit 5. The test results were considered significant at the 0.01 level for the multivariate analysis. Data are presented as means ± standard error (SE), if not specified. To account for multiple testing, we used the Bonferroni correction and all p values were two sided.
Results
The study population consisted of 44 patients (male:female = 20:24) from 41 families with SCA31. The demographic data and clinical scores at baseline are summarized in Table 1. The age at baseline was 73.6 ± 8.5 years (mean ± standard deviation [SD]). All patients presented with cerebellar ataxia (SARA score 4–31.5). By the Inventory of Non-Ataxia Signs (INAS) [25], increased ankle reflexes were seen in 12 (12/42, 28.6%), but only one patient (1/42, 2.4%) showed extensor plantar responses. Rigidity or involuntary movements were not observed in any patients, except for moderate facial dystonia in one patient. Dysphagia (16/42, 38.1%) and mild decrease of vibration sense (9/30, 30.0%) were relatively common in our cohort. Cognitive decline was noted in five patients (5/42, 11.9%). Seven patients (7/42, 16.7%) complained of mild double vision, but ophthalmoparesis or slowing of saccades was evident in only two patients. Only a few patients showed mild autonomic dysfunction, urinary dysfunction in 2 (2/42, 4.8%) and constipation in 6 (6/44, 13.6%). The INAS count was 0.93 ± 1.18 (mean ± SD, n = 42, 75.5 ± 8.3 years at evaluation). Ten patients (10/43, 23.3%) showed hearing disturbance.
Figure e-1 shows the detailed data of patients followed each year and the reasons for dropout. All 44 patients were examined at a follow-up visit after 1 year (visit 2), but the number of patients examined at visit 3 decreased to 41 (93.2%), 39 (88.6%) at visit 4, and 37 (84.1%) at the final visit after 4 years (visit 5). Of the total 176 follow-up visits during the study period, 161 (91.5%) were completed. Five patients (11.4%) died during the follow-up period, three of pneumonia, one of congestive heart failure, and one of asphyxia (age at death 86.0 ± 3.2 years, SARA score and BI at their last visits 26.4 ± 5.9 and 42.0 ± 28.9 (mean ± SD), respectively). Additionally, two patients were lost to follow-up because of admission to a nursing home. Eleven of 44 patients were admitted to a hospital 15 times during the study period because of comorbid diseases such as infection, trauma, and cardiovascular diseases.
At baseline (visit 1), the mean SARA score was 16.5 ± 6.9 points and the BI was 82.5 ± 21.4 points (mean ± SD). Disease duration correlated well with both the SARA score and BI (r = 0.699, p < 0.001; r = −0.620, p < 0.001), and the age at baseline correlated well with both the SARA score and BI (r = 0.629, p < 0.001; r = −0.477, p = 0.001). In contrast to the previous report [7], the TGGAA repeat length of the expanded allele was not correlated with age at onset in this study. Among the 44 patients in this study, male patients tended to be younger and have a shorter disease duration than female patients; however, the difference was not statistically significant. In multiple regression analysis, 65.4% of the variance of the SARA at baseline score were explained. The SARA score was influenced by age at onset and disease duration. Unlike SCA6 in a Japanese cohort [15], the SARA score was not influenced by sex and TGGAA repeat length in SCA31 (Table 2).
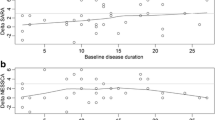
There was a strong correlation (r = −0.818, p < 0.001) between the total SARA score and BI (Figure e-2). In most patients, the SARA score and BI worsened from year to year. The progression rates of both the SARA score and BI were best fitted with a linear model. The total SARA score increased during the 4-year period by an average of 3.5 ± 0.5 points (p < 0.001 for the comparison with baseline) (Fig. 1a), and the BI decreased by an average of 10.3 ± 1.8 points (p < 0.001) (Fig. 1b). The annual progression of the SARA score and BI was 0.8 ± 0.1 and −2.3 ± 0.4 points/year, respectively.
Based on the progression of the SARA score, we calculated sample sizes needed for two-group interventional trials of 1-year duration. Estimated sample sizes per group to achieve 80 and 90% power with effect sizes ranging from 20 to 100% are shown in Figure e-3. In SCA31, a trial with 1112 patients (556 patients per group) would be able to detect a 50% reduction in progression of the SARA score. To identify factors that were independently associated with the ∆SARA score, we applied multiple regression analysis. In SCA31, shorter disease duration was associated with a larger ∆SARA score (−0.187 ± 0.65 per additional ∆SARA score; p = 0.007), but we did not identify any other factors for the decline in the SARA score (Table 3).
Among the subscores of SARA, the subscores for gait (baseline to 4-year visit 4.50 to 5.66 points, p < 0.001), stance (2.77 to 3.46 points, p = 0.001), finger chase (0.90 to 1.31, p < 0.001), fast alternating hand movements (1.53 to 2.21, p < 0.001), and heel-shin slide (2.35 to 2.71, p = 0.031) significantly declined over 4 years. The three other subscores, sitting (0.50 to 0.41), speech disturbance (2.74 to 2.92), and nose-finger test (1.25 to 1.27), showed no significant change over the same period. Table e-1 shows the results of subgroup analysis defined according to disease duration (<10, 10–19, ≥20 years). An annual decline of the SARA score was conspicuous in the subgroup with the shortest disease duration (<10 years). Among the subscores, changes to gait and stance constituted approximately 60% of the total changes in the subgroup with the shortest disease duration. However, this change was not significant when compared with the subgroup with the longest disease duration.
Of 44 patients with SCA31, 13 (29.5%) were confined to a wheelchair at their initial visit (age; 78.7 ± 6.1 years, disease duration; 21.6 ± 11.6 years, mean ± SD). Both their disease duration and age at baseline were different from those of the other 31 patients who were able to walk without a walking aid at their initial visit (age 71.5 ± 8.6 years, disease duration 12.4 ± 7.0 years, mean ± SD). During this 4-year prospective study, 15 of the 31 ambulatory patients (48.4%) became wheelchair dependent. Figure 2a shows the Kaplan-Meier curve for the time at which patients became wheelchair dependent. The estimated mean of age of becoming wheelchair bound was 79.4 ± 1.7 years (95% confidence interval [CI] 76.0–82.8 years) and that of disease duration from onset to becoming wheelchair bound was 19.3 ± 1.5 years (95% CI 16.3–22.2 years).
Survival curves were calculated for overall survival according to the Kaplan-Meier method. Figure 2b shows the survival curve for the age at which patients died during the 4-year study period. The estimated means of disease duration and age at death were 37.9 ± 6.1 years (95% CI 26.0–50.0 years) and 88.5 ± 0.7 years (95% CI 87.0–89.9 years), respectively.
Discussion
To our knowledge, this is the first report to assess a cohort of patients with SCA31 prospectively, providing quantitative evidence for the disease progression of SCA31 with a high follow-up rate. In this study, we completed a follow-up in 42 of 44 patients (95.5%), 37 until the last visit as initially planned and 5 until they died. Unfortunately, two patients dropped out from the study because they were institutionalized to a nursing home during the study period.
At baseline, the clinical features of the patients in this study were consistent with those of previous cross-sectional studies, such as a late-onset and relatively pure cerebellar form of ataxia [1–7]. It was evident that the SARA score at baseline correlated well with both age at baseline and disease duration in SCA31 (Table 2). Further, there was a strong correlation between the SARA score and BI. The decline in the level of the ADLs of the patients started with a SARA score of more than 10 points (Figure e-2). It was clear that disturbance of the ADLs worsened in proportion to the severity of ataxia in the SCA31 patients. The present data showed that SCA31 patients became wheelchair dependent at approximately 80 years of age, indicating that SCA31 patients will need a wheelchair at approximately 20 years after disease onset. The mean life span of SCA31 patients was as long as that of the averaged one of the Japanese population, which was based on the recent statistical report in Japan [26].
In the present study, the SARA score declined by 0.8 points/year, and the annual decline of the SARA score was linear, despite heterogeneity among the patients. The decline of the SARA score in SCA6, the other predominant ADCA subtype of relatively pure cerebellar ataxias in Japan, was reported to range from 0.87 to 1.33 [10–15]. Therefore, we conclude that the disease progression of SCA31 is similar to or slightly slower than that of SCA6.
Our subgroup analysis showed that the annual progression rate of the SARA score decreased with disease duration and that disease progression reached a plateau at approximately 20 years after onset. Yasui et al. have pointed out that the same problem applied to SCA6 patients with severe ataxia (SARA score ≥25 points) [15]. One plausible reason for this is that the SARA score is an ordinal scale limited by ceiling. The SARA score may not be sufficiently sensitive for patients in the advanced stage of the disease. For the second plausible reason, no further active cerebellar degeneration might occur because almost all Purkinje cells have already gone.
The molecular defect in SCA31 is suggested to be an intronic insertion containing abnormally expanded TGGAA repeats in both BEAN and TK2 [8, 9]. Therefore, as in SCA8 and myotonic dystrophy types 1 and 2 [27–29], a pathogenic RNA gain-of-function mechanism or repeat-associated non-ATG translation (RAN translation) mechanism may contribute to neurodegeneration in SCA31. Recently, the development of multiple strategies targeting the toxic RNA is in progress, and some clinical trials based on antisense oligonucleotides are ongoing [30]. Our data suggest that patients with a disease duration less than 10 years may be suitable candidates for clinical trials in SCA31, because an annual decline in the SARA subscores for gait and stance is clearly detected in patients at this stage. Even at this stage, however, the decline of the subscores for gait and stance was 0.61 (2.43/4) and 0.24 (0.96/4) points/year, respectively. Because of the subtle decline of the SARA score, we need a large number of patients to confirm the efficacy of interventional trials for SCA31 using SARA. Therefore, more sensitive and objective methods to assess the severity of gait and stance (for example, a triaxial accelerometer) is needed [31, 32].
For the study of the natural history of SCA31, we have to take some limitations into consideration. Firstly, the ages of the patients enrolled in this study were much older than those in other ADCA subtypes reported previously [6, 10–15]. Therefore, SCA31 patients are more likely to be complicated with other common diseases or age-dependent sarcopenia-frail syndrome. Actually, 25% of the patients needed to be hospitalized due to factors other than cerebellar ataxia in this study. Secondly, disease onset is quite insidious and sometimes difficult to recognize in SCA31, especially in late-onset patients. We were very careful with these factors when we enrolled the patients, but it is possible that they still influenced the SARA score and BI. Patient selection bias that SCA31 patients enrolled in this study were only from the limited area of Japan may not have a significant problem because almost all Japanese patients with SCA31 are known to be homogeneous genetically and phenotypically and share a similar lifestyle [1–8]. However, it is still unclear whether our longitudinal data can be applicable to SCA31 patients outside of Japan.
Conclusion
Our prospective cohort study confirmed a protracted clinical course and long life span in patients with SCA31. The average clinical course of SCA31 patients is as follows: they first notice ataxic symptoms at 58.5 ± 10.3 years of age (mean ± SD), become wheelchair bound at 79.4 ± 1.7 years of age (mean ± SE), and finally die at 88.5 ± 0.7 years of age (mean ± SE). The present findings will help us not only conduct clinical trials for SCA31 patients but also inform SCA31 patients and their family members of disease prognosis.
References
Ouyang Y, Sakoe K, Shimazaki H, Namekawa M, Ogawa T, Ando Y, et al. 16q-linked autosomal dominant cerebellar ataxia: a clinical and genetic study. J Neurol Sci. 2006;247:180–6.
Onodera Y, Aoki M, Mizuno H, Warita H, Shiga Y, Itoyama Y. Clinical features of chromosome 16q22.1 linked autosomal dominant cerebellar ataxia in Japanese. Neurology. 2006;67:1300–2.
Nozaki H, Ikeuchi T, Kawakami A, Kimura A, Koide R, Tsuchiya M, et al. Clinical and genetic characterizations of 16q-linked autosomal dominant spinocerebellar ataxia (AD-SCA) and frequency analysis of AD-SCA in the Japanese population. Mov Disord. 2007;22:857–62.
Basri R, Yabe I, Soma H, Sasaki H. Spectrum and prevalence of autosomal dominant spinocerebellar ataxia in Hokkaido, the northern island of Japan: a study of 113 Japanese families. J Hum Genet. 2007;52:848–55.
Hayashi M, Adachi Y, Mori M, Nakano T, Nakashima K. Clinical and genetic epidemiological study of 16q22.1-linked autosomal dominant cerebellar ataxia in western Japan. Acta Neurol Scand. 2007;116:123–7.
Yoshida K, Shimizu Y, Morita H, Okano T, Sakai H, Ohata T, et al. Severity and progression rate of cerebellar ataxia in 16q-linked autosomal dominant cerebellar ataxia (16q-ADCA) in the endemic Nagano area of Japan. Cerebellum. 2009;8:46–51.
Sakai H, Yoshida K, Shimizu Y, Morita H, Ikeda S, Matsumoto N. Analysis of an insertion mutation in a cohort of 94 patients with spinocerebellar ataxia type 31 from Nagano, Japan. Neurogenetics. 2010;11:409–15.
Sato N, Amino T, Kobayashi K, Asakawa S, Ishiguro T, Tsunemi T, et al. Spinocerebellar ataxia type 31 is associated with “inserted” penta-nucleotide repeats containing (TGGAA)n. Am J Hum Genet. 2009;85:544–57.
Niimi Y, Takahashi M, Sugawara E, Umeda S, Obayashi M, Sato N, et al. Abnormal RNA structures (RNA foci) containing a penta-nucleotide repeat (UGGAA)n in the Purkinje cell nucleus is associated with spinocerebellar ataxia type 31 pathogenesis. Neuropathology. 2013;33:600–11.
Jacobi H, du Montcel ST, Bauer P, Giunti P, Cook A, Labrum R, et al. Long-term disease progression in spinocerebellar ataxia types 1, 2, 3, and 6: a longitudinal cohort study. Lancet Neurol. 2015;14:1101–8.
Ashizawa T, Figueroa KP, Perlman SL, Gomez CM, Wilmot GR, Schmahmann JD, et al. Clinical characteristics of patients with spinocerebellar ataxias 1, 2, 3 and 6 in the US; a prospective observational study. Orphanet J Rare Dis. 2013;8:177.
Velázquez-Pérez L, Rodríguez-Labrada R, Canales-Ochoa N, Montero JM, Sánchez-Cruz G, Aguilera-Rodríguez R, et al. Progression of early features of spinocerebellar ataxia type 2 in individuals at risk: a longitudinal study. Lancet Neurol. 2014;13:482–9.
Tezenas du Montcel S, Charles P, Goizet C, Marelli C, Ribai P, Vincitorio C, et al. Factors influencing disease progression in autosomal dominant cerebellar ataxia and spastic paraplegia. Arch Neurol. 2012;69:500–8.
Lee YC, Liao YC, Wang PS, Lee IH, Lin KP, Soong BW. Comparison of cerebellar ataxias: a three-year prospective longitudinal assessment. Mov Disord. 2011;26:2081–7.
Yasui K, Yabe I, Yoshida K, Kanai K, Arai K, Ito M, et al. A 3-year cohort study of the natural history of spinocerebellar ataxia type 6 in Japan. Orphanet J Rare Dis. 2014;9:118.
Shintaku M, Kaneda D. Chromosome 16q-22.1-linked autosomal dominant cerebellar ataxia: an autopsy case report with some new observations on cerebellar pathology. Neuropathology. 2009;29:285–92.
Ishikawa K, Mizusawa H. The chromosome 16q-linked autosomal dominant cerebellar ataxia (16q-ADCA*): a newly identified degenerative ataxia in Japan showing peculiar morphological changes of the Purkinje cell. Neuropathology. 2010;30:490–4.
Yoshida K, Asakawa M, Suzuki-Kouyama E, Tabata K, Shintaku M, Ikeda S, Oyanagi K. Distinctive features of degenerating Purkinje cells in spinocerebellar ataxia type 31. Neuropathology. 2014;34:261–7.
Tada M, Nishizawa M, Onodera O. Redefining cerebellar ataxia in degenerative ataxias: lessons from recent research on cerebellar systems. J Neurol Neurosurg Psychiatry. 2015;86:922–8.
Ishikawa K, Dürr A, Klopstock T, Müller S, De Toffol B, Vidailhet M, et al. Pentanucleotide repeats at the spinocerebellar ataxia type 31 (SCA31) locus in Caucasians. Neurology. 2011;77:1853–5.
Lee YC, Liu CS, Lee TY, Lo YC, Lu YC, Soong BW. SCA31 is rare in the Chinese population on Taiwan. Neurobiol Aging. 2012;33:426. e423–4.
Pedroso JL, Abrahao A, Ishikawa K, Raskin S, de Souza PV, de Rezende Pinto WB, et al. When should we test patients with familial ataxias for SCA31? A misdiagnosed condition outside Japan? J Neurol Sci. 2015;355:206–8.
Schmitz-Hübsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, et al. Scale for the assessment and rating of ataxia—development of a new clinical scale. Neurology. 2006;66:1717–20.
Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61–5.
Jacobi H, Rakowicz M, Rola R, Fancellu R, Mariotti C, Charles P, et al. Inventory of non-ataxia signs (INAS): validation of a new clinical assessment instrument. Cerebellum. 2013;12:418–28.
Ministry of Health, Labour and Welfare. Japan demographics profile. 2014. (online) Available at: http://www.mhlw.go.jp/toukei/saikin/hw/life/life14/. Accessed 22 Feb 2016.
Koob MD, Moseley ML, Schut LJ, Benzow KA, Bird TD, Day JW, et al. An untranslated CTG expansion causes a novel form of spinocerebellar ataxia (SCA8). Nat Genet. 1999;21:379–84.
Harley HG, Brook JD, Rundle SA, Crow S, Reardon W, Buckler AJ, et al. Expansion of an unstable DNA region and phenotypic variation in myotonic dystrophy. Nature. 1992;355:545–6.
Liquori CL, Ricker K, Moseley ML, Jacobsen JF, Kress W, Naylor SL, et al. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science. 2001;293:864–7.
Bernat V, Disney MD. RNA structures as mediators of neurological diseases and as drug targets. Neuron. 2015;87:28–46.
Matsushima A, Yoshida K, Genno H, Murata A, Matsuzawa S, Nakamura K, et al. Clinical assessment of standing and gait in ataxic patients using a triaxial accelerometer. Cerebellum Ataxias. 2015;2:9.
Shirai S, Yabe I, Matsushima M, Ito YM, Yoneyama M, Sasaki H, et al. Quantitative evaluation of gait ataxia by accelerometers. J Neurol Sci. 2015;358:253–8.
Acknowledgements
The authors thank Drs. Jun Miki, Kazuma Kaneko, Akiyo Hineno, Daigo Miyazaki, Chinatsu Kobayashi, Ken Takasone, Kazuki Ozawa, Michiaki Kinoshita, and Ryuta Abe for their contribution to the inclusion of the patients in this study. The authors thank Ms. Emi Nomura and Ms. Sonomi Nagasaki for their technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Source of Funding
This study was supported in part by a grant from the Research Committee for Ataxic Diseases, the Ministry of Health, Labor, and Welfare, Japan (K. Yoshida).
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Figure e-1
Flowchart for the annual follow-up of patients with SCA31. (JPEG 47 kb)
Figure e-2
Correlation between the total SARA score and BI in patients with SCA31 (n = 205 follow-ups). (JPEG 35 kb)
Figure e-3
Required sample size per group in two-group interventional trials of 1-year duration in SCA31 for various effect sizes. (JPEG 36 kb)
Table e-1
Change in the SARA subscores from baseline to Visit 5. (DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Nakamura, K., Yoshida, K., Matsushima, A. et al. Natural History of Spinocerebellar Ataxia Type 31: a 4-Year Prospective Study. Cerebellum 16, 518–524 (2017). https://doi.org/10.1007/s12311-016-0833-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-016-0833-6