Abstract
Current research in cerebellar cognitive and linguistic functions makes plausible the idea that the cerebellum is involved in processing temporally contiguous linguistic input. In order to assess this hypothesis, a lexical decision task was constructed to study the effects of cerebellar transcranial magnetic stimulation on semantic noun-to-verb priming based on association (e.g. ‘soap–cleaning’) or similarity (e.g. ‘robbery–stealing’). The results demonstrated a selective increase in associative priming size after stimulation of a lateral cerebellar site. The findings are discussed in the contexts of a cerebellar role in linguistic expectancy generation and the corticocerebellar ‘prefrontal’ reciprocal loop.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While the primary motor cortex is reciprocally connected with lobules V, VI, VIIb and VIIIa of the cerebellar cortex, prefrontal cortical areas are reciprocally connected with neocerebellar compartments, such as the cerebellar cortical Crus I and II [30, 40]. This modular, reciprocal connectivity of the cerebellum with a broad range of cortical areas, along with its cytoarchitectural homogeneity, provides a solid foundation for the view that the cerebellar cortex applies its algorithms in a domain-general uniform fashion to all of its inputs, crucially involving those of higher cognitive processes (e.g. [54, 58]). In parallel, the cerebellum is indeed widely supported to instantiate computations of state estimation [48] and feedforward control [71]. These computations are fundamental for acquiring associations between and generating predictions about temporally contiguous events for stimuli in sensory, motor, emotional and cognitive processing (e.g. [5, 8, 63]). The cerebellum has indeed been established as a fundamental site for classical conditioning (e.g. [7]) in both comparative (e.g. [37]) and human clinical studies (e.g. [9]). Such findings have been extended with reports of cerebellar impairments in learning associations between visual cues and motor commands [6, 68] and, more recently, in nonmotor associative learning [13, 62, 64]. It has been recently proposed that, in language processing, multi-level relations between co-occurring linguistic events can be acquired, stored and adapted in neocerebellar circuitry [1, 3]. The hypothesis was first explored in Argyropoulos [2], where the cerebellum was shown to selectively affect phrasal associative priming, i.e. priming that occurs in processing words that are temporally contiguous in speech ([61], p. 440; e.g. ‘gift–horse’, ‘skeletons–closet’), while leaving semantic categorical priming, i.e. priming owed to the similarity of semantic properties of words that do not co-occur in speech (e.g. ‘chicken–hen’, ‘storm–weather’) unaffected. These findings were in line with those yielded by Leggio and colleagues, where cerebellar damage was demonstrated to impair verbal fluency by affecting phonemic rule-based word production, yet sparing semantic rule-based performance [35].
Stronger evidence is currently available for neocerebellar contributions to lexical semantic associations. Imaging [23, 52] and patient studies [20, 24] have strongly implicated neocerebellar involvement in non-motor aspects of verb generation, where subjects are asked to provide a related verb response (e.g. ‘eat’) for a noun-stimulus (e.g. ‘cake’). Importantly, responses of cerebellar patients are inappropriate but remain categorically related (e.g. ‘small’, instead of take or ‘swallow’, in response to ‘pill’), supporting the idea that cerebellar damage leaves semantic networks intact [21]. Gebhart et al. [24] have demonstrated two suggestive dissociations in patients’ performance, as they performed poorly in generating appropriate verbs for nouns, but selected the correct verb for a noun from a list of alternative verb responses. This suggests that the lexical semantic/syntactic representations of noun–verb associations were preserved in a conceivably cortex-based memory; however, the online cerebellar-based ‘internal generation’ of verb responses to noun stimuli was compromised. The patients were also able to produce appropriate subordinate term (e.g. ‘apple’) responses to a superordinate term (‘fruit’) presented to them, suggesting that ‘[t]he right posterolateral cerebellum may be more involved in associative semantics than in categorical semantics’ ([24], p. 332).
However, there is currently no evidence that the same disruptions may be found in applying semantic associations in language comprehension. Moreover, there is no TMS study examining cerebellar involvement in semantic associations in either production or comprehension. Encouragingly, though, recent TMS evidence suggests cerebellar involvement in other related semantic aspects of verb processing. Oliveri and colleagues [46] examined whether processing the tense of a verb is influenced by the space where response takes place and by the semantics of the verb. Indeed, responses to past tense were facilitated in the left space while responses to future tense were facilitated in the right space. Above all, right cerebellar stimulation selectively decelerated responses to future tense of action verbs, while stimulation of both cerebellar hemispheres compromised response accuracy to past tense in the left space and to future tense in the right space for non-verbs and to future tense in the right space for state verbs. Their results thus suggested that right cerebellar–left motor networks could be involved in using past experiences to anticipate events, while both cerebellar hemispheres could participate in establishing grammatical rules for verb conjugation [46].
Furthermore, a limitation of the study in Argyropoulos [2] was that the two priming types, i.e. phrasal associative (e.g. ‘pony–tail’) and semantic categorical (e.g. ‘church–building’) differed in two separate axes, namely the level of language processing, i.e. phonological vs semantic, and the alleged mechanism, associative vs categorical. On the contrary, the study designed here involved two semantic priming types that differed only in one axis, namely the alleged mechanism. One stimulus set thus pertained to semantic associative priming, where the denotation of the noun is associated with a particular action denoted by the following verb (e.g. ‘chef–cooking’), and the other set involved semantic categorical relations, where the noun denotes an action that is semantically similar with the action denoted by the verb (e.g. ‘theft–stealing’). Moreover, the two cerebellar linguistic TMS studies conducted so far [2, 4] have involved repeated sessions within subjects, thus unavoidably inducing learning effects. Indeed, depending on the nature of the task, the repetition of trials and stimuli across repeated sessions for the same participant may involve learning effects that the experimenter may wish to avoid (e.g. [65]).
The Present Study
A priming paradigm was found appropriate for two basic reasons. First, there was a need to demonstrate that the cerebellum is involved in processing semantic associations in language comprehension apart from its well-documented involvement in language production. Second, priming paradigms are of significant methodological value in drawing inferences on mechanisms of sentence comprehension, since ‘the priming task […] represents a lower bound on the information available to the comprehender by eliminating constraints offered by the other nouns in the sentence, case marking, context and so on. Word–word priming, as a measure of what is activated in the absence of other constraints, thus offers a stringent test for studying these phenomena’ ([38], p. 1176).
Lexical decision tasks are ideal for studying effects of lexical priming, semantic or phrasal, categorical or associative in nature (e.g. [41]). Following a typical lexical decision task setup, two-letter strings appeared at the centre of the screen in quick succession in each trial. Participants were asked to silently read the first letter string (‘the prime’) and judge whether the second one (‘the target’) was a word of the English language or not. Prime words could be related or unrelated to the target word (see below). We thus investigated whether stimulation of different cerebellar sites had an impact on associative as opposed to categorical noun-to-verb priming.
Stimuli
Each session consisted of a total of 500 trials/pairs: 250 filler word–nonword pairs and 250 word–word pairs; the latter consisted of 200 test pairs (100 related, 100 unrelated) and 50 unrelated filler word–word pairs. Thus, the relatedness ratio was kept low (1:5), as in other studies, to minimize the involvement of strategic effects (e.g. [2, 4, 55]). Related pairs were divided into two sets: one of categorically related pairs and one of associated pairs. In the semantic categorical set, the related pairs included prime nouns denoting actions that were virtually identical or similar to those denoted by the target verbs (e.g. ‘robbery–stealing’, ‘applause–clapping’). Thus, facilitation in processing such related pairs would be a case of semantic categorical priming, in which the two terms are synonymous, or, at least, coordinate exemplars within the same category. Semantic similarity was determined using the ‘Path Length’Footnote 1 measure of ‘WordNet: Similarity software’ [49] Footnote 2 computed over ‘WordNet’ [14]. Categorically related noun–verb pairs were by far more semantically similar (priming type: F (1, 198) = 553.5, MSe = 0.03, p < 0.0005; mean = 0.73, SD 0.25, min 0.5, max 1; Table 1) than associatively related ones (mean = 0.12, SD = 0.06, min 0.05 max 0.33; Table 2). In addition, it was ensured that the noun–verb pairs did not immediately co-occur in speech, as assessed in the BNC (British National Corpus; written part [34]). Co-occurrence strength was thus kept low and matched for the two sets (mean 0.07, SD 0.27; priming type: F < 0.1), in order to emphasize the different aspects of semantic priming.
In the ‘semantic associative’ set, related pairs involved nouns that were appropriate thematic (‘θ’) role slot fillers projected by the corresponding verb. All four major θ-role-based subtypes were employed here, involving agent- (e.g. ‘butcher–carving’), patient- (e.g. ‘lawn–mowing’), instrument- (e.g. ‘scissors–cutting’) and location- (e.g. ‘casino–gambling’) based priming. Since there was no a priori hypothesis about effects on the particular strengths of different θ-role relations and given that the experiment examined θ-role priming in general as opposed to semantic categorical priming, pairs of the four basic θ-role relations were evenly distributed across the four lists. Each list contained nine agent–, five instrument–, four location– and seven patient–verb pairs. The pairs were taken from the stimulus sets used in McRae et al. [38] and Ferretti et al. [15, 16, 17]. In addition, as with the semantic categorical set, it was ensured that the words of the pairs did not co-occur in an immediate fashion. Whereas McRae and colleagues [38] attribute such priming effects to phrasal co-occurrence and/or semantic association, the emphasis here was given only to the latter, and to that end, only pairs with low phrasal co-occurrence were selected. Thus, pairs like ‘wine–tasting’, ‘ballroom–dancing’ that combine phrasal and semantic associative relations or pairs such as ‘toy–playing’ that combine phrasal-associative and semantic categorical relations were excluded.Footnote 3 Several more pairs from the sets of McRae and colleagues were excluded due to extremely frequent/infrequent words in the BNC (e.g. ‘patrolman–searching’), or due to coincidences of the same phoneme in the starting position between prime and target word (e.g. ‘crayon–colouring’), which is avoided in lexical decision setups [22].
As in McRae et al. [38] and other studies (e.g. [4]), four lists were generated, across which pairs and participants were rotated. The stimuli of the second half of the first list were the stimuli of the first half of the second list and vice versa, while each target word in the two sets appeared with a related prime in one list and with an unrelated prime in another (Table 3). Both categorical and associative sets of related and unrelated pairs were separately balanced across the four lists in prime/target word length/frequency (list: for categorical and associative F’s, F < 1; Tables 1 and 2).
Task Procedure
Prime and target stimuli were presented in black colours at the centre of a white screen of a laptop using the ‘DirectRT’ software [28]. Each trial consisted of the following sequence of three stimuli presented on the same screen location. First a fixation point (a cross ‘+’) was presented for 250 ms, immediately followed by presentation of the prime for 150 ms, which was followed immediately by the presentation of the target word in the same location as the prime. The targets remained on the screen until participants responded. No masking mediated the presentation of the prime and the target word, i.e. the stimulus-onset asynchrony value was confounded with the one of the prime duration (Fig. 1). Such short durations of prime presentation were preferred, as asynchrony values longer than 200–250 ms are generally considered to involve strategic effects in lexical decisions (e.g. [51]) and are thus often not preferred in studying automatic lexical priming. The inter-trial interval lasted 500 ms. Primes and targets were always presented in lowercase letters. Participants were instructed to focus on the fixation point, silently read the first letter string and respond only to the second one. Stimulus presentation was randomized, with a different order for each subject. Subjects were instructed to press one of two buttons on the keyboard (‘j’ for yes and ‘f’ for no) to indicate whether the target letter string was an English word or not, as rapidly and accurately as possible. They used their dominant right-hand index for the word responses. When the subject responded, the target disappeared from the screen. In order to encourage fast lexical decisions, a message appeared on the screen (‘Please try to respond faster!’) after each trial in which the subject would respond after 1,500 ms. Each subject received a total of 20 practice trials prior to the 500 experimental trials. Participants were tested individually in a silent, brightly lit room. They received written instructions explaining the task, as well as compensation for their participation.
TMS Experiment
The TMS experiment was conducted in the Institute of Cognitive Neuroscience (University College, London).
TMS Apparatus
Upon completion of the first half of the session, TMS was delivered via a 70-mm figure-of-eight-shaped coil connected to a Magstim Super Rapid Transcranial Magnetic Stimulator (Magstim Company, Whitland, UK). The coil was positioned tangentially to the scalp, with the handle pointing superiorly. The current in the coil was directed upward, which induced downward current in the cerebellar cortex. This coil position was found to be optimal for suppressing the contralateral motor cortex in single pulse TMS investigations (e.g. [44]) and to interfere with cognitive processes in 1 Hz rTMS (e.g. [66]) and continuous theta-burst stimulation paradigms (e.g. [2, 4]).
TMS Coordinates
As in other TMS studies (e.g. [2, 4, 60]), the experiment involved the stimulation of a (right) medial and a (right) lateral cerebellar site. As in previous studies conducted [2, 4], a disadvantage here was that no neuronavigational software was used to individuate the exact site of stimulation for each participant. The sites were instead defined on the basis of reliable scalp-based coordinates, as in many other cerebellar TMS studies (e.g. [2, 4, 31, 39, 44]). The right medial site was located 1 cm below and 1 cm laterally to the right from the inion. This location was selected as it is likely that the underlying cerebellar compartments would involve the right posterior superior (i.e. neocerebellar) vermis. Indeed, previous investigations have demonstrated that cerebellar stimulation predominantly affects posterior and superior lobules [26, 69], corresponding to lobules VII and VIII [33]. Moreover, the neocerebellar vermis is one of the compartments closest to the TMS coil [39], while TMS on the same coordinates has induced behavioural effects with high spatial precision and has been argued to affect lobules VI and VII [26, 43] in Larsell and Jansen’s [33] nomenclature. Cognitive effects induced by neocerebellar vermal stimulation have been shown in studies employing MRI- [11] and scalp-based coordinates [2, 4]. In order to estimate the depth of this site, a volunteer was employed whose brain image was already registered with the Brainsight TMS-MRI co-registration system (Rogue Research, Montreal, QC, Canada). This site was found to be at a depth of 21 mm from the scalp surface. The right lateral site was localized on the basis of non-motor-related activations of the right neocerebellar Crus I in fMRI studies (Talairach space) of verb generation ([23]; x = 48 mm, y = −60 mm, z = −30 mm) and stem completion ([47]; x = 41 mm, y = −55 mm, z = −18 mm). Both coordinates were registered and converted into scalp coordinates using the Brainsight™ TMS-MRI co-registration system (Rogue Research, Montreal, QC, Canada) in one volunteer and were found to correspond to 10 cm laterally to the right from the inion. Both sites were marked using non-permanent colour markers.
TMS Protocol
The rTMS protocol used was continuous theta-burst stimulation [27], employing a brief burst of three low-intensity, high-frequency (50 Hz) TMS pulses delivered at a 5-Hz rhythm. This protocol provides a rapid and reliable stimulation method increasingly used in current TMS research (e.g. [56]). Encouragingly, theta-burst stimulation of the cerebellar vermis has recently been proven safe and well-tolerated, offering the potential to modulate cognition and affect in schizophrenia [11]. Similarly, theta-burst stimulation has been recently applied on right posterolateral cerebellar loci, inducing changes in the excitability of the contralateral primary motor cortex in healthy subjects [31] and Parkinsonian patients [32]. The experiment employed the offline continuous theta-burst stimulation procedure, which is used to suppress cortical activity in cognitive studies (e.g. [70]): After completion of the first half of each session, 40 s (600 pulses) of continuous cerebellar theta-burst stimulation was applied on the cerebellum. Before cerebellar TMS, all subjects were first administered a 3-s continuous theta-burst stimulation. All subjects but one tolerated theta-burst stimulation well, reporting but mild discomfort due to muscle twitching in the right-side neck and face muscles. The subject that did not find the 3-s test stimulation tolerable was excused from the rest of the session. The session lasted approximately 35 min.
TMS Intensity
As in other studies (e.g. [2, 4]), stimulus intensity was kept constant across participants to 45 % of maximum machine output. A fixed stimulation level was employed, as it has proven successful and replicable in a wide range of studies and tasks (e.g. [36] and references therein). While many studies of cerebellar stimulation define the intensity on the basis of individual subjects’ motor thresholds, these are not particularly appropriate, since motor cortical excitability is not a good index of TMS thresholds in other cortical areas—for instance, there is no systematic relationship between the threshold needed to evoke a motor-evoked potential and the threshold needed to evoke phosphenes in visual cortical TMS [12, 59]. A fortiori for a subcortical and even more distant locus like the cerebellum, motor thresholds were seen as even less significant, and thus, a fixed stimulus intensity was employed across participants, also economizing on the duration of each session. Indeed, the applicability of motor threshold-based definitions of stimulation amplitude to the cerebellum remains debatable and needs to be treated by further research (see also [10, 11]). The 45 % of maximum machine output here represented a strong intensity level for continuous theta-burst stimulation, as compared with the 40 % commonly used in studies employing fixed intensities (e.g. [29, 36] and references therein).
Subjects
Fifty subjects were initially recruited, all being right-handed native speakers of English, with normal or corrected-to-normal vision and no known reading, attention or motoric deficits. They were divided into four groups: two groups that received cerebellar stimulation after completing the first phase of the session (henceforth, ‘TMS groups’) and two groups that received no TMS (henceforth, ‘no TMS groups’). Twenty-four subjects were initially recruited for the TMS groups. Twelve participated in the stimulation of the lateral cerebellum (henceforth, ‘lateral TMS group’) and 12 in the stimulation of the medial cerebellum (henceforth, ‘medial TMS group’). From the medial TMS group, one participant was excluded as she could not tolerate the intensity of stimulation in either site and was thus excused from further participation. From the no TMS groups (n = 26), one group (n = 13) completed the session with no mediating break between the first and second phase (henceforth, ‘no TMS group 1’), while group 2 (n = 13) were asked to complete the session with a 7-min’ break mediating between the first and the second half of the session (the equivalent time for the preparation and the application of TMS for the other two groups). Two subjects were excluded from the first no TMS group and one subject from the second one, as they were found to have completed earlier versions of the same task before this experiment.Footnote 4 The groups did not differ in age (lateral TMS group: mean 25.58 years, SD 7.79; medial TMS group: mean 22.00 years, SD 4.22; no TMS group 1: mean 21.67 years, SD 2.57; no TMS group 2: mean 21.73 years, SD 7.17; group 4: p > 0.3; post hoc comparisons employing Tukey’s honestly significant difference (HSD): all p’s, p > 0.35). Ethical approval was granted by the local ethics committee.
Design and Statistical Analysis
A 4 × 2 × 2 × 2 mixed design was employed, with the following independent variables: ‘Group’ (four; lateral TMS, medial TMS, no TMS 1, no TMS 2), ‘Priming Type’ (two; associative, categorical), ‘Phase’ (two; pre-/post-TMS) and ‘Relatedness’ (two; unrelated, related). After the end of the first half of the session, subjects were stimulated either in the medial (‘medial TMS’ group) or in the lateral cerebellar site (‘lateral TMS’ group), or they received no TMS at all, with no break time mediating between the two phases (‘no TMS group 1’), or with a 7-min break time mediating (‘no TMS group 2’). They encountered either associatively (‘gun–shooting’, ‘chef–cooking’) or categorically (‘robbery–stealing’, ‘applause–clapping’) related pairs or their corresponding unrelated versions (e.g. associative set: ‘chef–shooting’, ‘gun–cooking’; categorical set: ‘robbery–clapping’, ‘applause–stealing’), and the priming sizes were assessed before and after the half-time break (phase: pre-/post-TMS; Table 4).
In a subsequent analysis (“Analysis of Thematic Roles” section), the associative priming set was subdivided into four subsets according to the θ roles that the nouns would be assigned by the verb (“Stimuli” section) in the related condition, yielding a 4 × 2 × 2 × 4 design (Association Type, Phase, Relatedness, Group). This was done in order to examine whether any changes induced by cerebellar TMS on associative priming sizes was selective for Association Type or not (Table 5).
The primary dependent measure was lexical decision latencies, but accuracy rates were also analysed. Reaction times were trimmed by condition, excluding any trials receiving latencies exceeding 3 SDs above the mean. In all cases, this trimming excluded trials receiving latencies longer than 1,200 ms, which are taken to reflect the subject’s low familiarity with the stimulus or distraction, rather than lexical access [50]. No subject exceeded 3 SDs above the mean for the percentage of excluded trials for latencies (min 0 %; max 3 %; mean 1.10 %; SD 0.80 %) or for mistakes (min 0 %; max 8.50 %; SD 2.20; mean 4.30 %). Two pairs/trials were also deleted from the categorical priming set, an unrelated and a related pair version with ‘ousting’ as a target verb, since over 50 % of the participants erred on this particular lexical decision. In an alternative analysis, the List the subjects were assigned to (A–D) was also included as a between-subjects dummy variable, in order to also assess whether this interacted with any of independent variables [53]. This was important, since, due to availability constraints for participants, their complete rotation across the four lists was not possible.Footnote 5 In another analysis, latencies were also log-transformed without applying the 3 SD cutoff point. The results yielded did not compromise the significance of those reported below. Accuracy rates were submitted to a separate analysis. Finally, the same results could be demonstrated by employing the ratio/difference between the latencies and/or accuracy rates of the unrelated pairs with those of the related ones as a dependent measure, as often practiced in neuropsychological research (e.g. [2, 42, 55]).
Results
Automatic and Strategic Priming Effects
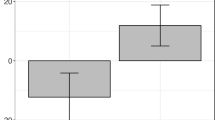
We first investigated the priming effects observed across groups. While there was a significant main effect of priming, with related pairs receiving shorter latencies than unrelated ones, there was also a selective occurrence of associative priming only in the second phase of the session. Categorical priming, on the other hand, occurred across phases (Fig. 2; Table 6).
Mean lexical decision latencies (milliseconds) for unrelated and related pairs of the associative and categorical priming sets pre- and post-TMS for the four subject groups (lateral TMS, medial TMS, no TMS 1, no TMS 2). Categorical priming is observed across groups and phases, while associative priming reaches significance across groups only in the second phase. Crucially, the increase in associative priming is significantly stronger after lateral cerebellar TMS; REL related pairs, UNR unrelated pairs; *p < 0.05; **p < 0.01; ***p < 0.005; *****p < 0.000005; NS: F < 1 or p > 0.07; see Table 7 for the rest of the effects and interactions
Effects of Lateral Cerebellar TMS on Semantic Associative Priming
Above all, however, the four-way ANOVA above yielded a stronger Priming Type × Phase × Relatedness × Group interaction. As demonstrated by Table 7 and Fig. 2, this was owed to the size of associative priming after lateral TMS. Indeed, a series of three-way ANOVAs conducted separately for each group showed that the Priming Type × Phase × Relatedness interaction reached significance only for the lateral TMS group. Furthermore, a three-way ANOVA on associative priming sizes demonstrated that, while priming only occurred post-TMS, the sizes observed for the lateral TMS group were significantly larger. On the contrary, there was no change in categorical priming sizes. Moreover, a Priming Type × Relatedness × Group interaction was only observed post-TMS, and not pre-TMS, suggesting that the four-way interaction was owed to TMS effects and not to differences in baseline conditions. A series of two-way ANOVAs demonstrated that lateral TMS selectively enhanced associative priming, since only the lateral TMS group showed a selective increase in associative priming. Indeed, the lateral TMS group showed significantly larger associative priming post-TMS than the medial TMS group, the no TMS group 1 and the no TMS group 2. Comparisons of the other groups in pairs showed no other significant difference in associative priming. Finally, only the lateral TMS group showed significant associative priming in the second phase, while the other groups showed but non-significant trends.
A similar analysis on accuracy rates (Table 8) did not replicate any such findings. Notably, however, the no TMS group 2 showed significant associative priming in the second phase (higher accuracy rates for related than for unrelated pairs) and so did the lateral TMS group for categorical priming post-TMS. The latter finding thus demonstrated that categorical priming was still preserved after lateral cerebellar TMS. However, neither of these effects was selective. Finally, the results above could only be captured as effects of lateral TMS on the difference between unrelated and related pairs of the associative priming set, and not selectively on unrelated or related pairs of that set. Comparing the latencies and the accuracy rates of the four groups per condition showed no significant differences (for all p’s, p > 0.14; for all post hoc comparisons Tukey’s HSD: p > 0.12).
Analysis of Thematic Roles
As described above, we also examined whether the increase in associative priming size after lateral TMS pertained selectively to one of the four different association types employed for the construction of the associative priming set (“Stimuli” and “Design and Statistical Analysis” sections). An analysis of latencies across conditions only demonstrated the increase in associative priming sizes after stimulation of the lateral cerebellar site. This increase was not selective or stronger for one of the four subtypes (Table 9). A similar analysis of accuracy rates across conditions showed no effect of TMS on priming sizes.
Discussion
The results above demonstrate that lateral cerebellar TMS selectively enhanced semantic associative noun-to-verb priming. While an increase in associative priming sizes was also observed across groups, those found for the lateral cerebellar TMS group were significantly stronger. This pattern could not be explained in terms of changes in the speed/accuracy trade-off, as no selective effects were observed for a similar analysis in accuracy rates. Moreover, categorical priming was still significant in the post-TMS phase for the lateral TMS group in the form of accuracy rates. The fact that the effect of lateral cerebellar TMS was selective for semantic associative and not for semantic categorical priming is in line with the findings in Gebhart et al. [24], where cerebellar patients showed spared performance in generating appropriate subordinate terms for given superordinate ones (e.g. stimulus: ‘fruit’; response: ‘apple’) but were able to select the appropriate verb for a given noun from a list, despite their deficits in verb generation.
The selective nature of this effect for TMS group, phase and priming type demonstrates that this pattern cannot be attributed to effects of cerebellar stimulation on reading or processing the semantic properties of noun primes or verb targets. Importantly, the fact that the priming enhancement could not be attributed to selective accelerations on processing related pairs or to decelerations on processing the unrelated pairs of the associative priming set would suggest that cognitive disruptions are at least as plausible as cognitive enhancements. In other words, the selective boost in associative priming may not necessarily result from a cognitive enhancement in processing semantic associates (e.g. ‘chef–cooking’) but from disruptions in processing the semantics of a non-associated event (e.g. cooking′) instead of an associated one (e.g. shooting′) on the basis of predictions generated upon processing the semantics of the prime word (e.g. pistol′).
Moreover, the fact that associative priming was only observed in the second phase of the experimental session across groups might suggest that the large increase in associative priming size for the lateral TMS group may also be strategic in nature. Thus, instead of a direct enhancement of non-attentional semantic associative priming, lateral cerebellar TMS might have arguably disrupted automatic access to associative memory. In so doing, it might have prompted heavier involvement of the strategic component, which may arguably be at work here. In order to validate this interpretation, tasks showing priming independently of phase should be used instead. Indeed, in a recent cerebellar TMS study [67], cerebellar stimulation resulted in selective facilitation of contralateral M1 excitability during procedural learning. While the effects were manifested when motor learning was obtained by actual execution of the task or by observation, they disappeared if learning had already been acquired by preceding observation. It would be thus tempting to examine whether the effects of lateral cerebellar TMS observed here were more related with such procedural learning aspects rather than associative priming per se.
An important question would be whether the behavioural disruptions induced are owed to an indirect effect on prefrontal cortical loci with which the cerebellum is connected in a reciprocal long distance fashion (see “Introduction” for references) or whether these changes are exclusively owed to disruptions in cerebellar function. In support of the former, cerebellar TMS has indeed been shown to indirectly modulate the excitability of the contralateral primary motor cortex ([19, 31, 44]; also [45] for references and discussion). A highly plausible mechanism may thus be the transient depression of the excitability of Purkinje cells of the right lateral cerebellar cortex, which may release from inhibition the excitatory output of the ventral, ‘neodentate’ nucleus to its contralateral (left, especially prefrontal, here) cerebrocortical output loci via dentatothalamic pathways. However, as in other cerebellar TMS studies (e.g. [39]), the experimental design here does not suffice to address these questions, and the addition of more conditions (e.g. prefrontal cortical TMS sites) would be necessary to establish any dissociations. Characteristically, though, in the cerebellar transcranial direct current stimulation study of Ferrucci et al. [18], contrastive stimulation of the prefrontal cortex induced an immediate change in task performance, while sparing the practice-related proficiency changes in the working memory task used, which were selectively affected after cerebellar stimulation. Observing such a pattern here would further corroborate hypotheses on distinct contributions of cerebellar computations to those of the ‘prefrontal’ corticocerebellar circuit [54], as also studied in language processing [1, 2]. It thus remains to be clarified whether these associative computations are performed exclusively by the cerebellum or whether they are performed in a broader fashion by corticocerebellar circuits, with the cerebellum being just one of conceivably more loci undertaking such operations. A related question that should be addressed as well would be whether the left prefrontal/parietal cerebrocortical outputs of the cerebellar areas of interest here are inhibitory or excitatory. For instance, cerebellar projections to the motor cortex terminate on both excitatory and inhibitory interneurons and cerebellar TMS results in changes to both inhibitory and excitatory neurons in the contralateral motor cortex (see [45] for references and discussion). Answering this question would be of fundamental significance for explaining the directionality of the cerebellar TMS effects observed. With respect to the present findings, though, while the possibility of the disinhibition of prefrontal cortical outputs following cerebellar continuous TBS cannot be excluded as the underlying neurofunctional explanation of enhanced associative priming, cerebral language pathology has yet to establish disruptions of associative priming as outcomes of damage to a particular cerebrocortical locus. For instance, patients with Alzheimer’s disease and semantic dementia show preserved associative priming but impaired semantic categorical priming (e.g. [55]), while Broca’s or Wernicke’s aphasics also show no evidence for disrupted lexical semantic or associative priming (e.g. [25]).
Another issue is the lack of any non-sensorimotor-related effects after medial cerebellar TMS. While a previous study on phrasal associative priming demonstrated selective enhancements after right medial cerebellar TMS [2], there were no changes in priming sizes observed here. A plausible explanation would be that, while the previous study had investigated phrasal associative priming between immediately co-occurring nouns (e.g. ‘gift–horse’), the study here investigated semantic associative priming between non-immediately co-occurring nouns and verbs (e.g. ‘gift–accepting’). However, the medial TMS group also failed to demonstrate categorical priming after stimulation, and thus, a sensorimotor disruption should rather be promoted as an explanation, in terms of a disruption in perceiving the prime word. Vermal pathology is indeed strongly associated with occulomotor deficits that may compromise reading (see Argyropoulos [2, 4]) for references). In fact, the study here used visual settings significantly different from those in Argyropoulos [2]: Both prime and target words here appeared in lowercase black letters at the centre of a white screen, and participants were sat in a brightly lit room. On the contrary, the visual settings of the Argyropoulos [2] study did not impose such heavy perceptual demands: Prime words appeared in lowercase, while target words appeared in uppercase letters; stimuli appeared in green fonts against a black background and were perceived in a dimly lit room; thus, perceptual disruptions accompanying medial cerebellar TMS might have been alleviated to a considerable extent. Hence, it remains an open question whether medial cerebellar TMS in contexts of lower perceptual demands would induce cognitive effects in the same direction as those of lateral cerebellar TMS. Admittedly, selecting this medial locus as a second (‘control’) site was a methodological weakness of the experiment, since there is evidence for the involvement of posterior superior vermal compartments in both occulomotor and cognitive aspects of linguistic task performance (see Argyropoulos [2, 4]) for references). In this study, then, reliable control conditions were generated by the related or unrelated pairs of the categorical priming set. A fortiori, given the evidence on the participation of both cerebellar hemispheres in at least certain aspects of cognitive/language processes (e.g. [46]; see [45] for discussion and references), the availability of a larger subject pool would also allow us to investigate whether stimulation of left cerebellar cortical loci may result in qualitatively different changes in performance or not.
Finally, compared with scalp landmark-based coordinates, the fMRI-guided neuronavigation approach in positioning the TMS coil is well-established to require much fewer subjects for yielding significant effects, as well as to yield effect sizes significantly stronger than those of scalp-based coordinates [57]. Conceivably, then, adopting a more accurate coil positioning approach may have allowed to clarify whether the increase in semantic associative priming after lateral cerebellar stimulation is owed to faster lexical decisions on related pairs or to slower lexical decisions on unrelated pairs of the semantic associative priming stimulus set. A fortiori, between-subjects designs, which are necessary for avoiding learning effects unavoidably occurring between sessions (e.g. [65]), involve smaller statistical power than within-subjects designs. Above all, though, the change in priming sizes observed here selectively after lateral cerebellar TMS for the associative priming type corroborates the idea promoted elsewhere [1, 3] that the neocerebellum is involved in linguistic associative processes.
Conclusion
The present study assessed semantic categorical and semantic associative noun-to-verb priming before and after TMS of different cerebellar compartments and before and after no TMS at all. Stimulation of a lateral right cerebellar site was shown to selectively enhance semantic associative priming to a significantly larger extent than that observed in all the other groups, while semantic categorical priming was maintained. The present study thus provided some first TMS evidence for the selective involvement of neocerebellar loci in semantic associative computations. While further work is required to clarify the exact nature of the TMS effect observed, the results yielded corroborate the findings on the involvement of neocerebellar associative computations in language processing.
Notes
A simple node-counting scheme (path). The relatedness score is inversely proportional to the number of nodes along the shortest path between the synsets. The shortest possible path occurs when the two synsets are the same, in which case the length is 1. Thus, the maximum relatedness value is 1.
As the software cannot assess the semantic relatedness of words of different grammatical categories (here, nouns and verbs), it was ensured that, in each pair, the noun was homonymous with a verb of the same basic semantic properties, e.g. ‘release’, ‘fear’.
Of course, semantic associative relatedness implies that the forms may co-occur, be it in a non-immediate fashion. However, such loose co-occurrences could easily be found in the categorically related pairs as well. For example, in (1) below, the semantic associatively related pair ‘stripper-entertaining’ (a) can loosely co-occur in speech, like the semantic categorically related one ‘murder-killing’ (b).
(a)‘[…] being a male stripper isn’t just about taking clothes off, it’s about entertaining people […]’
(b)‘It would seem that one is guilty of murder through killing someone by chance […]’ (Google search)
Indeed, the priming sizes of the three participants that had participated in earlier versions of this task were significantly larger overall than those of the rest of the no TMS subjects (Relatedness × Experience with task: F (1, 43) = 5.09, MSe = 342.85, p < 0.03; a large priming effect for the Experienced group: Relatedness: F (1, 1) = 19,063.58, MSe = 0.20, p < 0.005).
Distribution of subjects across lists: list A: n = 12; list B: n = 13; list C: n = 11; list D: n = 10; lateral group: A 3; B 3; C 3; D 3; medial group: A 3; B 4; C 2; D 2; no TMS group 1: A 3; B 3; C 3; D 3; no TMS group 2: A 3; B 3; C 3; D 2.
Abbreviations
- ANOVA:
-
Analysis of variance
- cm:
-
Centimetre
- mm:
-
Millimetre
- ms:
-
Millisecond(s)
- (r)TMS:
-
(Repetitive) transcranial magnetic stimulation
- SD:
-
Standard deviation
- SEM:
-
Standard error of the mean
- BNC:
-
British National Corpus
References
Argyropoulos GP. Neocerebellar emulation in language processing. In: Alter K, Horne M, Lindgren M, Roll M, von Koss Torkildsen J, editors. Brain talk: discourse with and in the brain. Papers from the first Birgit Rausing language program conference in linguistics. Lund: Lund University, Media Tryck; 2009. p. 193–206.
Argyropoulos GP. Cerebellar theta-burst stimulation selectively enhances lexical associative priming. Cerebellum. 2011;10(3):540–50.
Argyropoulos GP. Cortico-cortical and cortico-cerebellar computations in language change. In: Scott-Phillips TC, Tamariz M, Cartmill EA, Hurford JR, editors. The evolution of language: proceedings of the 7th international conference on the evolution of language. Singapore: World Scientific; 2012. p. 11–8.
Argyropoulos GP, Kimiskidis V, Papagiannopoulos S. Theta-burst stimulation of the right neocerebellar vermis selectively disrupts the practice-induced acceleration of lexical decisions. Behav Neurosci. 2011;125(5):724–34.
Bellebaum C, Daum I. Mechanisms of cerebellar involvement in associative learning. Cortex. 2011;47:128–36.
Canavan AG, Sprengelmeyer R, Diener HC, Hömberg V. Conditional associative learning is impaired in cerebellar disease in humans. Behav Neurosci. 1994;108:475–85.
Christian KM, Thompson RF. Long-term storage of an associative memory trace in the cerebellum. Behav Neurosci. 2005;119:526–37.
Courchesne E, Allen G. Prediction and preparation, fundamental functions of the cerebellum. Learn Mem. 1997;4:1–35.
Daum I, Ackermann H, Schugens MM, Reimold C, Dichgans J, Birbaumer N. The cerebellum and cognitive functions in humans. Behav Neurosci. 1993;107:411–9.
Del Olmo MF, Cheeran B, Koch G, Rothwell JC. Role of the cerebellum in externally paced rhythmic finger movements. J Neurophysiol. 2007;98:145–52.
Demirtas-Tatlidede A, Freitas C, Cromer JR, Safar L, Ongur D, Stone WS, Seidman LJ, Schmahmann JD, Pascual-Leone A. Safety and proof of principle study of cerebellar vermal theta burst stimulation in refractory schizophrenia. Schizophr Res. 2010;124:91–100.
Devlin JT, Watkins KE. Stimulating language: insights from TMS. Brain. 2007;130:610–22.
Drepper J, Timmann D, Kolb FP, Diener HC. Non- motor associative learning in patients with isolated degenerative cerebellar disease. Brain. 1999;122:87–97.
Fellbaum C (ed) (1998) WordNet: an electronic lexical database. Cambridge: MIT. http://wordnet.princeton.edu/
Ferretti TR, McRae K, Hatherell A. Integrating verbs, situation schemas, and thematic role concepts. J Mem Lang. 2001;44:516–47.
Ferretti TR, Gagné C, McRae K. Thematic role focusing by participle inflections: evidence from conceptual combination. J Exp Psychol Learn Mem Cogn. 2003;29(1):118–27.
Ferretti TR, Kutas M, McRae K. Verb aspect and the activation of event knowledge. J Exp Psychol Learn Mem Cogn. 2007;33(1):182–96.
Ferrucci R, Marceglia S, Vergari M, Cogiamanian F, Mrakic-Sposta S, Mameli F, Zago S, Barbieri S, Priori A. Cerebellar transcranial direct current stimulation impairs the practice-dependent proficiency increase in working memory. J Cogn Neurosci. 2008;20:1687–97.
Fierro B, Giglia G, Palermo A, Pecoraro C, Scalia S, Brighina F. Modulatory effects of 1 Hz rTMS over the cerebellum on motor cortex excitability. Exp Brain Res. 2007;176:440–7.
Fiez JA, Petersen SE, Cheney MK, Raichle ME. Impaired non-motor learning and error detection associated with cerebellar damage. A single case study. Brain. 1992;115:155–78.
Fiez JA, Raichle M. Linguistic processing. Int Rev Neurobiol. 1997;41:233–54.
Forster KI, Davis C. The density constraint on form-priming in the naming task: interference from a masked prime. J Mem Lang. 1991;30:1–25.
Frings M, Dimitrova A, Schorn CF, Elles H-G, Hein-Kropp C, Gizewski ER, Diener HC, Timmann D. Cerebellar involvement in verb generation: an fMRI study. Neurosci Lett. 2006;409:19–23.
Gebhart AL, Petersen SE, Thach WT. Role of the posterolateral cerebellum in language. Ann NY Acad Sci. 2002;978:318–33.
Hagoort P. Semantic priming in Broca’s aphasics at a short SOA: no support for an automatic access deficit. Brain Lang. 1997;56:287–300.
Hashimoto M, Ohtsuka K. Transcranial magnetic stimulation over the posterior cerebellum during visual saccades in man. Brain. 1995;118:1185–93.
Huang Y, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–6.
Jarvis BG. DirectRT (Version 2008.1.0.11) [Computer software]. New York: Empirisoft Corporation; 2008.
Kalla R, Muggleton NG, Cowey A, Walsh V. Human dorsolateral prefrontal cortex is involved in visual search for conjunctions but not features: a theta TMS study. Cortex. 2009;45:1085–109.
Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–44.
Koch G, Mori F, Marconi B, Codecà C, Pecchioli C, Salerno, Torriero S, Lo Gerfo E, Mir P, Oliveri M, Caltagirone C. Changes in intracortical circuits of the human motor cortex following theta burst stimulation of the lateral cerebellum. Clin Neurophysiol. 2008;119:2559–69.
Koch G, Brusa L, Carrillo F, Lo Gerfo E, Torriero S, Oliveri M, Mir P, Caltagirone C, Stanzione P. Cerebellar magnetic stimulation decreases levodopa-induced dyskinesias in Parkinson disease. Neurology. 2009;73:113–9.
Larsell O, Jansen J. The comparative anatomy and histology of the cerebellum, Vol 3 III: the human cerebellum, cerebellar connections, and cerebellar cortex. Minneapolis: University of Minnesota Press; 1972.
Leech G. 100 million words of English: the British National Corpus. Lang Res. 1992;28(1):1–13.
Leggio MG, Silveri MC, Petrosini L, Molinari M. Phonological grouping is specifically affected in cerebellar patients: a verbal fluency study. J Neurol Neurosurg Psychiatry. 2000;69:102–6.
Liu CL, Tseng P, Chiau HY, Liang WK, Hung DL, Tzeng OJ, Muggleton NG, Juan CH. The location probability effects of saccade reaction times are modulated in the frontal eye fields but not in the supplementary eye field. Cereb Cortex. 2010;21(6):1416–25.
McCormick DA, Thompson RF. Cerebellum: essential involvement in the classically conditioned eyelid response. Science. 1984;223:296–9.
McRae K, Hare M, Elman JL, Ferretti T. A basis for generating expectancies for verbs from nouns. Mem Cognit. 2005;33:1174–84.
Miall RC, King D. State estimation in the cerebellum. Cerebellum. 2008;7:572–6.
Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001;21:700–12.
Moss HE, Ostrin RK, Tyler LK, Marslen-Wilson WD. Accessing different types of lexical semantic information: evidence from priming. J Exp Psychol Learn Mem Cogn. 1995;21(4):863–83.
Ober B. RT and non-RT methodology for semantic priming research with Alzheimer’s disease patients: a critical review. J Clin Exp Neuropsychol. 2002;24:883–911.
Ohtsuka K, Enoki T. Transcranial magnetic stimulation over the posterior cerebellum during smooth pursuit eye movements in man. Brain. 1998;121:429–35.
Oliveri M, Koch G, Torriero S, Caltagirone C. Increased facilitation of the primary motor cortex following 1 Hz repetitive transcranial magnetic stimulation of the contralateral cerebellum in normal humans. Neurosci Lett. 2005;376:188–93.
Oliveri M, Torriero S, Koch G, Salerno S, Petrosini L, Caltagirone C. The role of transcranial magnetic stimulation in the study of cerebellar cognitive function. Cerebellum. 2007;6:95–101.
Oliveri M, Bonni S, Turriziani P, Koch G, Lo Gerfo E, Torriero S, Vicario CM, Petrosini L, Caltagirone C. Motor and linguistic linking of space and time in the cerebellum. PLoS One. 2009;4(11):e7933.
Palmer ED, Rosen HJ, Ojemann JG, Buckner RL, Kelley WM, Petersen SE. An event-related fMRI study of overt and covert word stem completion. NeuroImage. 2001;14:182–93.
Paulin M. Neural representations of moving systems. In: Schmahmann JD, editor. The cerebellum and cognition, international review of neurobiology, vol. 41. San Diego: Academic; 1997. p. 515–33.
Pedersen T, Patwardhan S, Michelizzi J. WordNet::similarity—measuring the relatedness of concepts. AAAI. 2004;04:1024–5.
Perea M, Gotor A. Associative and semantic priming effects occur at very short stimulus-onset asynchronies in lexical decision and naming. Cognition. 1997;62:223–40.
Perea M, Rosa E. The effects of associative and semantic priming in the lexical decision task. Psychol Res. 2002;66:180–94.
Petersen SE, Fox PT, Posner ML, Mintun M, Raichle ME. Positron emission tomographic studies of the processing of single words. J Cogn Neurosci. 1989;1:153–70.
Pollatsek A, Well AD. On the use of counterbalanced designs in cognitive research: a suggestion for a better and more powerful analysis. J Exp Psychol Learn Mem Cogn. 1995;21:785–94.
Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7:511–22.
Rogers SL, Friedman RB. The underlying mechanisms of semantic memory in Alzheimer’s disease and semantic dementia. Neuropsychologia. 2008;46:12–21.
Sack AT. Transcranial magnetic stimulation, causal structure–function mapping and networks of functional relevance. Curr Opin Neurobiol. 2006;16:593–9.
Sack AT, Kadosh RC, Schuhmann T, Moerel M, Walsh V, Goebel R. Optimizing functional accuracy of TMS in cognitive studies: a comparison of methods. J Cogn Neurosci. 2008;21(2):207–21.
Schmahmann JD. The role of the cerebellum in affect and psychosis. J Neurolinguistics. 2000;13:189–214.
Stewart LM, Walsh V, Rothwell JC. Motor and phosphine thresholds: a transcranial magnetic stimulation correlation study. Neuropsychologia. 2001;39:415–9.
Théoret H, Haque J, Pascual-Leone A. Increased variability of paced finger tapping accuracy following repetitive magnetic stimulation of the cerebellum in humans. Neurosci Lett. 2001;306:29–32.
Thompson-Schill SL, Kurtz KJ, Gabrieli JDE. Effects of semantic and associative relatedness on automatic priming. J Mem Lang. 1998;38:440–58.
Timmann D, Drepper J, Calabrese S, Bürgerhoff K, Maschke M, Kolb FP, Daum I, Diener HC. Use of sequence information in associative learning in control subjects and cerebellar patients. Cerebellum. 2004;3:75–82.
Timmann D, Drepper J, Frings M, Maschke M, Richter S, Gerwig M, Kolb FP. The human cerebellum contributes to motor, emotional and cognitive associative learning. A review. Cortex. 2010;46:845–57.
Timmann D, Drepper J, Maschke M, Kolb FP, Böring D, Thilmann AF, Diener HC. Motor deficits cannot explain impaired cognitive associative learning in cerebellar patients. Neuropsychologia. 2002;40:788–800.
Torriero S, Oliveri M, Koch G, Caltagirone C, Petrosini L. Interference of left and right cerebellar rTMS with procedural learning. J Cogn Neurosci. 2004;16:1605–11.
Torriero S, Oliveri M, Koch G, Lo Gerfo E, Salerno S, Petrosini L, Caltagirone C. Cortical networks of procedural learning: evidence from cerebellar damage. Neuropsychologia. 2007;45:1208–14.
Torriero S, Oliveri M, Koch G, Lo Gerfo E, Salerno S, Ferlazzo F, Caltagirone C, Petrosini L. Changes in cerebello-motor connectivity during procedural learning by actual execution and observation. J Cogn Neurosci. 2010;23(2):338–48.
Tucker J, Harding AE, Jahanshahi M, Nixon PD, Rushworth M, Quinn NP, Thompson PD, Passingham RE. Associative learning in patients with cerebellar ataxia. Behav Neurosci. 1996;110:1229–34.
Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation over the cerebellum in humans. Ann Neurol. 1995;37:703–13.
Vallesi A, Shallice T, Walsh V. Role of the prefrontal cortex in the foreperiod effect: TMS evidence for dual mechanisms in temporal preparation. Cereb Cortex. 2007;17:466–74.
Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci. 1998;2:338–47.
Acknowledgments
The authors would like to thank Dr. Thomas Bak, Prof. Simon Garrod, Prof. Jim Hurford, Prof. Vassilios Kimiskidis, Prof. Maria Leggio, Prof. Ken McRae, Prof. Massimo Oliveri, Prof. Martin Pickering, Prof. Richard Shillcock, Prof. Steve Small, Prof Ana Solodkin, Dr. Patrick Sturt, as well as the reviewers of the journal for their precious support and encouragement. NGM was supported by the UK Medical Research Council and by the National Science Council, Taiwan (100-2410-H-008-074-MY3).
Conflict of Interest
There were no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Argyropoulos, G.P., Muggleton, N.G. Effects of Cerebellar Stimulation on Processing Semantic Associations. Cerebellum 12, 83–96 (2013). https://doi.org/10.1007/s12311-012-0398-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-012-0398-y