Abstract
For many decades, the predominant view in the cerebellar field has been that the olivocerebellar system’s primary function is to induce plasticity in the cerebellar cortex, specifically, at the parallel fiber-Purkinje cell synapse. However, it has also long been proposed that the olivocerebellar system participates directly in motor control by helping to shape ongoing motor commands being issued by the cerebellum. Evidence consistent with both hypotheses exists; however, they are often investigated as mutually exclusive alternatives. In contrast, here, we take the perspective that the olivocerebellar system can contribute to both the motor learning and motor control functions of the cerebellum and might also play a role in development. We then consider the potential problems and benefits of it having multiple functions. Moreover, we discuss how its distinctive characteristics (e.g., low firing rates, synchronization, and variable complex spike waveforms) make it more or less suitable for one or the other of these functions, and why having multiple functions makes sense from an evolutionary perspective. We did not attempt to reach a consensus on the specific role(s) the olivocerebellar system plays in different types of movements, as that will ultimately be determined experimentally; however, collectively, the various contributions highlight the flexibility of the olivocerebellar system, and thereby suggest that it has the potential to act in both the motor learning and motor control functions of the cerebellum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The cerebellum is one of the key brain regions involved in motor coordination. To perform its role(s), the cerebellum receives information from two main sets of afferents, the mossy fiber and climbing (olivocerebellar) systems. The predominant view over the past several decades has been that under most circumstances, mossy fiber, and not olivocerebellar, activity is responsible for shaping the ongoing outflow from the cerebellum (i.e., for generating motor commands). In contrast, olivocerebellar activity has been proposed to serve primarily a motor learning function; specifically, it is hypothesized to gate synaptic plasticity such that future instances of the ongoing motor command are modified so that any movement errors resulting from the current command will have been eliminated. However, the olivocerebellar system has also been proposed to be directly involved in generating ongoing motor commands, based in part on its ability to generate synchronous activity [1].
Historically, studies have tended to focus on only one or the other of these roles. Indeed, the motor learning and motor control roles have often been considered as mutually exclusive, or at least that the olivocerebellar system’s role in one or the other function is not of major significance (e.g., see [2, 3]). Nevertheless, as expanded upon below, there is evidence consistent with the olivocerebellar system having significant roles both in modulating synaptic plasticity and in directly influencing ongoing cerebellar output. Here, we explore the possibility of the olivocerebellar system playing a significant role in both functions by asking how the distinctive organization of the olivocerebellar system would allow such dual functionality, and what would be the potential benefits and difficulties of having this system contribute to both motor learning and motor control.
The idea that olivocerebellar activity relates to motor learning processes and that the actual motor commands are driven by mossy fiber activity stems largely from the proposals of Marr [4] and Albus [5], which were based on some of the marked differences in the anatomical and physiological characteristics of these two afferent systems. Of particular importance was the contrast between the enormous convergence and divergence in the mossy fiber-granule cell-Purkinje cell pathway, which suggests that each Purkinje cell is influenced by many mossy fibers but only weakly so by any given one, with the singular and massive climbing fiber input to each Purkinje cell. The much higher average simple spike rates displayed by Purkinje cells, and their greater range of modulation by mossy fiber-driven activity, compared to complex spikes, which average only ~1 Hz and rarely exceed 2–3 Hz, under physiological conditions, have also been used as arguments against the olivocerebellar system having a significant direct contribution to motor commands [2, 6].
However, it is worth noting here that both Marr and Albus actually assumed that olivocerebellar activity could significantly alter the ongoing output of the cerebellar nuclei in addition to triggering changes in synaptic strengths [4, 5]. In Marr’s formulation, it was the complex spike itself that affected cerebellar nuclear output, whereas Albus focused on the effect of the pause in simple spikes that follow each complex spike, because of the discovery of the inhibitory nature of the Purkinje cell. Nevertheless, even though the motor learning hypotheses as originally formulated did allow for olivocerebellar activity to shape ongoing motor commands, the clear implication was that this function would have reduced significance as each motor act is associated with additional contexts through the learning process. Thus, ultimately, they imply that the mossy fiber and olivocerebellar systems will primarily function in two separate domains, ongoing motor control and motor learning, respectively.
Experimental findings, however, have increasingly suggested that a functional dichotomy between the two cerebellar afferent systems is not necessarily correct. Results have shown the existence of a greater diversity of the types of synaptic plasticity than envisioned by the original motor learning theories, not all of which are driven by olivocerebellar activity (for review, see [7, 8]). Moreover, olivocerebellar activity can modulate plasticity within the cerebellar nuclei [9], where the olivocerebellar axon collaterals end in typical synaptic arrangements rather than the specialized climbing fiber termination onto the Purkinje cell. This diversity suggests that modulation of synaptic plasticity is not the exclusive province of the olivocerebellar system, and in particular, that the special synaptic arrangement of the climbing fiber and Purkinje cell is not required for mediating all forms of synaptic plasticity. Moreover, recent work has shown that motor error-related information, originally conceived of as being carried by the olivocerebellar system, is also present in simple spike activity, and thus conveyed to the cerebellum via the mossy fiber system as well [10]. In sum, such results do not deny that the olivocerebellar system has a role in motor learning, but they do weaken the rationale for believing its anatomical and physiological characteristics are specialized for it to serve only a motor learning function. The sections by Ebner and Popa, by Reeves and Otis, and Jaeger expand on these issues.
Moreover, as mentioned earlier, evidence indicates that the olivocerebellar system has a significant direct role in generating ongoing motor commands, irrespective of any role it plays in motor learning. Lesions of the olivocerebellar system produce significant lasting motor coordination deficits that are similar to those following direct damage to the cerebellum itself [11–16]. One caveat is that the deficits observed after olivary lesions may reflect the direct loss of olivocerebellar activity or the alteration of spontaneous Purkinje cell simple spike activity that follows such lesions [17–22]. Nevertheless, the lesion results do suggest that normal ongoing functioning of the cerebellum requires an intact olivocerebellar system (i.e., olivocerebellar activity is not simply modulating plasticity).
Furthermore, the olivocerebellar system can dynamically form large ensembles of Purkinje cells whose complex spike activity is synchronized [23–26], and this ability has been suggested as a mechanism by which olivocerebellar activity can significantly influence ongoing motor commands [1], obviating the argument that the low complex spike firing rate makes it a priori unsuitable for controlling movement. Experimental support consistent with this idea comes from studies showing a correlation of synchronous complex spike activity with movement [26–31]. Significant effects of complex spike activity on cerebellar nuclear activity have also been shown [32–34], further raising the possibility that complex spike activity can have a major effect on cerebellar output. Finally, it is worth noting that synchronous olivocerebellar activity may also drive plasticity in the nuclei [35], further blurring the separation of motor learning and coordination functions of this system.
Such results immediately raise the question of how olivocerebellar activity can contribute significantly to the ongoing output of the cerebellum, given the much higher firing rates resulting from the latter system. In fact, several possible mechanisms by which olivocerebellar activity can exert a powerful influence despite its low average firing rate exist. These include synchronization, the burst nature of the complex spike, and complex spike-associated influences on simple spike activity. The roles these various mechanisms may play in increasing the influence of olivocerebellar activity on cerebellar output are discussed in the sections by Bengtsson and Jorntell, Heck, Jaeger, Cerminara et al., and Lang.
Finally, the possibility that the olivocerebellar system contributes to both motor learning and motor control brings with it both potential problems and benefits. Among the potential difficulties is the issue of how and whether olivocerebellar activity can be selectively directed toward one or the other of these functions. The sections by Schweighofer, Lang, and Kawato, and by Lang address this issue and suggest possible solutions based on the distinctive characteristics of complex spike synchrony and waveform. Finally, given that the motor plant of animals has ever changing properties that reflect its history, both long and short term, the contribution by De Zeeuw and Ozyildirim gues that an adaptable control system may not only be beneficial, but necessary for optimal motor coordination, and that evolution has efficiently combined both functions in the same output structure, i.e., the olivocerebellar system.
In sum, the sections below take as a starting point the possibility that the olivocerebellar system contributes in multiple ways to the motor control function of the cerebellum, to both generating ongoing motor commands and to adapting the system to optimize future motor performance. They then explore how the distinct characteristics of olivocerebellar activity may make this possible.
Do Purkinje Cell Simple Spikes Encode Motor Errors Better Than Complex Spikes? (T.J. Ebner, L.S. Popa)
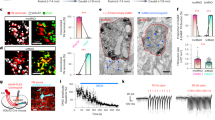
Error processing is essential for online control of movements and motor adaptation (for review see [36, 37]). Early motor control theories emphasized closed-loop control in which the ongoing motor commands are updated continuously by sensory feedback; however, closed-loop control relying on delayed feedback is inadequate and even unstable [36, 37]. Forward internal models provide a solution to this problem by predicting the sensory consequences of a motor command (see Fig. 1a) and extensive evidence suggests the CNS implements forward internal models. The internal predictions are compared with actual sensory feedback to compute sensory prediction errors that are used to control movements and drive learning (Fig. 1a).
a Schematic of motor control based on a forward internal model and sensory prediction errors. b Example of SS modulation with position errors, XE and YE. The firing rate is color coded relative to overall mean firing and in relation to the target (white circle). c, d Example temporal R 2 (c) and regression coefficient profiles (d) as a function of lead/lag (τ) for an individual error parameter (XE) from a single Purkinje cell. Error bars in d represent the confidence intervals at the times of the R 2 maxima in c. Note the reverse in sign of regression coefficients from lead to lag. e Decoding accuracy of the SSs to predict the upcoming XE from a population of Purkinje cells based on the feed-forward modulation
Traditionally, it has been assumed that the cerebellum detects and corrects for movement errors [38] and, more recently, processes sensory prediction errors [36]. The dominant hypothesis, incorporated into many models of cerebellar synaptic plasticity and learning (for review see [8]), is that the error signals are encoded exclusively by the complex spike (CS) discharge of Purkinje cells [2]. For example, this view informs the sections by Reeves and Otis and Schweighofer and Kawato. Support for this hypothesis is the CS modulation observed during eye movements in relation to retinal slip, smooth pursuit adaptation, and induced saccade errors [39–43]. During reaching, CSs are evoked by end point errors, redirection, unexpected loads, and adaptation to visuomotor transformations (for review, see [44]). However, numerous other studies found no clear relationship between motor errors and CS activity or the discharge of neurons in the inferior olive, the origin of the climbing fiber projection, either during eye or limb movements (see [45]). A very recent study of adaptation of reaching movements to a mechanical perturbation demonstrated that the perturbation evoked a CS response in a very small percentage of Purkinje cells while the simple spike (SS) firing adapted in a majority of the neurons [46]. Also, new findings show that climbing fiber activation occurs and is correlated with changes in SS firing during increases in vestibulo-ocular reflex (VOR) gain, but climbing fiber activation does not play a role in modifying SS firing during decreases in VOR gain [47, 48]. Although CSs have been strongly implicated in parallel fiber-Purkinje cell synaptic plasticity as proposed in the Marr-Albus-Ito hypothesis [2], both long-term depression and potentiation can be evoked by parallel fiber stimulation alone [49–52].
These conflicting observations and the inherent low bandwidth of the CS discharge, warrant a fresh perspective on whether error signaling in the cerebellum involves the SS discharge. Until recently, there was very limited support for the presence of error signals in the SS activity. For example, during circular tracking, SS discharge is correlated with direction and speed errors [53]; however, the interpretation was confounded by the lack of statistical independence between error and kinematic parameters. Instructive signals independent of CS activity contribute to cerebellar-dependent learning in the VOR [54], also suggesting the presence of error signals in the SS discharge.
Our recent work demonstrates that SS firing encodes performance errors during a manual task in which monkeys are required to track an unpredictable target [10, 55]. Performance errors were quantified by four measures based on cursor movement relative to target center, including position (XE, YE), distance (i.e., RE), and direction (i.e., PDE) errors. The properties of the SS firing in relation to error parameters revealed several features consistent with sensory prediction errors and a forward internal model (Fig. 1b–e) [10]. First, the SS discharge is robustly modulated with the four error parameters, independent of each other and of kinematic modulation. Second, the correlation of SS firing with an individual error parameter exhibits a bimodal temporal R 2 profile, with maxima at both predictive and feedback timing (e.g., XE in Fig. 1c). The bimodal profiles suggest that individual cells process both predictive and feedback information about an error parameter. Third, the regression coefficients for the predictive and feedback maxima reverse sign (Fig. 1d). Therefore, the predictive and feedback representations of the same error parameter counter each other, one increasing and the other decreasing the SS firing (Fig. 1d). These opposing SS modulations are precisely the signals required to compute the sensory prediction errors as the difference between a prediction of the motor command consequences and the sensory feedback (Fig. 1a). Fourth, decoding analyses confirm the SS discharge conveys highly accurate predictions of the upcoming errors (Fig. 1e), consistent with the output of a forward internal model [10, 56].
Simple spikes encode a rich repertoire of error signals needed to accurately track the target. Importantly, the findings go against the dominant view that only CS discharge represents motor errors. Decoding demonstrates the exquisite quality of these SS error signals in the population of Purkinje cells. To our knowledge, no other study has demonstrated similar accuracy of decoding errors by CSs. Nor have CSs been demonstrated to be predictive of upcoming errors. Further, the dual temporal coding of the SSs is consistent with sensory prediction error signals required for motor learning. Given these new findings on SS error signals, it may be time to rethink the role of CSs. Major alternative theories of CS function are that the olivocerebellar system is central to controlling motor timing [3] or acts to initiate intracellular signaling mechanisms controlling synaptic plasticity [2, 8].
Intriguingly, recent experiments show that local activation of Purkinje cells also triggers a delayed CS response mediated by a bi-synaptic inhibitory projection to inferior olive, suggesting that CS activity is strongly modulated by cerebellar cortical output [57–59]. It is possible that rather than only encoding motor errors, CS activity reflects integration between behavioral signals and information on the local level of activity.
In summary, our results show that the SS firing of Purkinje cells encodes rich and highly accurate representations of performance errors, both predictions and feedback. Clearly, CSs are not the sole provider of error signaling in the cerebellar cortex, and the SS discharge is a strong contender for higher quality error encoding. The challenge is to integrate this contrarian view with more long-held postulates of error signaling in the cerebellar cortex.
Contribution of Climbing Fibers to Cerebellar Cortex and Cerebellar Nuclei Activities (A.M.B. Reeves, T.S. Otis)
Extensive evidence suggests that climbing fibers (CFs) play a pivotal role in cerebellar-dependent forms of associative learning due to changes in circuit function driven by CF activity [41, 42, 60–62]. These findings raise a number of critical questions. What patterns of CF activity lead to learning? Which circuit elements do CFs alter? Are these alterations dependent on the stage or circumstance of learning?
Based on our own recent published [22, 63, 64] and unpublished work, as well as published work from many other labs [65–69], we favor the “trigger and storage” hypothesis of cerebellar learning [70]. It posits that CFs trigger plasticity at distinct sites within the cerebellar cortex and cerebellar nuclei in separate stages: a rapid plasticity in the cortex followed by a slower plasticity in the nuclear cells driven by the changes that have occurred in the cortex. The following section summarizes evidence and ideas pertaining to the role of error-associated CF activity in the trigger and storage hypothesis of cerebellar learning.
Originally, the trigger and storage hypothesis explained the mechanics of consolidation for eyeblink learning [71]. Since then, the hypothesis has expanded to include other forms of cerebellar-dependent learning [66, 68, 72]. In all of these forms of learning, the Purkinje cell (PC) and CF play central roles, although the mechanisms involved in the initial plasticity and consolidation remain incompletely understood.
The “trigger and storage” hypothesis treats the cerebellum as an error-correcting machine, where the CF is a source for error information. Errors can be viewed as arising from a difference between expected and actual outcome of a sensory prediction or motor command, as unexpected events that pertain to poorly calibrated sensorimotor function, or simply as negative sensory events to be avoided [73–75]. For example, retinal slip, corneal airpuffs, and periorbital stimulation are maladaptive or aversive sensory stimuli that in associative learning paradigms, the animal learns to anticipate and avoid.
Such errors evoke CF activity which is conveyed to the PCs as a complex spike—a salient, cell-wide signal—increasing calcium throughout the PC dendritic tree and cell soma [76, 77]. Since we can record a CF’s activity in the post-synaptic PC, we can study its effects on PC excitability. Evoked CF activity differs from spontaneous CF activity in its firing rate, population activity [23, 25–27, 30, 57, 78, 79], and capacity for altering circuit function [41, 42, 60–62].
CFs can drive associative decreases in PC firing [41, 60, 80]. Several studies have established correlational relationships of CF activity to long-term changes in PC firing. Some of the best evidence comes from studies of decerebrate ferret in which co-activation of CF and mossy fiber (MF) input gradually leads to CS-evoked PC pauses in firing [60]. Lisberger and colleagues have developed a smooth pursuit learning task in which the occurrence of a complex spike on one trial led to significant decreases in PC firing on the subsequent trial [41], and recent work using this paradigm demonstrates that the strength of complex spikes shows slight gradation and this is correlated with the magnitude of trial by trial learning [42]. Strikingly, this “analog teaching signal” must be correlated at a population level because the strength of behavioral learning can be predicted based on recordings from a single PC. These and other findings are consistent with a unique effect of evoked, population CF activity to drive circuit changes in cerebellar cortex. Such associative decreases in PC firing are hypothesized to drive increases in nuclear cell activity, allowing the cerebellum to exert control over descending motor pathways.
Once learning has occurred, the expression of learned pauses in PC activity requires MF activity but not CF activity. Typically, MFs convey external stimuli like auditory or visual cues to evoke learned pauses in PCs. However, it is conceivable that internal activity, replay patterns of MF activity that occurred during conditioning, could later drive learned pauses and promote consolidation to the cerebellar nuclei (CN). Support for this idea is provided by a study done on human participants in which blood oxygen level-dependent (BOLD) signals were measured during rest periods in between bouts of motor training [81]. Resting state activity in fronto-parietal and cerebellar networks were significantly elevated after motor learning but not after sham learning (i.e., motor performance without training). This suggests that motor learning, but not motor performance, specifically alters a cerebellar resting state network which then remains active offline.
Such activity is a candidate mechanism for replay-mediated consolidation in the cerebellum. Models of cerebellum energy use suggest that BOLD signals are chiefly the result of activity in the granule cells [82]. Thus, elevated BOLD signals during resting state may indicate self-generated replay of task-relevant granule cell activity. Replay would elicit learned pauses in PCs in the absence of external cues and promote transfer of motor memories from PC to the CN. Consistent with this idea, lesioning or inactivating cerebellar cortex shortly after motor training disrupts consolidation of motor memories [68]. More work remains to be done in order to better understand the role of learned PC pauses in motor memory consolidation.
What are the candidate circuit mechanisms underlying such CF-driven, learned reductions in PC firing? Parallel fiber long-term depression (PF LTD) is one proposed mechanism for associative decreases in PC firing [83–85]; however, the necessity of this form of plasticity in associative learning is under debate [86, 87]. In addition to PF LTD, some of the original theories of cerebellar function posited other sites of plasticity in cerebellar cortex [5], suggesting that CFs could drive LTP of PF inputs to molecular layer interneurons. Evidence in support of this mechanism is indirect. In vivo recordings show that CF stimulation leads to a strong increase in inhibitory receptive fields in PCs [61], and genetic deletion of GABAA receptors from PCs leads to deficits in memory consolidation in associative learning tasks [72].
The trigger and storage hypothesis predicts circuit changes downstream of the PC in the CN [70, 88, 89] and there is considerable evidence supporting the proposal that learning-related plasticity occurs in the CN [65, 90, 91]. Does the CF play a pivotal role in instructing learning-related plasticity in the CN?
Perhaps. In addition to the learned reductions in PC firing discussed above, the CF can elicit acute, non-associative decreases in PC firing, termed post-complex spike pauses, that could in principle modulate CN activity and drive plasticity [35]. Even if there is no overt pause (i.e., an increase in inter-spike interval beyond the pause duration predicted by the average baseline ISI), complex spikes reset the period of simple spike firing in PCs. Thus, given error-associated synchronous CF input to functional microzones, there will be a synchronous pause that could drive CN excitability.
It is in this context that we interpreted experiments indicating that pharmacological prolongation of the post-complex spike pause enhances rate of eyeblink acquisition but not extinction [63]. More recent evidence has suggested that post-complex spike pauses are regulated in an activity-dependent manner [92], which could affect the rate of CN plasticity. These findings support the idea that post-complex spike pauses train circuit changes in the CN by selectively enhancing plasticity at MF to CN synapses. In this mechanism, the PC is less of a trigger cell and more a mouthpiece for CF instructions, providing a pathway for the error information to reach the CN. Thus, both PC and CN plasticity could occur simultaneously but at different rates [71].
CFs convey errors to cerebellar cortex, but it is unknown whether the inferior olive or some upstream structure actually computes the error. Pharmacologically blocking synaptic inhibition of the inferior olive prevents extinction; conversely, pharmacologically blocking synaptic excitation of the inferior olive initiates extinction [71]. Importantly, these conditions maintain spontaneous CF activity suggesting that only evoked CF output serves as an acquisition signal, and that perhaps spontaneous CF activity can serve as an extinction signal. Recent findings indicate that projections from the CN inhibit gap junction coupling between IO neurons as well as their individual intrinsic oscillations [93]. This would prevent spatiotemporal synchrony among CFs within a single microzone as well as affect spontaneous PC firing rates.
One corollary of the trigger and storage hypothesis of cerebellar learning is that CF error signals do not alter nuclear synapses. Instead, errors adjust cortical synapses until the animal learns to avoid the error via disinhibition of its cerebellar nuclei. Successful patterns of nuclear disinhibition then consolidate by altering the strength of MF collaterals to the CN. Either acute or learned pauses in PC activity could drive MF-CN plasticity; however, we favor the notion that CF-instructed learned pauses in PC firing drive CN plasticity.
To summarize, in the initial stage of associative learning, error-associated population activity in CFs leads to learned pauses in PC firing in response to the conditioned sensory stimulus. Climbing fiber error signals may also be relayed to the CN via acute actions on PCs, which could instruct changes in PC and CN excitability to occur simultaneously, albeit at different rates. During consolidation, motor memories induced as pauses in PCs can then be transferred to the CN in a CF-independent, serial manner via externally or internally evoked PC pauses instructing LTP of collateral MF inputs to CN neurons.
The Mystery of Olivary Input to the Cerebellar Nuclei (D. Jaeger)
There is clear evidence for a direct pathway from the inferior olive to the cerebellar nuclei, which is formed by collaterals of climbing fibers projecting to the cerebellar cortex [94–96]. Specific distinguishing characteristics of the synaptic ultrastructure or postsynaptic receptors of the olivary nuclear synapses have not been determined, but in comparison to mossy fiber synapses, they are sparse and show a predominant termination on distal dendrites [97].
The electrophysiological effect of olivary inputs to CN neurons has been hard to examine with traditional methods because electrical stimulation in brain slices in sites where this projection can be activated may easily cause co-activation of mossy fibers. Even in vivo electrical stimulation of the olive does not fully circumvent this problem, as axons projecting to the olive may be stimulated that also end up as mossy fibers in the CN. Nevertheless, several studies have carried out such olivary electrical stimulation in cats [98–100], rats [101], and mice [102]. The results consistently show a subpopulation of CN neurons with a short-latency excitatory response with a broader population of CN neurons showing a pronounced longer latency inhibition, which is due to Purkinje cell input following climbing fiber activation. The subpopulation of early excitation in mice in recent study was found to be 31 of 66 units [102], and such excitation is characterized by a well-timed single spike in vivo.
To circumvent the possible contamination of mossy fiber activation, a very elegant approach was pursued by Blenkinsop and Lang in which simultaneous dual recordings from CF responses in Purkinje cells and CN neurons were used to study the direct excitatory input to CN neurons correlated with spontaneous CF activation of PCs. Out of 100 positive complex spike-CN unit response correlations, they found purely inhibitory CN responses in 70 cases, short-latency excitation followed by inhibition in 24 cases, and weak short-latency excitation alone in 6 cases. New optogenetic methods have very recently been tested for a direct olivary fiber activation of CN neurons in a transgenic strain with Channelrhodopsin-2 expression in the olive [103]. Consistent with the previous lines of evidence, only infrequent excitatory responses were found, which in slice recordings were determined to consist of relatively small EPSPs seen in 5 of 21 recordings. In contrast, large inhibitory responses were observed in the CN following optogenetic olivary stimulation in vivo. Therefore, the overall electrophysiological results point to relatively weak direct excitatory responses of olivary input in CN neurons that are dominantly overridden by climbing fiber-elicited Purkinje cell inhibition.
What then could the functional significance of these relatively weak direct excitatory olivo-nuclear inputs be? At this point, all answers to this question are highly speculative, but two interesting hypotheses offer themselves for further study. First, this system could be stronger early during early postnatal life and be a vital component to align cerebellar-olivo-nuclear microzones during development. Published studies indicate that an impressive alignment of inputs from the olive to the Purkinje cells, Purkinje cells to the CN, and feedback back from the CN to the olive exists in adult animals [94, 104]. In fact the study of Blenkinsop and Lang would not have been possible without the common occurrence of convergent input from Purkinje cells driven by the same climbing fibers that also project to the CN neurons receiving output from these Purkinje cells, which is quite remarkable. Such convergence could have been stabilized and pruned from a wider projection pattern through synapse elimination as observed in the cerebellar nuclei [105] through correlation-based plasticity rules.
A second and not mutually exclusive hypothesis is that the olivo-nuclear connection is involved in functional plasticity in adult life that may play a role in motor learning. Interestingly, plasticity rules governing LTP of Purkinje cell input in the CN have been described to be dependent on preceding excitatory input [106, 107]. While this excitatory input may also be from mossy fibers, the strong temporal correlation between olivary input to the CN and subsequent Purkinje cell inhibition seems ideally suited for this type of plasticity and would promote strengthening input from Purkinje cells that may have received themselves a training signal via climbing fibers, possibly related to motor errors.
In conclusion, while the direct olivo-nuclear connection has not been incorporated in most concepts about cerebellar function, future studies may yet reveal an important role of this pathway in developmental or functional plasticity mechanisms, but identifying experimental procedures that could isolate such a role remain highly challenging.
Generation of Motor Regulatory Signals in CN Neurons (F. Bengtsson, H. Jörntell)
The organization of the olivo-cerebellar projection forms the basis of the microzones [108] and combined with the organization in the cortico-nuclear projection, it forms the basis of the cerebellar microcomplex [109]. The microzone can be mapped out as a longitudinal strip of Purkinje cells (PCs) that receive similar afferent input through the climbing fiber (CF) pathway and in turn project to a specific set of cells in the cerebellar nuclei (CN) [110]. In addition to the indirect input from the mossy fibers (MFs) and CFs via the PCs, the CN cells receive direct input through MF and CF collaterals [111]. As the approximately 200–600 PCs of a microzone converge onto a common group of neurons in the CN [33], the microcomplex can be argued to be the smallest functional unit of the cerebellum. The output of the CN is conveyed primarily to motor or premotor areas of the cerebral cortex as well as to various motor nuclei in the brainstem (e.g., the red nucleus). However, how the motor control signal, issued by the cerebellum through the CN cells, is generated is still a matter of debate.
We recently studied the effect of direct and indirect MF and CF inputs to the CN cells in in vivo whole cell recordings from the anterior interposed nucleus [33, 112]. We found that the spontaneous synaptic activity of the CN cells primarily consisted of two alternating patterns. Most of the time, the membrane potential was dominated by extremely small unitary IPSPs (<<0.1 mV) driven at very high frequencies (>10 kHz) from the spontaneously active PCs. In addition, we recorded intermittent bursts (8–17 Hz) of giant IPSPs (of peak amplitudes of 3–10 mV) with activation dynamics that were consistent with a CF-driven synchronization of the activation of a large number of PCs [23]. This is consistent with a role of coupled activation between adjacent climbing fibers within a microzone. The giant IPSPs consisted of an initial small EPSP (0.5–1.5 mV) that likely represented the direct CF input to the CN neuron, followed by a large IPSP (3–10 mV). However, despite the substantial inhibition, the spontaneous giant IPSPs never resulted in a postinhibitory rebound response [33].
We also used electrical stimulation to directly activate MF and CF inputs, respectively. Electrical as well as manual stimulation of the cutaneous MF receptive fields of the CN neurons [112] generated substantial excitatory modulations of their membrane potential. Based on the latency times of these responses and the fact that electrical stimulation of a known source of MF-CN synapses evoked monosynaptic EPSPs in these neurons, the responses could be ascribed to the direct excitatory synapses formed by MF collaterals [112]. This input modulated the CN cell activity in an apparently linear fashion, and the firing rate modulations were of similar magnitudes as those observed during behavioral recordings of interpositus neurons [113]. From other parts of the skin, inhibition through the indirect MF activation of PCs was observed.
Electrical activation of a specific subset of cells in the inferior olive (IO), as determined by the limited number of cortical microzones activated, resulted in a large IPSP with the same temporal topography as the spontaneous giant IPSPs. However, the magnitude of a full IO-evoked IPSP was about twice as large (up to 20 mV) as the largest spontaneous giant IPSPs, and full IO-evoked IPSPs were, as a rule, followed by a postinhibitory rebound response [33]. They were defined as full responses as they represented saturated responses (i.e., an increase in the stimulation intensity in the IO did not result in a corresponding increase of the IPSP amplitude). In contrast, submaximal activation through the IO did not result in a rebound response. This suggested that a prerequisite for rebound responses in vivo (under non-anesthetized conditions) is that there is a synchronous activation of essentially all olivary cells projecting to the microzone(s) that innervate the CN neuron.
Rebound responses have previously been recorded in numerous in vitro studies [114–117]. However, here, the conditions differ from those in vivo, for example the tonic PC inhibition of the CN cells is removed, which probably alters the activation properties of the conductances. Rebound responses have previously also been reported in vivo [102, 118, 119], but recent studies have questioned how easily they are induced [58, 120]. Also, in the studies reporting rebounds, the stimulations used most probably activated the PCs in a similar fashion as the synchronous CF activation of all the PCs to a CN cell that we found to be a requirement to evoke CN neuron rebound responses [33]. The question is how likely rebound responses occur under normal circumstances. Studies of CF activation in the corresponding part of the cerebellum during movements have shown that activation of the IO is inhibited during the execution phase [121]. In conclusion, altogether, these findings suggest that CN output during behavior is primarily governed by the combination of MF collateral input and the level of modulation of the inhibition from the PCs, linearly combined by the CN neuron.
The Coordination of Rhythmic Orofacial Movements: a Proposed New Function of the Olivo-Cerebellar System (D.H. Heck)
In a series of elegant experiments using multiple-electrode recordings from the cerebellum of awake and behaving rats, Llinás and colleagues showed that neurons in the inferior olive (IO) synchronize their activity in phase with rhythmic licking movements rats perform when drinking water [23, 27]. IO activity was not monitored directly but through the characteristic complex spikes elicited in Purkinje cells by IO climbing fiber inputs. Since each Purkinje cell in the healthy adult cerebellum receives input from only one IO neuron, the observations of complex spikes in up to 29 different Purkinje cells reflected the activity of as many different IO neurons.
These population recordings showed that distinct groups or assemblies of IO neurons dynamically synchronized their spiking phase-locked to the rhythm of fluid licking. IO neurons fire at an average rate of one per second while licking occurs at a rate of about 10 Hz. Thus, complex spikes did not occur at each licking cycle, but the synchronization events were phase-locked to licking with millisecond precision, and different groups of IO neurons synchronized independently during different lick cycles.
In earlier studies, Shambes and colleagues had mapped sensory representations in the same area of the rat cerebellar hemisphere (folia Crus I and II) where Llinás and colleagues had recorded complex spike synchrony during licking. The mapping studies revealed a strong representation of facial and oral tactile inputs to the cerebellum, represented in a seemingly unstructured spatial pattern which the authors described as “fractured somatotopy” [122]. More recent work also shows motor representation of orofacial movements other than licking in the cerebellum. Chen and colleagues showed that the position of mystacial vibrissae in the mouse is represented in the simple spike activity of individual Purkinje cells in Crus I [123]. We reported representations of respiratory and whisker movements in the anterior vermis [124–126].
These electrophysiological and mapping studies strongly implicated the cerebellum in orofacial behavior. However, what exactly the cerebellum contributes to such behavior remains unclear. Most rhythmic orofacial movements such as breathing, licking, or swallowing are controlled by pattern-generating circuits in the brainstem [127–129]. Pattern-generating circuits are by definition able to generate the motor-controlling neuronal patterns autonomously. Why would the cerebellum be involved? Here, I propose that the cerebellum is involved in the coordination of fluid licking with breathing and swallowing movements in order to make fluid intake faster. Behavioral evidence for this proposed role of the cerebellum comes from our own work in mice and that of Vajnerova et al. in rats [124, 125, 130, 131], showing that loss of cerebellar output results in a slowing of the licking rhythm by 15–19 %.
What are possible neuronal mechanisms and pathways for this coordination and what are the respective roles of simple and complex spikes? We have shown that fluid licking movements are represented in the simple spike activity of large, distributed populations of Purkinje cells in Crus I/II of the mouse cerebellum [124]. Most Purkinje cells showed a rhythmic modulation of simple spike rate on a lick-by-lick basis. However, we also found a smaller set of Purkinje cells whose simple spike activity was modulated during licking, but in an arrhythmic way. These cells thus seemed to occasionally modulate their firing phase-locked to licking but not on a cycle-by-cycle basis (Fig. 3e, f in [124]). The firing pattern of these cells fits with the assumption that they generate a signal involved in the coordination of fluid licking with respiration and/or swallowing movements, as explained below.
Behavioral studies have shown that rats swallow water during licking without stopping to lick [132]. The water accumulating in the mouth is swallowed every six to eight licks. During swallowing, inspiration must be suppressed and a corresponding coordination of licking with respiration has been shown as well [133]. While complex spikes may not be involved in the generation and control of licking on a cycle-by-cycle basis they are ideally suited to signal the timing and possible motor errors related to swallowing movements. The complex spike population synchrony observed by Llinás and colleagues may thus have provided the timing signals allowing the precise coordination of licking with respiration and swallowing movements. Such coordination could be accomplished by small adjustments to the phase relationships between the respiratory, licking, and swallowing pattern generators. All three of these movements are controlled by brain stem pattern-generating circuits [127–129]. The cerebellum projects broadly to the brain stem [134] with projections to areas containing respiratory pattern generators originating from the medial cerebellar nucleus [126]. Neurons in the medial cerebellar nucleus in mice represent multiple orofacial movements, including licking and breathing [126]. I propose that the purpose of these cerebellar brain stem projections is the temporal coordination of multiple pattern generators possibly by modulating the phases of pattern generator cycles (Fig. 2).
Schematic diagram of the hypothesized cerebellar role in coordinating brainstem pattern generators. The brainstem contains autonomous pattern generators for respiration and orofacial movements, including those involved in fluid licking, which involves the coordination of tongue, jaw, and respiratory movements. Efferent projections from the cerebellar nuclei reach many areas of the brain stem, including areas containing pattern-generating circuits for respiratory and orofacial movements. The hypothesis I put forward is that those efferents include projections that play a key role in optimizing the temporal coordination of brain stem pattern-generating circuits involved in licking movements
I further propose that the involvement of the inferior olive in this process lies in providing the training signal that shapes Purkinje cell firing in order to optimize the temporal coordination and ultimately increase the speed of water intake. This would provide an evolutionary advantage as time spent drinking is usually time during which the animal is more vulnerable to being detected by predators. Loss of the cerebellum does not seem to eliminate licking/breathing/swallowing coordination but it reduces the speed of licking. Coordination without a cerebellum may thus be accomplished by less efficient “back up” mechanisms residing in the brain stem. Being able to drink water 15–19 % faster with an intact cerebellum could have provided “fast drinkers” with a significant advantage over slower drinking conspecifics. Over the course of evolution, seemingly small increases in the probability of survival to reproduction can significantly improve the success of a species.
Purkinje Cell Complex Spike Firing Patterns and Their Relationship to Zebrin Banding (N.L. Cerminara, J. Xiao, I. Sugihara, E.C. Lang, R. Apps)
Purkinje cells are the principal computational units of the cerebellar cortex. They receive and integrate two main types of excitatory inputs: mossy fibers and climbing fibers, which generate simple spikes and complex spikes, respectively (for review see Ito; [135]). Purkinje cells are the sole output of the cerebellar cortex and their axons form inhibitory synapses in the cerebellar nuclei [136, 137].
Purkinje cells are heterogeneous in terms of phenotype, with the most comprehensively studied molecular marker being zebrin II [138]. Zebrin II is expressed by subsets of Purkinje cells and in many areas of the cerebellar cortex, zebrin-positive cells alternate with those that do not express zebrin II, forming an array of rostrocaudally oriented zebrin-positive and zebrin-negative bands. A cloning study has shown that the zebrin II antigen is the respiratory isoenzyme aldolase C [139].
In addition to zebrin II, numerous other molecular markers have also been shown to be expressed heterogeneously in Purkinje cells with many co-expressed with zebrin II (e.g., phospholipase Cβ3 [140] and excitatory amino acid transporter 4 [141]). This raises the question of whether distinct functional classes of Purkinje cells exist that are related to phenotypic signature. In vitro studies have revealed that Purkinje cells can differ in their biophysical properties (e.g., [142–147]); however, it is unknown if this translates to differences in firing properties in vivo, or whether such differences are related to molecularly defined compartments within the cerebellar cortex. In particular, recent work has found that the zebrin banding pattern closely matches the topography of olivo-cortico-nuclear microcircuits [148]. This raises the possibility that differences in complex spike activity exist that are related to the expression of zebrin by Purkinje cells.
In ketamine/xylazine-anesthetized rats, Purkinje cells from identified zebrin-positive and zebrin-negative bands in Crus II of the same animal displayed a significant difference in their complex spike firing rates, with Purkinje cells located in zebrin-negative bands firing, on average, at higher rates (Fig. 3a, see also [149, 150]). We also examined whether the number of spikelets per complex spike varied between zebrin-positive and zebrin-negative bands. In contrast to previous findings in vitro [151], the number of spikelets in vivo does not vary systematically between Purkinje cells located in zebrin-positive and zebrin-negative bands [152]. On the other hand, in awake head-fixed mice, complex spikes in zebrin-positive Purkinje cells differ in waveform from those recorded in zebrin-negative Purkinje cells (e.g., they have a greater spike area) [150]. However, the extent to which this measure relates to spikelet number is not clear, so whether complex spikes differ systematically in spikelet number between zebrin bands remains to be established.
a Dendritically recorded complex spikes (CS) from histologically identified zebrin-negative (Z−) bands showed higher firing rates than those in zebrin-positive (Z+) ones within individual animals. The median firing rate across zebrin-positive Purkinje cells was plotted against the zebrin-negative median for each individual animal. b Cumulative distribution functions for the duration of the simple spike (SS) post-CS pauses. Purkinje cells recorded from Z− bands (top, blue) or C1 zone (bottom, blue) have shorter post-CS pauses in their SS activity than from Z+ bands (top, red) or A2 zone (bottom, red). The median of the duration was calculated as the median interval between the CS and its following SS for each cell. c Cells recorded from Z− bands showed stronger post-CS increase in their SS activity than from Z+ bands. The cumulative distribution of the ratio of increase (calculated as the SS firing rate within 100 ms immediately following each CS divided by the overall SS firing rate) plotted for Z− or C1 zone (in blue) and Z+ or A2 zone (in red)
Another consideration is the interaction between complex spikes and simple spikes since this is thought to be important for cerebellar information processing and motor learning. In particular, the complex spike-induced pause in simple spike activity varies; pauses were found to be longer in zebrin-positive than zebrin-negative bands (Fig. 3b; see also [149, 150]). At a zonal level of resolution, the A2 zone in Crus II/paramedian lobule in rats is mainly if not exclusively zebrin positive, while the neighboring C1 zone is mainly zebrin negative [153]. Consistent with the zebrin band data, complex spike-induced pauses in simple spike activity were longer in Purkinje cells recorded in the A2 zone.
Following a complex spike-induced pause, a transient increase or decrease in simple spike activity relative to baseline rates has also been reported [154]. In the current experiments we found that modulation of simple spike activity in a 100 ms time window following a complex spike was greater in Purkinje cells located in zebrin positive bands and the A2 zone than zebrin negative/C1 zone Purkinje cells (Fig. 3c).
Our results therefore suggest that Purkinje cells are functionally heterogeneous in firing patterns, both in terms of complex spike rates and also in the influence that complex spikes have on subsequent simple spike activity (and vice versa; see also [150]). These systematic differences in Purkinje cell firing properties have implications for information processing at a microcircuit level of operation. For example, in terms of associative learning, climbing fibers and the complex spikes they generate are generally thought to convey teaching signals that drive plasticity in cerebellar circuits [60, 155, 156]. Complex spike-induced simple spike pauses could result in an increase in cerebellar nuclear activity through disinhibition. As a result, the pause could be used as an instruction signal for associative learning at this level of the circuit [22]. Although speculative, variation between zebrin bands in pause duration may reflect differences between cerebellar olivo-cortico-nuclear microcircuits in their ability to contribute to this learning process.
Coordinated Modulation of Complex Spike Synchrony and Waveform: a Mechanism for Flexibly Linking the Motor Control and Plasticity Functions of the Olivocerebellar System (E.J. Lang)
If olivocerebellar activity both modulates synaptic plasticity and contributes directly to ongoing cerebellar output, it is likely advantageous to link these two functions in many situations, as suggested in the section by De Zeeuw. However, it is also likely that there are times when only one of these functions is needed, or at least that it would be beneficial to be able to alter the relationship of olivocerebellar activity to each of these functions independently. For example, once a movement has been perfected, one would not want each subsequent use of the command that evoked that movement to alter the circuitry underlying the command, at least until changes in the state of the motor apparatus necessitated adjustments. Conversely, modifications of motor system circuitry can take place without actual movements being generated, as demonstrated by the improvement in motor performance following mental rehearsal [157]. Of course, the motor command may be blocked from expression at a site downstream from the cerebellum, or the sites affected by such rehearsal may not involve the cerebellum (however, some evidence exists for changes in cerebellar activity due to mental rehearsal of a motor task, e.g., [158]). Nevertheless, it seems reasonable that being able to direct the functional consequences of olivocerebellar activity would, in general, be beneficial.
Here, I propose that coordinated changes in two functional parameters of olivocerebellar activity, complex spike synchrony and complex spike waveform, may provide a basis for separable control of the motor control and synaptic plasticity gating functions of the olivocerebellar system. In line with previous ideas (e.g., [1]), synchrony is here assumed to be the primary mechanism for allowing olivocerebellar activity to influence motor output, but I will argue that it may also have a role in gating plasticity via its effect on complex spike waveform [159]. I will also argue that complex spike waveform can be altered by a second mechanism, molecular layer interneuron (MLI; basket and stellate cells) activity, and that having this dual control over complex spike waveform would allow for a flexible linkage between the actions of the olivocerebellar system in shaping cerebellar output and gating synaptic plasticity.
The idea that synchrony is a key mechanism whereby olivocerebellar activity can evoke movements originated from the demonstration that tremor results from harmaline’s action to synchronize olivocerebellar activity [160, 161]. Subsequently, correlation of synchronous complex spike activity with voluntary movements was also demonstrated [26, 27, 30], showing that the relationship between synchrony and movement holds under physiological conditions and not just under hypersynchronous states.
Synchronous complex spike activity is probably able to alter cerebellar nuclear output, and thus cause movements, because it occurs mainly among Purkinje cells located in the same zebrin compartment as each other [162], and the axons of Purkinje cells in the same zebrin compartment converge onto the same region of the cerebellar nuclei [163, 164]. Thus, synchronous complex spike activity among such Purkinje cell groups should lead to synchronous barrages of IPSPs in the target nuclear cells. Indeed, evidence of the predicted powerful inhibitory effect by spontaneous complex spike activity at physiological levels of synchronization has been obtained [32, 34, 165], and synchronous activation of the olivocerebellar system has been shown to evoke giant IPSPs [33].
Critically, although electrical coupling among inferior olivary neurons via gap junctions permits large-scale synchronization of complex spikes [166–169], the actual patterns and levels of synchrony are dynamically controlled by synaptic inputs to the inferior olive [170–174]. Thus, individual complex spikes may occur synchronously with those in neighboring Purkinje cells, likely causing a major effect on nuclear cell activity, or in relative isolation, and thus likely not to impact cerebellar output significantly.
Synchrony may also be an important parameter in regard to the olivocerebellar system’s role in gating synaptic plasticity, and I propose that this may be via its effect on complex spike waveform. We have recently shown that the complex spike waveform varies with synchrony, and that at least part of this variation is due to changes in spikelet number [159]. Specifically, highly synchronous complex spikes tend to have greater numbers of spikelets than less synchronous ones.
The mechanism underlying the relationship between complex spike synchrony and waveform is not known; however, one plausible possibility rests on the fact that olivary neurons can discharge both individual spikes and high frequency bursts of action potentials [175–178]. Although a single climbing fiber EPSP is capable of generating a complex spike [175], the number of spikelets it contains tends to increase in proportion to the number of action potentials in an olivary neuron burst [178]. Thus, if the olivary neuron burst size varies with synchrony, this variation would mediate a correlated change in the complex spike synchrony and waveform, explaining the relationship between these two parameters. If this is the case, then synchrony levels can potentially be linked to plasticity, because the number of spikes in an olivary burst and in the CS has been related to the degree and type of plasticity that occurs at the parallel fiber-Purkinje cell synapse [42, 178, 179]. For example, single discharges of olivary neurons induce LTP, whereas larger bursts produced increasingly stronger LTD [178] and other memory-related effects [42].
Together, the above results suggest that highly synchronous complex spike discharges would both produce a significant impact on cerebellar nuclear activity, because of convergence of synchronously active Purkinje cells onto individual nuclear cells, and activate plasticity mechanisms leading to LTD, because spikelet numbers tend to increase with synchrony. In contrast, less synchronized or asynchronous complex spike activity would have relatively weaker impact on cerebellar nuclear activity and on plasticity, or perhaps would induce LTP. Thus, by itself, modulation of synchrony levels would lead to a coordinated but relatively fixed relationship between the effects of olivocerebellar activity on plasticity and cerebellar nuclear activity.
An additional mechanism for shaping complex spike waveform could transform this relatively fixed relationship into a more flexible one. The molecular layer interneurons (MLIs) of the cerebellar cortex (basket and stellate cells) may provide such a mechanism. Activation of MLIs does not block the complex spike and thus would not prevent a synchronous complex spike discharge from affecting cerebellar nuclear activity; however, when complex spikes are conditioned by activation of MLIs, their duration (i.e., the number of spikelets comprising them) is reduced [180], as is the associated calcium entry into the Purkinje cell [76, 181]. Moreover, activation of MLIs can block the LTD that normally results from paired inferior olive and parallel fiber stimulation [182]. Thus, a number of scenarios can be imagined. High complex spike synchrony could occur in the setting of either low, intermediate, or high levels of MLI activity, leading to motor output and either LTD, no plasticity, or LTP, respectively (depending on the spikelet composition of the complex spikes, as shaped by MLI activity). In contrast, at low synchrony levels, no motor output would result, but MLI levels could still regulate the type and strength of the plasticity induced by these complex spikes.
In sum, the interaction of the state of the inferior olive (electrical coupling level) and cerebellar cortex (level of MLI activity, in particular) is proposed to form a mechanism for allowing a dynamic and flexible coupling of the plasticity and motor control functions of the olivocerebellar system, and would represent a novel way for the mossy fiber and olivocerebellar systems to interact. In this regard, it is interesting to note in closing that simple spike activity can influence the level of synchronization of impending complex spike activity [57], suggesting that a very tight coordination of mossy fiber and olivocerebellar activity is required for both the motor learning and motor control functions of the cerebellum.
Synchronization and De-synchronization of Inferior Olive Neurons for Learning and Control (N. Schweighofer, E.J. Lang, M. Kawato)
The cerebellum learns internal models of our own body or of the external world for prediction and control by minimizing performance errors, e.g., [183]. Such functions of the cerebellum are in agreement with known error-related signals carried by the inferior olive (IO), e.g., [184], and known plasticity at the parallel fiber-Purkinje cell synapses driven by IO firing—see [7] for review. For learning highly complex internal models, the IO must transmit error signals with high-temporal resolution [185, 186]. However, IO neurons fire at a low rate, between one and three spikes/s, thus information transmitted to Purkinje cells as complex spikes is limited. The low firing rates may be beneficial so that complex spikes do not interfere with simple spikes mainly carrying the functional cerebellar cortical output. To resolve this conundrum, we earlier proposed that high-frequency components of the error inputs are distributed to ensemble of functionally related Purkinje cells, via sporadic, irregular, and desynchronized spikes [187]. Desynchronization scatters the spike timings of each neuron to increase the time resolution of the population rate coding. Then, the continuous error signal can be reconstructed by spatial integration across functionally related Purkinje cells, as well as by temporal integration at each Purkinje cell via the cumulative effects of synaptic plasticity.
How can the IO achieve such desynchronization, however? The IO is electrotonically coupled by gap junctions, more extensively than any other region in the mammalian brain. Although the coupled IO system has the capability of generating widespread synchrony, it often does not do so [188, 189]. Indeed, models of coupled IO neurons can generate robust chaotic regime of spiking activity [187]. In such regimes, a “chaotic resonance” [190] enhances the transfer of error information over the network at each trial, and over each cell across trials.
For such chaotic resonance to emerge in our computer simulations, levels of electrical coupling must be in intermediate ranges [185, 187]. Inhibitory inputs from cerebellar nuclear neurons and excitatory inputs control the strength of electrical coupling between IO cells [93, 191, 192]. Because cerebellar nuclear neurons are targets of the Purkinje cells, the strength of effective coupling presumably depends on the modulation of the cerebellar neurons by Purkinje cells [57, 186]. In this scheme, the role of the IO–PC–cerebellar nucleus triangle is to control synchronous IO firing to optimize cerebellar learning [29, 193].
Besides its role on learning, and based on the traditional view that electrical coupling synchronizes neurons, it has also been proposed that the IO exerts its influence on motor control in real time via synchronous and rhythmic discharges [160]. It has notably been shown that changes in complex spike activity are associated with performance of well-learned movements, e.g., [27]. Because of the relatively few complex spikes compared to simple spikes, olivocerebellar system can only contribute to motor commands primarily when it is operating in a relatively synchronized state [165].
Yet, just as was the case with motor learning, the low firing rates of complex spikes presents a problem for the direct participation of the olivocerebellar system in motor control and coordination. Synchronized IO activity, together with the fact that Purkinje cells on average only fire a single complex spike during a typical movement, puts severe restrictions on the ability of the olivocerebellar system to code signals for on-line motor control and coordination in terms of individual cell firing rates. A possibility is that a large motor error would cause major volleys in excitatory afferent IO pathways, which then would lead to synchronous complex spikes, and then triggering an emergency or protective motor response in response to this error.
Cerebellar Learning and Timing Hypothesis Go Hand in Hand (C.I. De Zeeuw, O. Ozyildirim)
Even though the climbing fibers innervating the dendritic tree of Purkinje cells have been shown to originate in the inferior olive half a century ago [194], their function is still under debate. One line of theoreticians and experimentalists has advocated their potential role as a teacher in controlling plasticity in the molecular layer [4, 5, 195], whereas another line of researchers has claimed that they serve to directly control motor timing [27, 196–199]. Yet, these two functions are not mutually exclusive and here I want to propose that they in fact do go hand in hand in that proper motor learning requires timing of neuronal activity and motor activity and that proper motor timing requires learning and plasticity. Below, I will briefly summarize the essentials and development of both the learning and timing hypothesis, and subsequently try to explain why they are not independent from each other and why the system is in fact efficiently designed to combine both functions.
The learning hypothesis is largely based on the original concept that the climbing fibers may control plasticity at the parallel fiber to Purkinje cell synapse changing the synaptic weight so as to modify motor output [4, 5]. While Marr and Albus diverged as to whether long-term potentiation (LTP) or long-term depression (LTD) might be the main mechanism underlying motor learning, Ito and Kano provided the first experimental evidence in vitro that climbing fiber activity might indeed reduce the efficacy of this synapse by LTD [200]. Following up on the Marr-Albus-Ito hypothesis, Fujita, Dean, Jorntell, and colleagues argued and provided evidence that plasticity at the molecular layer interneurons might also contribute to cerebellar learning, operating together with the plasticity at the parallel fiber to Purkinje cell synapses as an adaptive filter for, for example, the removal of predictable sensory encoding signals [201, 202]. Subsequently, Gao and colleagues pointed out that the various forms of plasticity, including both synaptic and intrinsic, potentiation and depression in both the molecular layer and granular layer operate in a distributed and synergistic fashion, which is guided by not only the presence but also the absence of climbing fiber activity [8]. Finally, evidence is emerging that the mechanisms underlying learning in the cerebellar cortex are not as homogeneous as might be expected from its uniform and well-organized, matrix-like cyto-architecture; indeed, different modules with intrinsically different Purkinje cells operate at different firing frequencies possibly providing preferential tendencies for potentiation and suppression mechanisms [52, 149, 150, 203, 204].
The timing hypothesis is originally based on work by Llinás and Volkind [160] who showed that muscles can be activated on the beat of rhythmic olivary activity triggered by the tremorgenic drug harmaline. Indeed, several decades later Welsh, Lang, and colleagues demonstrated that particular patterns of complex spike activity distributed across various macrozones of Purkinje cells in Crus I and II can be correlated to retraction of tongue movements [27]. This concept was recently confirmed and refined by De Gruijl and colleagues who showed with the use of calcium imaging that such patterns can also occur within microzones in relation to limb movements and that these patterns can be accentuated following perturbation of movements [31, 205]. The occurrence of both the macro- and microzonal patterns as well as the timing of concomitant movements depend on the level of electrotonic coupling by connexin36 gap junction channels located between dendrodendritic spines in glomeruli [168, 169, 206].
Why does climbing fiber-dependent motor learning depend on climbing fiber-dependent timing of neuronal activity and motor activity? Climbing fiber-dependent motor learning has been extensively described for adaptation of eye movements in the floccular complex of the vestibulocerebellum and for classical Pavlovian eye blink conditioning in lobulus simplex in the cerebellar hemispheres [207–212]. Interestingly, these two areas are largely zebrin positive and zebrin negative, respectively [150, 213], and both types of learning may be dominated by different learning rules in that the zebrin-positive areas, which operate at relatively low simple spike firing frequency domains, may be prone for simple spike enhancing and/or potentiation mechanisms, whereas the zebrin-negative areas, which operate at relatively high simple spike firing frequency domains, appear to be more prone for suppression mechanisms [204]. Indeed, gain-increase learning of the vestibulo-ocular reflex (VOR) and conditioning eyeblink responses to a light or tone result in an increase and decrease of simple spike activity in the corresponding Purkinje cell zones, respectively [8, 48, 214]. Importantly, despite the possibly opposite dominant learning rules, the climbing fibers and the timing of their activity with respect to motor output play an essential role in both forms of learning. In case of VOR increase learning, the absence of climbing fiber activity at the appropriate part of the stimulus cycle is required to allow the potentiation mechanisms in the floccular cortex to take place [8, 215–217], which implies that inappropriate motor timing itself will also disturb motor learning of amplitude and direction, since motor timing will affect the level of retinal slip and thereby climbing fiber activity (see e.g., [208, 215]). In case of eyeblink conditioning, the presence of climbing fiber activity at the appropriate parts of the paired trials of both conditioned and unconditioned stimuli is required to allow the suppression mechanisms in the lobulus simplex to take place in optima forma [204, 214]. These data suggest that motor timing itself also strengthens the learning process in this paradigm, because the complex spikes associated with the conditioned response emerge and develop during the training and are phase locked to the initiation of the conditioned response, consolidating the learning process [204, 214]. Future studies will have to reveal to what extent the differences in simple spike modulations during these two forms of climbing fiber dependent motor learning mainly reflect the zebrin-positive and zebrin-negative character of the modules involved, whether they are due to the inherently different temporal character of the tonically driven VOR adaptation and the phasically driven eyeblink conditioning trials (see also [42, 218] for suppression mechanisms in trial by trial learning in zebrin-positive zones), and/or whether they are related to the direction of movements involved [27, 214, 215].
Why does climbing fiber-dependent motor timing require climbing fiber-dependent learning? Motor timing of the paradigms discussed above, including both the execution of compensatory eye movements and that of eyeblink responses, depends to a varying degree on the presence of climbing fiber activity. With respect to unconditioned reflex types of movements, the execution of a well-timed optokinetic reflex (OKR) or eyeblink response to an air puff on the eye depends on synchronized climbing fibers that carry visual signals from the accessory optic system to the flocculus or cutaneous information from the trigeminal nuclei to the lobules simplex, respectively [29, 207, 208, 219, 220]. This level of synchronization, which can determine the latency of the movements [31, 205, 206], has recently been shown to be influenced by the NMDA-dependent excitatory drive from the olivary afferents, regulating the level of gap junction coupling through presumably calcium-mediated plasticity mechanisms [192, 205, 206, 221]. With respect to the conditioned reflex types of movements, the execution of a well-timed adapted VOR or eyeblink response to a conditioned stimulus may depend equally well on synchronized climbing fiber inputs from the same olivary subnuclei as the unconditioned movements, but now the climbing fiber activity as well as its level of synchrony may be determined predominantly by a rebound following activation from the GABAergic input from the prepositus hypoglossi nucleus and dorsolateral hump, respectively [29, 93, 205, 206, 214, 222–227]. Importantly, these GABAergic cells in the hindbrain may themselves also be subject to plasticity in that their input from mossy fiber collaterals may be dramatically enhanced during the learning and thereby affect motor responses [214, 226, 228, 229]. Thus, in both cases, i.e., unconditioned and conditioned reflexes, climbing fiber-dependent motor timing may well depend on processes of plasticity.
Why is the olivocerebellar system efficiently designed to combine both learning and timing functions? Essential structural and cell physiological components subserving learning and/or timing functions, such as plasticity of chemical and electrical synapses, such as outgrowth of axonal fibers, and such as modification of intrinsic excitability and rebound excitation, are distributed throughout all three main elements of the olivocerebellar modules, including the cerebellar cortex, cerebellar nuclei, and olivary subnuclei [8, 93, 192, 205, 206, 214, 221, 226, 228–231]. These olivocerebellar modules as a whole are organized according to their output in that each module controls the amplitude and timing of a particular set of motor domains, such as eye or limb muscles [232]. Since these modules will be used for evoking, optimizing, and coordinating unconditioned reflexes of motor activity as well as for modifying the amplitude and timing of the same motor domains during conditioning, our brain, i.e., the cerebellum, uses the same outlets and reference frames for both short-term and long-term functions. Considering the high complexity in controlling movements with multiple degrees of freedom within a particular motor domain let alone in coordinating the activity across multiple motor domains [233], I would like to argue that this configuration is not only the most efficient way to organize the olivocerebellar system, but probably also the only way to do so. Moreover, given the wide and uniform distribution of this system in the animal kingdom, varying from fish and birds up to rodents and primates [234], and thus including all animals capable of adapting their motor timing, but excluding those with more rigid timing mechanisms such as insects that have a cerebellar-like structure lacking an inferior olive [235–237], it is evident from an evolutionary point of view that it was advantageous to combine learning and timing functions within the same olivocerebellar system.
Conclusions
For the past four to five decades, two lines of thought have dominated thinking concerning the role of the olivocerebellar system in the motor control function of the cerebellum. One is that the olivocerebellar system gates plasticity to improve future motor commands. The other is that olivocerebellar activity significantly contributes to the ongoing motor command being issued by the cerebellum. The general tendency has been to view these two roles as mutually (or at least largely) exclusive.
The question motivating the present paper—how can the characteristics of the olivocerebellar system potentially allow it to play roles in both the motor coordination and motor learning functions of the cerebellum—obviously takes a different perspective, one in which these roles are not viewed as mutually exclusive. Indeed, one may ask whether any brain system is solely dedicated to being a learning system rather than having the capability of learning being built into all brain systems. The hippocampus, for example, has long been thought to be an essential component of the system for forming declarative memories, but recently it has been proposed that its role in memory formation is but one instantiation of the hippocampus’s more general function to form cognitive maps [238]. In a similar vein, perhaps the olivocerebellar system’s role in motor learning is just one aspect of its more general role in motor function.
Interestingly, nothing in the original formulations of either the motor learning or motor control hypotheses proscribes the possibility of the olivocerebellar system having a role in the other, although, clearly, an implication of Marr’s paper (1969) is that the large majority of movements would be generated without a contribution from the olivocerebellar system in a well-trained animal. Of course this raises the issue of what is meant by well trained, and in particular, the issue of how generalizable are movement patterns. That is, do we mostly make trained movements, or, in fact, can most movements be generated without prior practice. If the latter is correct, Marr’s own formulation would imply that the olivocerebellar activity contributes to many movements.
It is worth noting that, similarly, the various articles in the present collection also present no logical reasons or experimental data that preclude the olivocerebellar system being involved in both the motor learning and motor control functions of the cerebellum. Rather, the various contributions show that the olivocerebellar system is a highly flexible and more subtle system than it is traditionally portrayed as being, one that might be easily capable of performing multiple functions. For example, the ability to form neuronal ensembles with synchronized activity, and the variability of the complex spike waveform represent just two mechanisms by which the olivocerebellar system’s activity may be subtly adjusted to perform different tasks.
Reaching a consensus on specifically how the olivocerebellar system could function in both motor learning and motor control was not attempted, because the answer to this question will ultimately be decided experimentally; however, it seems reasonable to state that much of the field has moved away from the view that these two roles are mutually exclusive. Furthermore, asking the question itself suggests a distinct perspective for thinking about cerebellar physiology that may be worthwhile because it suggests new questions to be investigated. For example, the idea that the olivocerebellar system functions in the motor learning and motor control realms suggests that the afferent systems to each cerebellar region (i.e., the mossy fibers and climbing fibers) have multiple functions, which raises the possibility that all (or at least many) cerebellar operations require the activity of both the mossy and climbing systems. This, in turn, raises the issue of how and where the activity of the two systems might interact in carrying out these functions. Although there are a number of potential sites for interaction, the Purkinje cell stands out because of its being the sole output from the cortex and its being the main target of both afferent systems.
The view that Purkinje cell simple and complex spikes functioned independently has a long history, and perhaps makes some sense under the assumption that the olivocerebellar and mossy fibers systems have independent roles. From the present perspective, however, the nature of their interaction becomes a central question. In fact, that simple and complex spikes can interact is well-known. Complex spike activity modulates simple spike activity in both a tonic and phasic manner [154, 239–253], and conversely, changes in simple spike activity can affect complex spike rates and synchrony levels via the cerebellum’s feedback to the inferior olive [57, 58]. Intriguingly, these interactions may vary between cerebellar cortical regions, as recent studies have shown that the firing patterns of simple and complex spikes, and some aspects of their interactions, vary between zebrin positive and negative compartments [149, 150].
However, despite a number of studies showing these various interactions, relatively little is known about simple spike-complex spike interactions during behavior, because studies on motor control have mainly focused on simple spike activity, and the relatively few studies that have analyzed complex spike activity during behavior have largely analyzed both spike types independently. Thus, understanding simple spike-complex spike interactions during behavior, a long understudied phenomenon, may prove to be a fruitful future line of enquiry, one which may be central to understanding cerebellar function if the perspective of this article is correct.
Statement on the Welfare of Animals
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed, and all procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Acknowledgments
Timothy J. Ebner6 and Laurentiu S. Popa6. Supported in part by NIH grant R01 NS18338.
Dieter Jaeger8. This study was supported by grants 1R21NS074296 and 1R01NS067201 from NINDS/NIH.
Fredrik Bengtsson3 and Henrik Jörntell3. This study was supported by grants from NINDS/NIH (R01 NS040863), The Hand Embodied (THE) (Integrated Project, EU FP7, project no. 248587), and the Swedish Research Council (VR Medicine).
Detlef H. Heck7. This study was supported by funding from the UTHSC Neuroscience Institute, from the UTHSC Dept. of Anatomy and Neurobiology, and by NIH grant R01NS060887.
Nadia L Cerminara2, Jianqiang Xiao1, Izumi Sugihara11, Eric J Lang1, Richard Apps2. This study was supported by grants from the Medical Research Council, UK (G1100626; NLC, RA); Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (KAKENHI), (25430032; IS); National Science Foundation (IOS-1051858; EJL) and the National Institute of Neurological Disorders (NS095089; EJL).
Eric J. Lang1. This study was supported by grants from the National Science Foundation (IOS-1051858) and the National Institute of Neurological Disorders (NS095089).
Nicolas Schweighofer11, Eric J. Lang1 and Mitsuo Kawato9. This study was supported by grants from the NSF BCS (1031899; NS), National Science Foundation (IOS-1051858; EJL) and the National Institute of Neurological Disorders (NS095089; EJL).
C. I. De Zeeuw4,5 and Ozgecan Ozyildirim5. We thank the Dutch Organization for Medical Sciences (ZonMw; C.I.D.Z.), Life Sciences (ALW; C.I.D.Z.), Senter (NeuroBasic; C.I.D.Z.), the Neurotime (Erasmus Mundus), the ERC-advanced and ERC-POC programs of the European Community (C.I.D.Z.).
References
Llinás R. The noncontinuous nature of movement execution. In: Humphrey DR, Freund H-J, editors. Motor control: concepts and issues. New York: Wiley; 1991. p. 223–42.
Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev. 2001;81:1143–95.
Llinás RR. The olivo-cerebellar system: a key to understanding the functional significance of intrinsic oscillatory brain properties. Front Neural Circuits. 2013;7:96.
Marr D. A theory of cerebellar cortex. J Physiol Lond. 1969;202:437–70.
Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61.
Keating JG, Thach WT. Nonclock behavior of inferior olive neurons: interspike interval of Purkinje cell complex spike discharge in the awake behaving monkey is random. J Neurophysiol. 1995;73:1329–40.
Hansel C, Linden DJ, D’Angelo E. Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat Neurosci. 2001;4:467–75.
Gao Z, van Beugen BJ, De Zeeuw CI. Distributed synergistic plasticity and cerebellar learning. Nat Rev Neurosci. 2012;13:619–35.
Aizenman CD, Manis PB, Linden DJ. Polarity of long-term synaptic gain change is related to postsynaptic spike firing at a cerebellar inhibitory synapse. Neuron. 1998;21:827–35.
Popa LS, Hewitt AL, Ebner TJ. Predictive and feedback performance errors are signaled in the simple spike discharge of individual Purkinje cells. J Neurosci. 2012;32:15345–58.
Wilson WC, Magoun HW. The functional significance of the inferior olive in the cat. J Comp Neurol. 1945;83:69–77.
Murphy MG, O’Leary JL. Neurological deficit in cats with lesions of the olivocebellar system. Arch Neurol. 1971;24:145–57.
Soechting JF, Ranish NA, Palminteri R, Terzuolo CA. Changes in a motor pattern following cerebellar and olivary lesions in the squirrel monkey. Brain Res. 1976;105:21–44.
Kennedy PR, Ross HG, Brooks VB. Participation of the principal olivary nucleus in neocerebellar motor control. Exp Brain Res. 1982;47:95–104.
Seoane A, Apps R, Balbuena E, Herrero L, Llorens J. Differential effects of trans-crotononitrile and 3-acetylpyridine on inferior olive integrity and behavioural performance in the rat. Eur J Neurosci. 2005;22:880–94.
Horn KM, Deep A, Gibson AR. Progressive limb ataxia following inferior olive lesions. J Physiol Lond. 2013;591:5475–89.
Colin F, Manil J, Desclin JC. The olivocerebellar system. I. Delayed and slow inhibitory effects: an overlooked salient feature of cerebellar climbing fibers. Brain Res. 1980;187:3–27.
Savio T, Tempia F. On the Purkinje cell activity increase induced by suppression of inferior olive activity. Exp Brain Res. 1985;57:456–63.
Batini C, Billard JM. Release of cerebellar inhibition by climbing fiber deafferentation. Exp Brain Res. 1985;57:370–80.
Barmack NH, Yakhnitsa V. Cerebellar climbing fibers modulate simple spikes in Purkinje cells. J Neurosci. 2003;23:7904–16.
Cerminara NL, Rawson JA. Evidence that climbing fibers control an intrinsic spike generator in cerebellar Purkinje cells. J Neurosci. 2004;24:4510–7.
Mathews PJ, Lee KH, Peng Z, Houser CR, Otis TS. Effects of climbing fiber driven inhibition on Purkinje neuron spiking. J Neurosci. 2012;32:17988–97.
Lang EJ, Sugihara I, Welsh JP, Llinas R. Patterns of spontaneous Purkinje cell complex spike activity in the awake rat. J Neurosci. 1999;19:2728–39.
Sasaki K, Bower JM, Llinas R. Multiple Purkinje cell recording in rodent cerebellar cortex. Eur J Neurosci. 1989;1:572–86.
Bell CC, Kawasaki T. Relations among climbing fiber responses of nearby Purkinje cells. J Neurophysiol. 1972;35:155–69.
Ozden I, Sullivan MR, Lee HM, Wang SS. Reliable coding emerges from coactivation of climbing fibers in microbands of cerebellar Purkinje neurons. J Neurosci. 2009;29:10463–73.
Welsh JP, Lang EJ, Sugihara I, Llinás R. Dynamic organization of motor control within the olivocerebellar system. Nature. 1995;374:453–7.
Lang EJ, Sugihara I, Llinás R. Olivocerebellar modulation of motor cortex ability to generate vibrissal movements in rats. J Physiol Lond. 2006;571:101–20.
Van Der Giessen RS, Koekkoek SK, van Dorp S, De Gruijl JR, Cupido A, Khosrovani S, et al. Role of olivary electrical coupling in cerebellar motor learning. Neuron. 2008;58:599–612.
Mukamel EA, Nimmerjahn A, Schnitzer MJ. Automated analysis of cellular signals from large-scale calcium imaging data. Neuron. 2009;63:747–60.
Hoogland TM, De Gruijl JR, Witter L, Canto CB, De Zeeuw CI. Role of synchronous activation of cerebellar Purkinje cell ensembles in multi-joint movement control. Curr Biol. 2015;25:1157–65.
Blenkinsop TA, Lang EJ. Synaptic action of the olivocerebellar system on cerebellar nuclear spike activity. J Neurosci. 2011;31:14708–20.
Bengtsson F, Ekerot CF, Jorntell H. In vivo analysis of inhibitory synaptic inputs and rebounds in deep cerebellar nuclear neurons. PLoS One. 2011;6, e18822.
Tang T, Suh CY, Blenkinsop TA, Lang EJ. Synchrony is key: complex spike inhibition of the deep cerebellar nuclei. Cerebellum. 2016;15:10–3.
Otis TS, Mathews PJ, Lee KH, Maiz J. How do climbing fibers teach? Front Neural Circuits. 2012;6:95.
Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci. 2010;33:89–108.
Wolpert DM, Miall RC. Forward models for physiological motor control. Neural Netw. 1996;9:1265–79.
Oscarsson O. Functional organization of olivary projection to the cerebellar anterior lobe. In: Courville J, De Montigny C, Lamarre Y, editors. The inferior olivary nucleus: anatomy and physiology. New York: Raven; 1980. p. 279–89.
Graf W, Simpson JI, Leonard CS. Spatial organization of visual messages of the rabbit’s cerebellar flocculus. II. Complex and simple spike responses of Purkinje cells. J Neurophysiol. 1988;60:2091–121.
Soetedjo R, Kojima Y, Fuchs AF. Complex spike activity in the oculomotor vermis of the cerebellum: a vectorial error signal for saccade motor learning? J Neurophysiol. 2008;100:1949–66.
Medina JF, Lisberger SG. Links from complex spikes to local plasticity and motor learning in the cerebellum of awake-behaving monkeys. Nat Neurosci. 2008;11:1185–92.
Yang Y, Lisberger SG. Purkinje-cell plasticity and cerebellar motor learning are graded by complex-spike duration. Nature. 2014;510:529–32.
Guo CC, Ke MC, Raymond JL. Cerebellar encoding of multiple candidate error cues in the service of motor learning. J Neurosci. 2014;34:9880–90.
Ebner TJ, Hewitt AL, Popa LS. What features of limb movements are encoded in the discharge of cerebellar neurons? Cerebellum. 2011;10:683–93.
Popa LS, Streng ML, Hewitt AL, Ebner TJ. The errors of our ways: understanding error representations in cerebellar-dependent motor learning. Cerebellum. 2016;15:93–103.
Hewitt AL, Popa LS, Ebner TJ. Changes in Purkinje cell simple spike encoding of reach kinematics during adaption to a mechanical perturbation. J Neurosci. 2015;35:1106–24.
Kimpo RR, Rinaldi JM, Kim CK, Payne HL, Raymond JL. Gating of neural error signals during motor learning. Elife. 2014;3, e02076.
Nguyen-Vu TD, Kimpo RR, Rinaldi JM, Kohli A, Zeng H, Deisseroth K, et al. Cerebellar Purkinje cell activity drives motor learning. Nat Neurosci. 2013;16:1734–6.
Hartell NA. Strong activation of parallel fibers produces localized calcium transients and a form of LTD that spreads to distant synapses. Neuron. 1996;16:601–10.
Han VZ, Zhang Y, Bell CC, Hansel C. Synaptic plasticity and calcium signaling in Purkinje cells of the central cerebellar lobes of mormyrid fish. J Neurosci. 2007;27:13499–512.
Wang X, Chen G, Gao W, Ebner T. Long-term potentiation of the responses to parallel fiber stimulation in mouse cerebellar cortex in vivo. Neuroscience. 2009;162:713–22.
Wang X, Chen G, Gao W, Ebner TJ. Parasagittally aligned, mGluR1-dependent patches are evoked at long latencies by parallel fiber stimulation in the mouse cerebellar cortex in vivo. J Neurophysiol. 2011;105:1732–46.
Roitman AV, Pasalar S, Ebner TJ. Single trial coupling of Purkinje cell activity to speed and error signals during circular manual tracking. Exp Brain Res. 2009;192:241–51.
Ke MC, Guo CC, Raymond JL. Elimination of climbing fiber instructive signals during motor learning. Nat Neurosci. 2009;12:1171–9.
Hewitt AL, Popa LS, Pasalar S, Hendrix CM, Ebner TJ. Representation of limb kinematics in Purkinje cell simple spike discharge is conserved across multiple tasks. J Neurophysiol. 2011;106:2232–47.
Popa LS, Hewitt AL, Ebner TJ. Purkinje cell simple spike discharge encodes error signals consistent with a forward internal model. Cerebellum. 2013;12:331–3.
Marshall SP, Lang EJ. Local changes in the excitability of the cerebellar cortex produce spatially restricted changes in complex spike synchrony. J Neurosci. 2009;29:14352–62.
Chaumont J, Guyon N, Valera AM, Dugue GP, Popa D, Marcaggi P, et al. Clusters of cerebellar Purkinje cells control their afferent climbing fiber discharge. Proc Natl Acad Sci U S A. 2013;110:16223–8.
Witter L, Canto CB, Hoogland TM, de Gruijl JR, De Zeeuw CI. Strength and timing of motor responses mediated by rebound firing in the cerebellar nuclei after Purkinje cell activation. Front Neural Circuits. 2013;7:133.
Jirenhed DA, Bengtsson F, Hesslow G. Acquisition, extinction, and reacquisition of a cerebellar cortical memory trace. J Neurosci. 2007;27:2493–502.
Jorntell H, Ekerot CF. Reciprocal bidirectional plasticity of parallel fiber receptive fields in cerebellar Purkinje cells and their afferent interneurons. Neuron. 2002;34:797–806.
Mauk MD, Steinmetz JE, Thompson RF. Classical conditioning using stimulation of the inferior olive as the unconditioned stimulus. Proc Natl Acad Sci U S A. 1986;83:5349–53.
Maiz J, Karakossian MH, Pakaprot N, Robleto K, Thompson RF, Otis TS. Prolonging the postcomplex spike pause speeds eyeblink conditioning. Proc Natl Acad Sci U S A. 2012;109:16726–30.
Lee KH, Mathews PJ, Reeves AM, Choe KY, Jami SA, Serrano RE, et al. Circuit mechanisms underlying motor memory formation in the cerebellum. Neuron. 2015;86:529–40.
Cooke SF, Attwell PJ, Yeo CH. Temporal properties of cerebellar-dependent memory consolidation. J Neurosci. 2004;24:2934–41.
Kassardjian CD, Tan YF, Chung JY, Heskin R, Peterson MJ, Broussard DM. The site of a motor memory shifts with consolidation. J Neurosci. 2005;25:7979–85.
Okamoto T, Shirao T, Shutoh F, Suzuki T, Nagao S. Post-training cerebellar cortical activity plays an important role for consolidation of memory of cerebellum-dependent motor learning. Neurosci Lett. 2011;504:53–6.
Shutoh F, Ohki M, Kitazawa H, Itohara S, Nagao S. Memory trace of motor learning shifts transsynaptically from cerebellar cortex to nuclei for consolidation. Neuroscience. 2006;139:767–77.
Titley HK, Heskin-Sweezie R, Chung JY, Kassardjian CD, Razik F, Broussard DM. Rapid consolidation of motor memory in the vestibuloocular reflex. J Neurophysiol. 2007;98:3809–12.
Medina JF. The multiple roles of Purkinje cells in sensori-motor calibration: to predict, teach and command. Curr Opin Neurobiol. 2011;21:616–22.
Medina JF, Nores WL, Mauk MD. Inhibition of climbing fibres is a signal for the extinction of conditioned eyelid responses. Nature. 2002;416:330–3.
Wulff P, Schonewille M, Renzi M, Viltono L, Sassoe-Pognetto M, Badura A, et al. Synaptic inhibition of Purkinje cells mediates consolidation of vestibulo-cerebellar motor learning. Nat Neurosci. 2009;12:1042–9.
Simpson JI, Alley KE. Visual climbing fiber input to rabbit vestibulo-cerebellum: a source of direction-specific information. Brain Res. 1974;82:302–8.
Gilbert PFC, Thach WT. Purkinje cell activity during motor learning. Brain Res. 1977;128:309–28.
Kim JJ, Krupa DJ, Thompson RF. Inhibitory cerebello-olivary projections and blocking effect in classical conditioning. Science. 1998;279:570–3.
Kitamura K, Hausser M. Dendritic calcium signaling triggered by spontaneous and sensory-evoked climbing fiber input to cerebellar Purkinje cells in vivo. J Neurosci. 2011;31:10847–58.
Tank DW, Sugimori M, Connor JA, Llinas RR. Spatially resolved calcium dynamics of mammalian Purkinje cells in cerebellar slice. Science. 1988;242:773–7.
Ghosh KK, Burns LD, Cocker ED, Nimmerjahn A, Ziv Y, Gamal AE, et al. Miniaturized integration of a fluorescence microscope. Nat Methods. 2011;8:871–8.
Schultz SR, Kitamura K, Post-Uiterweer A, Krupic J, Hausser M. Spatial pattern coding of sensory information by climbing fiber-evoked calcium signals in networks of neighboring cerebellar Purkinje cells. J Neurosci. 2009;29:8005–15.
Lisberger SG. Neural basis for motor learning in the vestibuloocular reflex of primates. III. Computational and behavioral analysis of the sites of learning. J Neurophysiol. 1994;72:974–98.
Albert NB, Robertson EM, Miall RC. The resting human brain and motor learning. Curr Biol. 2009;19:1023–7.
Howarth C, Gleeson P, Attwell D. Updated energy budgets for neural computation in the neocortex and cerebellum. J Cereb Blood Flow Metab. 2012;32:1222–32.
Ito M. The molecular organization of cerebellar long-term depression. Nat Rev Neurosci. 2002;3:896–902.
Safo P, Regehr WG. Timing dependence of the induction of cerebellar LTD. Neuropharmacology. 2008;54:213–8.
Wang SS, Denk W, Hausser M. Coincidence detection in single dendritic spines mediated by calcium release. Nat Neurosci. 2000;3:1266–73.
Schonewille M, Gao Z, Boele HJ, Veloz MF, Amerika WE, Simek AA, et al. Reevaluating the role of LTD in cerebellar motor learning. Neuron. 2011;70:43–50.
Welsh JP, Yamaguchi H, Zeng X-H, Kojo M, Nakada Y, Takagi A, et al. Normal motor learning during pharmacological prevention of Purkinje cell long-term depression. Proc Natl Acad Sci U S A. 2005;102:17166–71.
Medina JF, Nores WL, Ohyama T, Mauk MD. Mechanisms of cerebellar learning suggested by eyelid conditioning. Curr Opin Neurobiol. 2000;10:717–24.
Miles FA, Lisberger SG. Plasticity in the vestibulo-ocular reflex: a new hypothesis. Annu Rev Neurosci. 1981;4:273–99.
Ohyama T, Mauk M. Latent acquisition of timed responses in cerebellar cortex. J Neurosci. 2001;21:682–90.
Perrett SP, Ruiz BP, Mauk MD. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. J Neurosci. 1993;13:1708–18.
Grasselli G, He Q, Wan V, Adelman JP, Ohtsuki G, Hansel C. Activity-dependent plasticity of spike pauses in cerebellar Purkinje cells. Cell Rep. 2016;14:2546–53.
Lefler Y, Yarom Y, Uusisaari MY. Cerebellar inhibitory input to the inferior olive decreases electrical coupling and blocks subthreshold oscillations. Neuron. 2014;81:1389–400.
Ruigrok TJH, Voogd J. Organization of projections from the inferior olive to the cerebellar nuclei in the rat. J Comp Neurol. 2000;426:209–28.
van der Want JJ, Wiklund L, Guegan M, Ruigrok T, Voogd J. Anterograde tracing of the rat olivocerebellar system with Phaseolus vulgaris leucoagglutinin (PHA-L). Demonstration of climbing fiber collateral innervation of the cerebellar nuclei. J Comp Neurol. 1989;288:1–18.
Wiklund L, Toggenburger G, Cuenod M. Selective retrograde labelling of the rat olivocerebellar climbing fiber system with D-[3H]aspartate. Neuroscience. 1984;13:441–68.
van der Want JJ, Voogd J. Ultrastructural identification and localization of climbing fiber terminals in the fastigial nucleus of the cat. J Comp Neurol. 1987;258:81–90.
Kitai ST, McCrea RA, Preston RJ, Bishop GA. Electrophysiological and horseradish peroxidase studies of precerebellar afferents to the nucleus interpositus anterior. I. Climbing fiber system. Brain Res. 1977;122:197–214.
Ruigrok TJ. Cerebellar nuclei: the olivary connection. Prog Brain Res. 1997;114:167–92.
Armstrong DM, Cogdell B, Harvey RJ. Responses of interpositus neurones to nerve stimulation in chloralose anaesthetized cats. Brain Res. 1973;55:461–6.
Rowland NC, Jaeger D. Responses to tactile stimulation in deep cerebellar nucleus neurons result from recurrent activation in multiple pathways. J Neurophysiol. 2008;99:704–17.
Hoebeek FE, Witter L, Ruigrok TJ, De Zeeuw CI. Differential olivo-cerebellar cortical control of rebound activity in the cerebellar nuclei. Proc Natl Acad Sci U S A. 2010;107:8410–5.
Lu H, Yang B, Jaeger D. Cerebellar nuclei neurons show only small excitatory responses to optogenetic olivary stimulation in transgenic mice: in vivo and in vitro studies. Front Neural Circuits. 2016; in press.
Pijpers A, Voogd J, Ruigrok TJH. Topography of olivo-cortico-nuclear modules in the intermediate cerebellum of the rat. J Comp Neurol. 2005;492:193–213.
Fournier B, Lohof AM, Bower AJ, Mariani J, Sherrard RM. Developmental modifications of olivocerebellar topography: the granuloprival cerebellum reveals multiple routes from the inferior olive. J Comp Neurol. 2005;490:85–97.
Person AL, Raman IM. Deactivation of L-type Ca current by inhibition controls LTP at excitatory synapses in the cerebellar nuclei. Neuron. 2010;66:550–9.
Pugh JR, Raman IM. Mechanisms of potentiation of mossy fiber EPSCs in the cerebellar nuclei by coincident synaptic excitation and inhibition. J Neurosci. 2008;28:10549–60.
Andersson G, Oscarsson O. Climbing fiber microzones in cerebellar vermis and their projection to different groups of cells in the lateral vestibular nucleus. Exp Brain Res. 1978;32:565–79.
Ito M. The cerebellum and neural control. New York: Raven; 1984.
Garwicz M, Ekerot CF. Topographical organization of the cerebellar cortical projection to nucleus interpositus anterior in the cat. J Physiol Lond. 1994;474:245–60.
Shinoda Y, Sugihara I, Wu HS, Sugiuchi Y. The entire trajectory of single climbing and mossy fibers in the cerebellar nuclei and cortex. Prog Brain Res. 2000;124:173–86.
Bengtsson F, Jorntell H. Specific relationship between excitatory inputs and climbing fiber receptive fields in deep cerebellar nuclear neurons. PLoS One. 2014;9, e84616.
van Kan PL, Houk JC, Gibson AR. Output organization of intermediate cerebellum of the monkey. J Neurophysiol. 1993;69:57–73.
Jahnsen H. Electrophysiological characteristics of neurones in the guinea-pig deep cerebellar nuclei in vitro. J Physiol Lond. 1986;372:129–47.
Llinás R, Muhlethaler M. Electrophysiology of guinea-pig cerebellar nuclear cells in the in vitro brain stem-cerebellar preparation. J Physiol Lond. 1988;404:241–58.
Raman IM, Gustafson AE, Padgett D. Ionic currents and spontaneous firing in neurons isolated from the cerebellar nuclei. J Neurosci. 2000;20:9004–16.
McKay BE, Molineux ML, Mehaffey WH, Turner RW. Kv1 K+ channels control Purkinje cell output to facilitate postsynaptic rebound discharge in deep cerebellar neurons. J Neurosci. 2005;25:1481–92.
Armstrong DM, Rawson JA. Responses of neurones in nucleus interpositus of the cerebellum to cutaneous nerve volleys in the awake cat. J Physiol Lond. 1979;289:403–23.
Hesslow G. Inhibition of classically conditioned eyeblink responses by stimulation of the cerebellar cortex in the decerebrate cat. J Physiol Lond. 1994;476:245–56.
Alvina K, Walter JT, Kohn A, Ellis-Davies G, Khodakhah K. Questioning the role of rebound firing in the cerebellum. Nat Neurosci. 2008;11:1256–8.
Horn KM, Van Kan PL, Gibson AR. Reduction of rostral dorsal accessory olive responses during reaching. J Neurophysiol. 1996;76:4140–51.
Shambes GM, Gibson JM, Welker W. Fractured somatotopy in granule cell tactile areas of rat cerebellar hemispheres revealed by micromapping. Brain Behav Evol. 1978;15:94–140.
Chen S, Augustine GJ, Chadderton P. The cerebellum linearly encodes whisker position during voluntary movement. Elife. 2016;5.
Bryant JL, Boughter JD, Gong S, LeDoux MS, Heck DH. Cerebellar cortical output encodes temporal aspects of rhythmic licking movements and is necessary for normal licking frequency. Eur J Neurosci. 2010;32:41–52.
Cao Y, Maran SK, Dhamala M, Jaeger D, Heck DH. Behavior related pauses in simple spike activity of mouse Purkinje cells are linked to spike rate modulation. J Neurosci. 2012;32:8678–85.
Lu L, Cao Y, Tokita K, Heck DH, Boughter JD. Medial cerebellar nuclear projections and activity patterns link cerebellar output to orofacial and respiratory behavior. Front Neural Circuits. 2013;7:56.
Travers JB, Dinardo LA, Karimnamazi H. Motor and premotor mechanisms of licking. Neurosci Biobehav Rev. 1997;21:631–47.
Jean A. Brainstem control of swallowing: localization and organization of the central pattern generator for swallowing. In: Taylor A, editor. Neurophysiology of the jaws and teeth. London: Macmillan; 1990. p. 294–321.
Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–66.
Vajnerova O, Zhuravin IA, Brozek G. Functional ablation of deep cerebellar nuclei temporarily impairs learned coordination of forepaw and tongue movements. Behav Brain Res. 2000;108:189–95.
Hayar A, Bryant JL, Boughter JD, Heck DH. A low-cost solution to measure mouse licking in an electrophysiological setup with a standard analog-to-digital converter. J Neurosci Methods. 2006;2:203–7.
Weijnen JA, Wouters J, van Hest JM. Interaction between licking and swallowing in the drinking rat. Brain Behav Evol. 1984;25:117–27.
Welzl H, Bures J. Lick-synchronized breathing in rats. Physiol Behav. 1977;18:751–3.
Teune TM, van der Burg J, van der Moer J, Voogd J, Ruigrok TJ. Topography of cerebellar nuclear projections to the brain stem in the rat. Prog Brain Res. 2000;124:141–72.
Eccles JC, Ito M, Szentágothai J. The cerebellum as a neuronal machine. Berlin: Springer; 1967.
Ito M, Yoshida M, Obata K. Monosynaptic inhibition of the intracerebellar nuclei induced rom the cerebellar cortex. Experientia. 1964;20:575–6.
Ito M, Yoshida M, Obata K, Kawai N, Udo M. Inhibitory control of intracerebellar nuclei by the Purkinje cell axons. Exp Brain Res. 1970;10:64–80.
Gravel C, Hawkes R. Parasagittal organization of the rat cerebellar cortex: direct comparison of Purkinje cell compartments and the organization of the spinocerebellar projection. J Comp Neurol. 1990;291:79–102.
Ahn AH, Dziennis S, Hawkes R, Herrup K. The cloning of zebrin II reveals its identity with aldolase C. Development. 1994;120:2081–90.
Sarna JR, Marzban H, Watanabe M, Hawkes R. Complementary stripes of phospholipase Cbeta3 and Cbeta4 expression by Purkinje cell subsets in the mouse cerebellum. J Comp Neurol. 2006;496:303–13.
Dehnes Y, Chaudhry FA, Ullensvang K, Lehre KP, Storm-Mathisen J, Danbolt NC. The glutamate transporter EAAT4 in rat cerebellar Purkinje cells: a glutamate-gated chloride channel concentrated near the synapse in parts of the dendritic membrane facing astroglia. J Neurosci. 1998;18:3606–19.
Llinás R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol Lond. 1980;305:171–95.
Llinás R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol Lond. 1980;305:197–213.
McKay BE, Turner RW. Kv3 K+ channels enable burst output in rat cerebellar Purkinje cells. Eur J Neurosci. 2004;20:729–39.
Pouille F, Cavelier P, Desplantez T, Beekenkamp H, Craig PJ, Beattie RE, et al. Dendro-somatic distribution of calcium-mediated electrogenesis in purkinje cells from rat cerebellar slice cultures. J Physiol Lond. 2000;527(Pt 2):265–82.
Womack M, Khodakhah K. Active contribution of dendrites to the tonic and trimodal patterns of activity in cerebellar Purkinje neurons. J Neurosci. 2002;22:10603–12.
Kim CH, Oh SH, Lee JH, Chang SO, Kim J, Kim SJ. Lobule-specific membrane excitability of cerebellar Purkinje cells. J Physiol Lond. 2012;590:273–88.
Cerminara NL, Aoki H, Loft M, Sugihara I, Apps R. Structural basis of cerebellar microcircuits in the rat. J Neurosci. 2013;33:16427–42.
Xiao J, Cerminara NL, Kotsurovskyy Y, Aoki H, Burroughs A, Wise AK, et al. Systematic regional variations in Purkinje cell spiking patterns. PLoS One. 2014;9, e105633.
Zhou H, Lin Z, Voges K, Ju C, Gao Z, Bosman LW, et al. Cerebellar modules operate at different frequencies. Elife. 2014;3, e02536.
Paukert M, Huang YH, Tanaka K, Rothstein JD, Bergles DE. Zones of enhanced glutamate release from climbing fibers in the mammalian cerebellum. J Neurosci. 2010;30:7290–9.
Xiao J, Cerminara NL, Aoki H, Sugihara I, Wise A, Sanderson J, et al. Investigation of potential determinants of the complex spike waveform: Zebrin reactivity and simple spike activity. Society for Neuroscience Meeting Abstracts 2012;580.05.
Sugihara I, Shinoda Y. Molecular, topographic, and functional organization of the cerebellar cortex: a study with combined aldolase C and olivocerebellar labeling. J Neurosci. 2004;24:8771–85.
McDevitt CJ, Ebner TJ, Bloedel JR. The changes in Purkinje cell simple spike activity following spontaneous climbing fiber inputs. Brain Res. 1982;237:484–91.
Kim JJ, Thompson RF. Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends Neurosci. 1997;20:177–81.
Yeo CH, Hardiman MJ, Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit. II. Lesions of the cerebellar cortex. Exp Brain Res. 1985;60:99–113.
Allami N, Paulignan Y, Brovelli A, Boussaoud D. Visuo-motor learning with combination of different rates of motor imagery and physical practice. Exp Brain Res. 2008;184:105–13.
Lacourse MG, Turner JA, Randolph-Orr E, Schandler SL, Cohen MJ. Cerebral and cerebellar sensorimotor plasticity following motor imagery-based mental practice of a sequential movement. J Rehabil Res Dev. 2004;41:505–24.
Lang EJ, Tang T, Suh CY, Xiao J, Kotsurovskyy Y, Blenkinsop TA, et al. Modulation of Purkinje cell complex spike waveform by synchrony levels in the olivocerebellar system. Front Syst Neurosci. 2014;8:210.
Llinás R, Volkind RA. The olivo-cerebellar system: functional properties as revealed by harmaline-induced tremor. Exp Brain Res. 1973;18:69–87.
de Montigny C, Lamarre Y. Rhythmic activity induced by harmaline in the olivo-cerebello-bulbar system of the cat. Brain Res. 1973;53:81–95.
Sugihara I, Marshall SP, Lang EJ. Relationship of complex spike synchrony to the lobular and longitudinal aldolase C compartments in crus IIA of the cerebellar cortex. J Comp Neurol. 2007;501:13–29.
Chung SH, Marzban H, Hawkes R. Compartmentation of the cerebellar nuclei of the mouse. Neuroscience. 2009;161:123–38.
Sugihara I. Compartmentalization of the deep cerebellar nuclei based on afferent projections and aldolase C expression. Cerebellum. 2011;10:449–63.
Lang EJ, Blenkinsop TA. Control of cerebellar nuclear cells: a direct role for complex spikes? Cerebellum. 2011;10:694–701.
Llinás R, Baker R, Sotelo C. Electrotonic coupling between neurons in cat inferior olive. J Neurophysiol. 1974;37:560–71.
Sotelo C, Llinás R, Baker R. Structural study of inferior olivary nucleus of the cat: morphological correlates of electrotonic coupling. J Neurophysiol. 1974;37:541–59.
Blenkinsop TA, Lang EJ. Block of inferior olive gap junctional coupling decreases Purkinje cell complex spike synchrony and rhythmicity. J Neurosci. 2006;26:1739–48.
Marshall SP, van der Giessen RS, de Zeeuw CI, Lang EJ. Altered olivocerebellar activity patterns in the connexin36 knockout mouse. Cerebellum. 2007;6:287–99.
Llinás R, Sasaki K. The functional organization of the olivo-cerebellar system as examined by multiple Purkinje cell recordings. Eur J Neurosci. 1989;1:587–602.
Lang EJ. Organization of olivocerebellar activity in the absence of excitatory glutamatergic input. J Neurosci. 2001;21:1663–75.
Lang EJ. GABAergic and glutamatergic modulation of spontaneous and motor-cortex-evoked complex spike activity. J Neurophysiol. 2002;87:1993–2008.
Lang EJ, Sugihara I, Llinás R. GABAergic modulation of complex spike activity by the cerebellar nucleoolivary pathway in rat. J Neurophysiol. 1996;76:255–75.
Sugihara I, Lang EJ, Llinás R. Serotonin modulation of inferior olivary oscillations and synchronicity: a multiple-electrode study in the rat cerebellum. Eur J Neurosci. 1995;7:521–34.
Eccles JC, Llinas R, Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol Lond. 1966;182:268–96.
Crill WE. Unitary multiple-spiked responses in cat inferior olive nucleus. J Neurophysiol. 1970;33:199–209.
Armstrong DM, Rawson JA. Activity patterns of cerebellar cortical neurones and climbing fibre afferents in the awake cat. J Physiol Lond. 1979;289:425–48.
Mathy A, Ho SS, Davie JT, Duguid IC, Clark BA, Hausser M. Encoding of oscillations by axonal bursts in inferior olive neurons. Neuron. 2009;62:388–99.
Rasmussen A, Jirenhed DA, Zucca R, Johansson F, Svensson P, Hesslow G. Number of spikes in climbing fibers determines the direction of cerebellar learning. J Neurosci. 2013;33:13436–40.
Eccles JC, Llinás R, Sasaki K, Voorhoeve PE. Interaction experiments on the responses evoked in Purkinje cells by climbing fibres. J Physiol Lond. 1966;182:297–315.
Callaway JC, Lasser-Ross N, Ross WN. IPSPs strongly inhibit climbing fiber-activated [Ca2+]i increases in the dendrites of cerebellar Purkinje neurons. J Neurosci. 1995;15:2777–87.
Ekerot C-F, Kano M. Long-term depression of parallel fibre synapses following stimulation of climbing fibres. Brain Res. 1985;342:357–60.
Kawato M. Internal models for motor control and trajectory planning. Curr Opin Neurobiol. 1999;9:718–27.
Kitazawa S, Kimura T, Yin PB. Cerebellar complex spikes encode both destinations and errors in arm movements. Nature. 1998;392:494–7.
Tokuda IT, Han CE, Aihara K, Kawato M, Schweighofer N. The role of chaotic resonance in cerebellar learning. Neural Netw Off J Int Neural Netw Soc. 2010;23:836–42.
Tokuda IT, Hoang H, Schweighofer N, Kawato M. Adaptive coupling of inferior olive neurons in cerebellar learning. Neural Netw Off J Int Neural Netw Soc. 2013;47:42–50.
Schweighofer N, Doya K, Fukai H, Chiron JV, Furukawa T, Kawato M. Chaos may enhance information transmission in the inferior olive. Proc Natl Acad Sci U S A. 2004;101:4655–60.
Makarenko V, Llinas R. Experimentally determined chaotic phase synchronization in a neuronal system. Proc Natl Acad Sci U S A. 1998;95:15747–52.
Schweighofer N, Doya K, Kawato M. Electrophysiological properties of inferior olive neurons: a compartmental model. J Neurophysiol. 1999;82:804–17.
Nishimura H, Katada N, Aihara K. Coherent response in a chaotic neural network. Neural Process Lett. 2000;12:49–58.
Onizuka M, Hoang H, Kawato M, Tokuda IT, Schweighofer N, Katori Y, et al. Solution to the inverse problem of estimating gap-junctional and inhibitory conductance in inferior olive neurons from spike trains by network model simulation. Neural Netw. 2013;47:51–63.
Turecek J, Yuen GS, Han VZ, Zeng XH, Bayer KU, Welsh JP. NMDA receptor activation strengthens weak electrical coupling in mammalian brain. Neuron. 2014;81:1375–88.
Kawato M, Kuroda S, Schweighofer N. Cerebellar supervised learning revisited: biophysical modeling and degrees-of-freedom control. Curr Opin Neurobiol. 2011;21:791–800.
Desclin JC. Histological evidence supporting the inferior olive as the major source of cerebellar climbing fibers in the rat. Brain Res. 1974;77:365–84.
Ito M. Experimental verification of Marr-Albus’ plasticity assumption for the cerebellum. Acta Biol Acad Sci Hung. 1982;33:189–99.
Ivry RB, Spencer RM, Zelaznik HN, Diedrichsen J. The cerebellum and event timing. Ann N Y Acad Sci. 2002;978:302–17.
Lamarre Y, Chapman CE. Comparative timing of neuronal discharge in cortical and cerebellar structures during a simple arm movement in the monkey. Exp Brain Res Ser. 1986;15:14–27.
Llinás R. Eighteenth Bowditch lecture. Motor aspects of cerebellar control. Physiologist. 1974;17:19–46.
Mano Y, Funakawa I, Nakamuro T, Takayanagi T, Matsui K. The kinesiological, chemical and pathological analysis in pulsed magnetic stimulation to the brain. Rinsho Shinkeigaku. 1989;29:982–8.
Ito M, Kano M. Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci Lett. 1982;33:253–8.
Dean P, Porrill J, Ekerot CF, Jorntell H. The cerebellar microcircuit as an adaptive filter: experimental and computational evidence. Nat Rev Neurosci. 2010;11:30–43.
Fujita M. Simulation of adaptive modification of the vestibulo-ocular reflex with an adaptive filter model of the cerebellum. Biol Cybern. 1982;45:207–14.
Wadiche JI, Jahr CE. Patterned expression of Purkinje cell glutamate transporters controls synaptic plasticity. Nat Neurosci. 2005;8:1329–34.
De Zeeuw CI, Ten Brinke MM. Motor learning and the cerebellum. Cold Spring Harb Perspect Biol. 2015;7:a021683.
De Gruijl JR, Hoogland TM, De Zeeuw CI. Behavioral correlates of complex spike synchrony in cerebellar microzones. J Neurosci. 2014;34:8937–47.
De Gruijl JR, Sokol PA, Negrello M, De Zeeuw CI. Modulation of electrotonic coupling in the inferior olive by inhibitory and excitatory inputs: integration in the glomerulus. Neuron. 2014;81:1215–7.
Boele HJ, Koekkoek SK, De Zeeuw CI. Cerebellar and extracerebellar involvement in mouse eyeblink conditioning: the ACDC model. Front Cell Neurosci. 2010;3:19.
De Zeeuw C, Koekkoek B, van Alphen A, Luo C, Hoebeek F, van Der Steen J, et al. Gain and phase control of compensatory eye movements by the flocculus of the vestibulocerebellum. The vestibular system (Handbook of auditory research) 2004. 2004:375–423.
De Zeeuw CI, Yeo CH. Time and tide in cerebellar memory formation. Curr Opin Neurobiol. 2005;15:667–74.
Ito M. Synaptic plasticity in the cerebellar cortex and its role in motor learning. Can J Neurol Sci. 1993;20 Suppl 3:S70–4.
Jirenhed D-A, Hesslow G. Learning stimulus intervals—adaptive timing of conditioned Purkinje cell responses. Cerebellum. 2011;10:523–35.
Rambold H, Churchland A, Selig Y, Jasmin L, Lisberger SG. Partial ablations of the flocculus and ventral paraflocculus in monkeys cause linked deficits in smooth pursuit eye movements and adaptive modification of the VOR. J Neurophysiol. 2002;87:912–24.
Mostofi A, Holtzman T, Grout AS, Yeo CH, Edgley SA. Electrophysiological localization of eyeblink-related microzones in rabbit cerebellar cortex. J Neurosci. 2010;30:8920–34.
Ten Brinke MM, Boele HJ, Spanke JK, Potters JW, Kornysheva K, Wulff P, et al. Evolving models of Pavlovian conditioning: cerebellar cortical dynamics in awake behaving mice. Cell Rep. 2015;13:1977–88.
Badura A, Schonewille M, Voges K, Galliano E, Renier N, Gao Z, et al. Climbing fiber input shapes reciprocity of Purkinje cell firing. Neuron. 2013;78:700–13.
Coesmans M, Weber JT, De Zeeuw CI, Hansel C. Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron. 2004;44:691–700.
Piochon C, Kruskal P, Maclean J, Hansel C. Non-Hebbian spike-timing-dependent plasticity in cerebellar circuits. Front Neural Circuits. 2012;6:124.
Yang Y, Lisberger SG. Role of plasticity at different sites across the time course of cerebellar motor learning. J Neurosci. 2014;34:7077–90.
Kistler WM, De Zeeuw CI. Dynamical working memory and timed responses: the role of reverberating loops in the olivo-cerebellar system. Neural Comput. 2002;14:2597–626.
Welsh JP. Functional significance of climbing-fiber synchrony: a population coding and behavioral analysis. Ann N Y Acad Sci. 2002;978:188–204.
Mathy A, Clark BA, Hausser M. Synaptically induced long-term modulation of electrical coupling in the inferior olive. Neuron. 2014;81:1290–6.
De Zeeuw CI, Wentzel P, Mugnaini E. Fine structure of the dorsal cap of the inferior olive and its GABAergic and non-GABAergic input from the nucleus prepositus hypoglossi in rat and rabbit. J Comp Neurol. 1993;327:63–82.
De Zeeuw CI, Wylie DR, Stahl JS, Simpson JI. Phase relations of Purkinje cells in the rabbit flocculus during compensatory eye movements. J Neurophysiol. 1995;74:2051–64.
Bazzigaluppi P, De Gruijl JR, van der Giessen RS, Khosrovani S, De Zeeuw CI, de Jeu MT. Olivary subthreshold oscillations and burst activity revisited. Front Neural Circuits. 2012;6:91.
Bazzigaluppi P, Ruigrok T, Saisan P, De Zeeuw CI, de Jeu M. Properties of the nucleo-olivary pathway: an in vivo whole-cell patch clamp study. PLoS One. 2012;7, e46360.
Boele HJ, Koekkoek SK, De Zeeuw CI, Ruigrok TJ. Axonal sprouting and formation of terminals in the adult cerebellum during associative motor learning. J Neurosci. 2013;33:17897–907.
Winkelman BH, Belton T, Suh M, Coesmans M, Morpurgo MM, Simpson JI. Nonvisual complex spike signals in the rabbit cerebellar flocculus. J Neurosci. 2014;34:3218–30.
De Zeeuw CI, Hoebeek FE, Bosman LWJ, Schonewille M, Witter L, Koekkoek SK. Spatiotemporal firing patterns in the cerebellum. Nat Rev Neurosci. 2011;12:327–44.
Person AL, Raman IM. Purkinje neuron synchrony elicits time-locked spiking in the cerebellar nuclei. Nature. 2011;481:502–5.
Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4:885–900.
Bagnall MW, du Lac S. A new locus for synaptic plasticity in cerebellar circuits. Neuron. 2006;51:5–7.
Voogd J, Schraa-Tam CK, van der Geest JN, De Zeeuw CI. Visuomotor cerebellum in human and nonhuman primates. Cerebellum. 2012;11:392–410.
Vinueza Veloz MF, Zhou K, Bosman LW, Potters JW, Negrello M, Seepers RM, et al. Cerebellar control of gait and interlimb coordination. Brain Struct Funct. 2014.
Nieuwenhuys R, Puelles L. Towards a new neuromorphology. Heidelberg: Springer; 2016.
Devor A. Is the cerebellum like cerebellar-like structures? Brain Res Brain Res Rev. 2000;34:149–56.
Farris SM, Schulmeister S. Parasitoidism, not sociality, is associated with the evolution of elaborate mushroom bodies in the brains of hymenopteran insects. Proc Biol Sci. 2011;278:940–51.
Hashimoto M, Hibi M. Development and evolution of cerebellar neural circuits. Dev Growth Differ. 2012;54:373–89.
Buzsaki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16:130–8.
Granit R, Phillips CG. Excitatory and inhibitory processes acting upon individual Purkinje cells of the cerebellum in cats. J Physiol Lond. 1956;133:520–47.
Bell CC, Grimm RJ. Discharge properties of Purkinje cells recorded on single and double microelectrodes. J Neurophysiol. 1969;32:1044–55.
Murphy JT, Sabah NH. The inhibitory effect of climbing fiber activation on cerebellar purkinje cells. Brain Res. 1970;19:486–90.
Latham A, Paul DH. Spontaneous activity of cerebellar Purkinje cells and their responses to impulses in climbing fibres. J Physiol Lond. 1971;213:135–56.
Burg D, Rubia FJ. Inhibition of cerebellar Purkinje cells by climbing fiber input. Pflugers Arch. 1972;337:367–72.
Bloedel JR, Roberts WJ. Action of climbing fibers in cerebellar cortex of the cat. J Neurophysiol. 1971;34:17–31.
Bloedel JR, Ebner TJ, Yu Q. Increased responsiveness of Purkinje cells associated with climbing fiber inputs to neighboring neurons. J Neurophysiol. 1983;50:220–39.
Ebner TJ, Bloedel JR. Temporal patterning in simple spike discharge of Purkinje cells and Its relationship to climbing fiber activity. J Neurophysiol. 1981;45:933–47.
Ebner TJ, Bloedel JR. Role of climbing fiber afferent input in determining responsiveness of Purkinje cells to mossy fiber inputs. J Neurophysiol. 1981;45:962–71.
Ebner TJ, Yu Q, Bloedel JR. Increase in Purkinje cell gain associated with naturally activated climbing fiber input. J Neurophysiol. 1983;50:205–19.
Mano N, Kanazawa I, Yamamoto K. Complex-spike activity of cerebellar Purkinje cells related to wrist tracking movement in monkey. J Neurophysiol. 1986;56:137–58.
Sato Y, Miura A, Fushiki H, Kawasaki T. Short-term modulation of cerebellar Purkinje cell activity after spontaneous climbing fiber input. J Neurophysiol. 1992;68:2051–62.
Miall RC, Keating JG, Malkmus M, Thach WT. Simple spike activity predicts occurrence of complex spikes in cerebellar Purkinje cells. Nat Neurosci. 1998;1:13–5.
Montarolo PG, Palestini M, Strata P. The inhibitory effect of the olivocerebellar input on the cerebellar Purkinje cells in the rat. J Physiol Lond. 1982;332:187–202.
Rawson JA, Tilokskulchai K. Suppression of simple spike discharges of cerebellar Purkinje cells by impulses in climbing fibre afferents. Neurosci Lett. 1981;25:125–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed, and all procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Lang, E.J., Apps, R., Bengtsson, F. et al. The Roles of the Olivocerebellar Pathway in Motor Learning and Motor Control. A Consensus Paper. Cerebellum 16, 230–252 (2017). https://doi.org/10.1007/s12311-016-0787-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-016-0787-8