Abstract
Apraxic agraphia is a peripheral writing disorder caused by neurological damage. It induces a lack or loss of access to the motor engrams that plan and programme the graphomotor movements necessary to produce written output. The neural network subserving handwriting includes the superior parietal region, the dorsolateral and medial premotor cortex and the thalamus of the dominant hemisphere. Recent studies indicate that the cerebellum may be involved as well. To the best of our knowledge, apraxic agraphia has not been described on a developmental basis. This paper reports the clinical, neurocognitive and (functional) neuroimaging findings of a 15-year-old left-handed patient with an isolated, non-progressive developmental handwriting disorder consistent with a diagnosis of "apraxic dysgraphia". Gross motor coordination problems were objectified as well but no signs of cerebellar, sensorimotor or extrapyramidal dysfunction of the writing limb were found to explain the apraxic phenomena. Brain MRI revealed no supra- and infratentorial damage but quantified Tc-99m-ECD SPECT disclosed decreased perfusion in the anatomoclinically suspected prefrontal and cerebellar brain regions crucially involved in the planning and execution of skilled motor actions. This pattern of functional depression seems to support the hypothesis that "apraxic dysgraphia" might reflect incomplete maturation of the cerebello-cerebral network involved in handwriting. In addition, it is hypothesized that “apraxic dysgraphia” may have to be considered to represent a distinct nosological category within the group of the developmental dyspraxias following dysfunction of the cerebello-cerebral network involved in planned actions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agraphia is a collective term used to denote various types of writing disorders resulting from acquired neurological damage. Agraphic manifestations can be classified as either of the central (linguistic) or the peripheral (non-linguistic) type on the basis of their semiological characteristics [1]. The central agraphias consist of lexical (or surface) agraphia, phonological agraphia, deep agraphia, semantic agraphia and agraphia due to impairment of the graphemic buffer and they involve disruption of the linguistic system: they are characterized by qualitatively similar spelling errors across all output modalities (e.g. in written as well as in oral spelling, typing, letter selection and sequencing). The peripheral agraphias on the other hand consist of allographic agraphia, apraxic agraphia, motor execution agraphia (micrographia and megalographia), hemianoptic agraphia and afferent or neglect dysgraphia: these do not result from damage to the linguistic system itself but from neurological problems (motor or sensory deficits) which primarily compromise the ability to correctly execute the manual production of letters. As a result, the peripheral agraphias are characterized by a clear qualitative dissociation between inferior handwritten and superior non-handwritten forms of spelling (e.g. mental spelling, typing, letter selection).
Within the group of the peripheral agraphias, apraxic or pure agraphia represents an acquired writing disorder following disruption of the skilled movement plans of writing. In this condition, distorted letter formation and disruption of the graphomotor trajectory does not follow from sensorimotor, extrapyramidal or cerebellar dysfunction affecting the writing limb but from distortion of the mental representation, planning and execution of graphomotor letter formation. Disruption of the spatiotemporal features of handwriting typically results in hesitant, awkward, and imprecise letter formation or even in illegible scrawls [1, 2]. However, in spite of letter deformation, the distinction between upper- and lowercase forms may be preserved [3, 4], the formation of single letters may be significantly superior to letter sequencing in words [5] and in some patients, the inability to write letters sharply contrasts with an intact ability to produce written numbers [6]. Grapheme formation in apraxic agraphia typically improves during the copying of letters and words [1]. Oral spelling, type-writing and the use of anagram letters are well-preserved in patients with apraxic agraphia [2, 7, 8].
Apraxic agraphia has been documented in a variety of etiologically heterogeneous neurological conditions and is typically associated with causative lesions located in the superior parietal lobe (storage of graphomotor plans) or the dorsolateral and medial part of the prefrontal cortex (conversion of graphomotor plans to motor commands) of the language dominant hemisphere; e.g. [6, 7, 9, 10]. In a recent review of 25 cases of vascular apraxic agraphia—published since the first description of the condition by Heilman et al. in 1973 [7]—De Smet et al. [11] confirmed that apraxic agraphia may be associated with lesions outside the language dominant parietal and frontal region.
In school-aged children difficulties in the acquisition of the mechanical aspects of handwriting are very common, i.e. the estimated incidence is 27 % in grade one to 13 % at the end of grade five, e.g. [12]. In addition, bad handwriting is often considered a hallmark feature of children with neurodevelopmental disorders such as dyslexia, attentional deficit and hyperkinetic disorder [13], developmental coordination disorder [14, 15], developmental right hemisphere syndrome [16] and autism spectrum disorders [17]. Cratty, for instance, showed that handwriting difficulties were present in 30–40 % of the surveyed children with learning disabilities [18]. Although legible handwriting remains an important skill to achieve academic success, the clinical assessment of handwriting performance remains a challenge. Many tests are available to evaluate graphomotor performance but the scarcity of valid and reliable handwriting tools limits the application of standardized assessments in a clinical setting [19]. In addition, little is known about the responsible neurobiological mechanisms subserving distorted handwriting. Gubbay et al. studied 259 children with an average age of 13.6 years attending high school in the central metropolitan area in Perth (Australia) in order to provide a neurological classification of the developmental dysgraphias [20]. Their survey disclosed only children with dysgraphia of the linguistic type and a ‘non-significant incidence of children with apraxic or mechanical dysgraphia’. However, no in-depth description of both forms of peripheral writing disorders was provided.
To the best of our knowledge, there have been no detailed studies of children in whom disrupted development of handwriting skills was identified to reflect an underlying apraxic deficit. This report describes for the first time the clinical, neurocognitive and (functional) neuroimaging characteristics in a 15-year-old left-handed patient who presented with a non-progressive graphomotor disturbance, unaccompanied by any neurocognitive dysfunction or a cerebellar, extrapyramidal or sensorimotor deficit affecting the writing limb.
Case Report
A 15-year-old left-handed patient was referred to the department of Clinical Neurolinguistics of ZNA Middelheim Hospital because of persisting problems with handwriting. The boy was born at term after normal gestation. According to ‘WHO child growth standards’, acquisition of gross motor milestones was slightly delayed. He could sit without support at 7 months (mean = 6.0; SD = 1.1), he could stand with assistance at 8 months (mean = 7.6; SD = 1.4) but did not crawl, nor could he walk independently before the age of 17 months (mean = 12; SD = 1.8 months). He was able to independently ride a bicycle without support at the age of 4.6 years. By the age of 4–5 years he had developed a clear left-hand preference. In kindergarten he was briefly treated with physiotherapy and psychomotor therapy because of poor gross coordination skills. Cognitive and speech/language development were entirely normal. At the age of 7 years, Asperger’s syndrome was suspected because of a failure to establish and maintain peer relationships and an apparently inflexible adherence to routine behaviour. Formal psychiatric assessments, however, were normal and excluded a diagnosis of Asperger’s syndrome. Medical history was unremarkable. Family history was negative for developmental disorders and learning disabilities. The patient had successfully finished primary school and obtained above average results in the 3rd grade of secondary school (science–economics).
Detailed clinical neurological examination in which cerebellar functionality was studied in detail by means of the Brief Ataxia Rating Scale (BARS) revealed no signs of ataxia (total BARS score of 0/30) [21]. The Körperkoordinations Test für Kinder (KTK) was also administered to assess gross motor coordination skills [22]. The following four movement tasks were completed by the patient: (1) walking backwards along a balance beam of different widths (number of successful steps are recorded), (2) hopping for height (the patient hops on one foot over a pile of foam the height of which is raised after each successful jump, the height of the last successful jump is recorded), (3) jumping sideways as fast as possible with both feet together (timing of 15 consecutive sideway jumps) and (4) moving sideways on boxes (the patient stands on one box and moves sideways onto another box he has put down himself. The sequences of picking up and placing down boxes leading to a correct movement are counted). The patient obtained a normal motor quotient (MQ) for the subtests ‘hopping for height’ (MQ = 90; mean = 100; SD = 15) and ‘jumping sideways’ (MQ = 96). An MQ below percentile 15 was found for the subtest ‘walking backwards’ (MQ = 76) and an MQ below the third percentile was found for ‘moving sideways on boxes’ (MQ = 60). A total MQ of 72 is consistent with a diagnosis of gross motor coordination disorder.
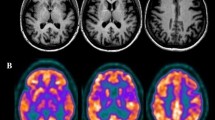
The EEG was normal. MRI of the brain revealed no lesions at the supra- and infratentorial level. A quantified Tc-99m-ECD SPECT study was carried out; 740 MBq (20 mCi) Tc-99m-ECD was administered to the patient by means of a previously fixed butterfly needle while he was sitting in a quiet and dimmed room, eyes open and ears unplugged. Acquisition was started 40 min after injection using a three-headed rotating gamma camera system (Triad 88; Trionix Research Laboratory, Twinsburg, Ohio, USA) equipped with lead super-fine fanbeam collimators with a system resolution of 7.3 mm FWHM (rotating radius 13 cm). Projection data were accumulated in a 128 × 64 matrix, pixel size 3.56 mm, 15 s/angle, 120 angles for each detector (3° steps, 360° rotation). Projection images were rebinned to parallel data, smoothed and reconstructed in a 64 × 64 matrix, using a Butterworth filter with a high cut frequency of 0.7 cycles/cm and a roll-off of 5. No attenuation or scatter correction was performed. Trans-axial images with a pixel size of 3.56 mm were anatomically standardized using SPM and compared to a standard normal and SD image obtained from ECD perfusion studies in a group of 15 normally educated healthy adults consisting of eight men and seven women with an age ranging from 45 to 70 years. This normal image was created by co-registration of each normal study to the SPECT template image of SPM using the “normalize” function in SPM. At the same time, the global brain uptake of each study was normalized. On the mean image, 31 ROI’s were drawn and a 31 ROI template was created. Using the normalized studies and the 31 ROI template, the mean normal uptake and SD value (=1 Z score) in each ROI was defined. Patient data were normalized using SPM in the same way and the perfusion uptake in each ROI was calculated. From this uptake, the mean uptake and SD value of the normal database, the Z score for each region can be calculated. A regional Z score of >2.0 is considered significant. The quantified Tc-99m-ECD SPECT study of our patient showed a significant decrease of perfusion in both cerebellar hemispheres (right, −2.86 SD; left, −2.18 SD) as well as a hypoperfusion in the medial prefrontal left hemisphere (−2.18 SD; Fig. 1).
Neuropsychological Investigations
Neuropsychological assessment consisted of a range of formal test batteries including the Wechsler Intelligence Scale for Children, third edition (WISC-III) [23], the Bourdon–Vos Test [24], the Wisconsin Card Sorting Test (WCST) [25], the Stroop Colour–Word test [26], the Trail Making Test (TMT) [27], the Rey–Osterrieth figure [28], the praxis subtests of the Hierarchic Dementia Scale (HDS) [29], the Beery Developmental Test of Visual–Motor Integration [30] and the Test of Visual–Perceptual Skills, third edition (TVPS-3) [31]. Neurolinguistic assessments consisted of the Boston Naming Test [32], subtests of the Akense Afasie Test (AAT) [33], the Dudal Spelling Tests [34, 35], a selection of written language tests of the Psycholinguistic Assessment of Language Processing in Aphasia (PALPA) [36] and a semantic controlled oral word association task (unpublished norms).
A strong and consistent left-hand preference was confirmed by a laterality quotient of -100 on the Edinburgh Handedness Inventory [37]. As shown in Table 1, general cognitive skills which were measured by the WISC-III, showed superior verbal and performance IQ levels. No discrepancy was found between the verbal IQ and the performance IQ and there were no inconsistencies in the distribution of subtest scores at both the verbal and performance level. Visuomotor attention (Bourdon–Vos Test; WISC-III digit symbol substitution), visual search and sequencing (TMT) and digit span (WISC-III) scored well within the normal range. As evidenced by the Stroop Colour–Word test, the ability to inhibit a competing and more automatic response set was normal. Abstract concept formation, shifting and maintaining of goal-oriented cognitive strategies in response to changing environmental contingencies was normal (WCST). Constructional (HDS item 12; WISC-III block design), ideational (HDS item 5), ideomotor (HDS item 3), drawing (Rey–Osterrieth figure; HDS item 15) and bucco-labio-lingual praxis were normal. On the Beery Developmental Test of Visual–Motor Integration low average results for visual–motor integration skills and average results for visual–motor coordination were found. In addition to a superior visuo-perceptual quotient, the TVPS-3 subtests scored within the normal range.
Articulation and prosody in conversational and spontaneous speech were normal. No grammatical errors or lexical retrieval difficulties were observed. Visual confrontation naming (BNT) and semantic verbal fluency were normal as well. Repetition of phonemes, morphemes and sentences and reading of words and sentences were normal (AAT). Spelling of words and sentences (Dudal spelling) was assessed by means of a portable computer since the patient’s handwriting was not legible (Fig. 2). Oral spelling was also used to avoid illegible handwriting quality. No evidence was found for an underlying linguistic spelling disorder. PALPA subtests, administered to investigate a possible impact of linguistic parameters on spelling were normal and revealed no influence of word length (PALPA 37), imageability (PALPA 38), word frequency (PALPA 38), grammatical class (PALPA 39), and imageability (PALPA 40) or spelling-sound regularity (PALPA 42). Writing of nonwords (PALPA 43), morphological suffixes (PALPA 41) and homophones (PALPA 44) were normal as well. Discrimination of mirror reversed graphemes (PALPA 17), case matching (PALPA 18–19) and letter discrimination (PALPA 20) were intact (Table 2).
Handwriting sample demonstrating the characteristic features of apraxic agraphia: writing of lowercase sentences to dictation. [Target text for figure 2, De publicatie staat sinds gisteren ook online. Ze baseerden zich voor hun onderzoek op de gezinsdemografische trend. Onderzoek naar depressiviteit binnen een bepaalde bevolkingsgroep is zeldzaam en het is zeker het eerste in België zegt Bracke. We kunnen het nu bevestigen wat voorheen enkel een vermoeden was dat de klachten over depressiviteit over een tijdsspanne van acht jaar verdubbeld zijn]
Handwriting skills were formally investigated by means of the “Systematische Opsporing Schrijfproblemen” (SOS; Systematic Detection of Writing Problems) [38]. In this test, the patient is asked to copy a printed text for a period of 5 minutes. Only the first five lines are used for analysis. A score between 0 and 2 is attributed to fluency, transition between letters, mean letter size, regularity of size, blank spaces between words and line trajectory. Score zero stands for a normal performance, higher scores indicate (greater) problems. He obtained a severely defective total score of 8/12 (mean 1.7; SD = 1.32; below percentile 3) with graphomotor distortions affecting fluency (score = 2), letter transition (score = 2), mean letter size (score = 1), regularity of letter size (score = 2) and blank spaces between words (score = 1) (Fig. 2). Copying of letters, words and sentences significantly improved the quality of handwriting. Formation of uppercase letters was superior to lowercase forms and letters written in isolation were better preserved than letters written in sequences. Writing with the non-dominant hand and writing with no visual support (eyes closed) induced an aggravation of symptoms. The patient held the pencil with a mature pattern and in a typical fashion (dynamic tripod grasp pattern, fixating the pen between the thumb and index finger, with the pencil resting against the side of the middle finger). During the act of writing with either the left of right hand he only moved the wrist joint, while the other joints of the left arm remain in a fixed position.
At the social level, no significant difficulties were mentioned in establishing and maintaining social contacts with peers. No evidence for affective problems or emotional instability was recorded after careful inquiry of the patient and his parents.
Discussion
Handwriting is a highly complex skill which requires the mastery and integration of a range of subskills involving cognitive operations, linguistic processing and sensorimotor functioning. In this 15-year-old left-handed patient detailed cognitive and linguistic investigations identified a pattern of graphomotor abnormalities consistent with a diagnosis of "apraxic dysgraphia". Depressed writing fluency, defective transition between letters, abnormal and irregular letter size, awkward and imprecise letter formation, abnormal blank spaces and illegible scrawls were the spatiotemporal features of a deviant and deformed written output. The failure to develop normal handwriting not only markedly contrasted with the absence of a linguistic disturbance of spelling, reading or a selective language impairment, but it also contrasted with the patient's normal development of general cognitive skills and normal academic achievements. Indeed, aside from apraxic disruption of handwriting, in-depth neuropsychological investigations formally ruled out any associated cognitive impairment, even in closely related domains of the motor-praxis system (such as ideomotor, ideational, constructional, oral, drawing and dressing praxis), the executive system of planning and organisation, the visuomotor integration system, attention, or in visuospatial and nonverbal cognition. As demonstrated by a borderline result on the subtest ‘walking backwards’ and a profoundly defective performance on ‘moving sideways on boxes’ of the KTK, clinical investigations revealed evidence of neurodevelopmental immaturity confined to gross motor coordination skills. The BARS and the KTK did not reveal any formal neurological signs or symptoms of cerebellar, sensorimotor, extrapyramidal dysfunction of the writing limb to explain the apraxic symptoms. Handwriting was not better when the patient wrote with the non-dominant, right hand or when he was blindfolded. This indicates efficient monitoring of visual and proprioceptive feedback processes during the execution of graphomotor patterns.
Apraxic agraphia is typically caused by acquired neurological damage to the processing components involved in the programming of writing movements. Mechanisms responsible for this disruption include the destruction or disconnection of stored graphomotor engrams or damage to the processes associated with translating the graphomotor engrams into graphic innervatory patterns to muscles involved in the motor execution of handwriting [1]. Studies indicate that the graphomotor engrams are stored in the parietal lobe of the hemisphere contralateral to the hand preferred for writing whereas the prefrontal areas (dorsolateral premotor cortex and the supplementary motor area) of the same hemisphere subserve the translation of these programmes into graphomotor innervatory patterns [1]. In a review of vascular apraxic agraphia, De Smet et al. [11] confirmed the view that in the majority of cases, apraxic agraphia is due to a cerebral hemisphere lesion contralateral to the preferred hand. In 17 out of the 22 right-handers, apraxic agraphia was caused by a lesion involving the left (language dominant) hemisphere while the small number of only two left-handed patients included in the review developed the condition after a unilateral right (non-dominant) hemisphere lesion. Although more substantial anatomoclinical evidence is needed to ground the view of an intrinsic correlation between the neurobiology of manual preference and graphomotor planning, the absence of associated linguistic deficits in these two left-handed cases might indicate that the neurobiological mechanisms of planning and execution of writing might be more closely linked to the neural basis of manual dominance than to the neural basis of language dominance. In addition, anatomoclinical studies of patients with apraxic agraphia show that the condition may also occur after isolated subcortical lesions (for a review see [11]). Watson and Heilman, for instance, described a 43-year-old right-handed woman who presented with apraxic agraphia due to vascular damage of the corpus callosum in addition to transcortical motor aphasia and ideomotor, ideational and buccofacial apraxia [39]. Croisile et al. [40] and Nagaratnam et al. [41] observed the condition following vascular damage to the centrum semiovale of the left hemisphere. In a 78-year-old right-handed patient described by Ohno et al., apraxic agraphia resulted from isolated vascular damage to the dorsomedial part of the left thalamus [42]. Mariën et al. [43] and De Smet et al. [11] recently identified apraxic agraphia in patients with causative lesions located in the right cerebellar hemisphere. In this patient, though, structural imaging of the brain revealed no supra- or infratentorial abnormalities and EEG recordings were normal as well. However, a quantified Tc-99m-ECD SPECT study showed a pattern of significant perfusion deficits in the structurally unaffected anatomical regions crucially implicated in the distributed neural network subserving the planning and execution of skilled graphomotor actions. Indeed, in addition to a significant perfusion deficit involving the medial prefrontal region of the left hemisphere, a hypoperfusion affected both cerebellar hemispheres (right more pronounced than left). These findings are in line with recent anatomoclinical evidence indicating that the cerebellum and prefrontal region are implicated in the distributed network of planning and organisation of skilled motor actions such as oral-motor speech production [44, 45], and handwriting [11, 43]. However, it remains to be settled whether the lateralized distribution of supratentorial abnormalities reflecting disrupted graphomotor planning relates to language dominance rather than to manual preference. Although functional neuroimaging studies [46, 47] confirm involvement of the cerebellum in the general neural network of writing, neurobehavioural evidence for a crucial role of this network in peripheral handwriting disorders is rare. Indeed, apart from the few patients who presented with apraxic agraphia [11, 43] only a handful of additional cases with cerebellar induced peripheral agraphia have been documented in the literature. Silveri et al. reported an adult patient who showed typical features of acquired spatial agraphia after vascular ischemic damage of the left cerebellar hemisphere [48]. This type of peripheral writing disorder, usually following posteriorly located lesions of the non-dominant hemisphere, was also found in a patient with cerebellar atrophy [49]. The same type of written language impairment was reported by Fournier del Castillo et al. [50]. They described an 8-year-old patient who developed apraxic agraphia in a context of cerebellar atrophy secondary to acute cerebellar swelling at the age of 4.6 years. Frings et al. identified megalographia in six children with chronic surgical cerebellar lesions following posterior fossa tumour resection [51]. To the best of our knowledge, apraxia of the graphomotor skills in the adult and paediatric population has not been observed as a developmental phenomenon without any demonstrable cerebral damage.
Haggard et al. suggested that in patients with cerebellar lesions deficient motor programming may cause peripheral writing problems [52]. Disruption of the feedforward–feedbackward mechanisms may lead to a decomposition of movement due to over-dependence on high-level cortical feedback loops in controlling the movements of the affected hand. Fabbro et al. suggested that the right cerebellar hemisphere and portions of the vermis may control written language processes by integrating their activity with the “frontal lobe system” [53]. In our patients with apraxic agraphia following focal cerebellar damage [11, 43], the hypothesis of a functional disruption of the cerebello-cerebral network subserving the planning and execution of skilled motor actions was supported by SPECT findings which revealed cerebellar-cerebral diaschisis, reflecting the functional impact of the cerebellar lesion on a distant supratentorial region crucially involved in handwriting [54, 55]. Insufficient maturation or underdevelopment of the distributed cerebro-cerebellar network subserving coordinated motor skills might account for the symptoms characterizing “apraxic dysgraphia”. As a result, anatomoclinical findings in this patient with a selective impairment affecting handwriting not only seem to confirm that the integrity of the cerebello-cerebral network is crucially important in the planning and execution of skilled actions [56], but also seems to demonstrate that apraxic handwriting may be of developmental origin. The exact mechanisms, by which the cerebello-cerebral network modulates handwriting and other skilled motor actions remain to be elucidated. Given its cardinal role in the coordination of motor functioning it might be speculated that in addition to the integration and processing of sensory-motor signals within the motor system the close interplay between cerebellum, thalamus and prefrontal areas may subserve a higher order level of movement planning that requires timing and coordination across multiple effectors [57].
Following these observations, it is hypothesized that “apraxic dysgraphia” may have to be considered to represent a distinct nosological entity within the group of the developmental dyspraxias that might result from immaturity of the cerebello-cerebral network involved in planned actions. Future research is needed to identify the role of this network in skilled actions.
References
Rapcsak SZ, Beeson PM. Agraphia. In: Crosson B, Rothi LJG, Nadeau S, editors. Aphasia and language: theory and practice. New York: Guilford; 2000. p. 184–220.
Valenstein E, Heilman KM. Apraxic agraphia with neglect-induced paragraphia. Arch Neurol. 1979;36:506–8.
Margolin DI, Binder L. Multiple component agraphia in a patient with atypical cerebral dominance: an error analysis. Brain Lang. 1984;22:26–40.
Roeltgen DP, Heilman KM. Apractic agraphia in a patient with normal praxis. Brain Lang. 1983;18:35–46.
Zettin M, Cubelli R, Perino C, Rago R. Impairment of letter formation: the case of ‘ideomotor’ apraxic agraphia. Aphasiology. 1995;9:283–94.
Hodges JR. Pure apraxic agraphia with recovery after drainage of the left frontal cyst. Cortex. 1991;27:469–73.
Heilman KM, Coyle JM, Gonyea EF, Geschwind N. Apraxia and agraphia in a left-hander. Brain. 1973;96:2–28.
Heilman KM, Gonyea EF, Geschwind N. Apraxia and agraphia in a right-hander. Cortex. 1974;10:284–8.
Alexander MP, Fischer RS, Friedman R. Lesion localization in apractic agraphia. Arch Neurol. 1992;49:246–51.
Sakurai Y, Matsumura K, Iwatsubo T, Momose T. Frontal pure agraphia for kanji or kana: dissociation between morphology and phonology. Neurol. 1997;49:946–52.
De Smet HJ, Engelborghs S, Paquier PF, De Deyn PP, Mariën P. Cerebellar-induced apraxic agraphia: a review and three new cases. Brain Cogn. 2011;76:424–34.
Karlsdottir R, Stefansson T. Problems in developing functional handwriting. Percept Mot Skills. 2002;94:623–62.
Racine M, Majnemer A, Shevell M, Snider L. Handwriting performance in children with attention deficit hyperactivity disorder (ADHD). J Child Neurol. 2008;23(4):399–406.
Miller L, Missiuna C, Macnab J, Malloy-Miller T, Polatajko H. Clinical description of children with developmental coordination disorder. Can J Occup Ther. 2001;68(1):5–15.
Rosenblum S, Livneh-Zirinski M. Handwriting process and product characteristics of children diagnosed with developmental coordination disorder. Hum Movement Sci. 2008;27(2):200–14.
Gross-Tsur V, Shalev RS, Manor O, Amir N. Developmental right-hemisphere syndrome: clinical spectrum of the nonverbal learning disability. J Learn Disabil. 1995;28(2):80–6.
Fuentes C, Mostofsky S, Bastian A. Children with autism show specific handwriting impairments. Neurol. 2009;73(19):1532–7.
Cratty BJ. Clumsy child syndromes: description, evaluation and remediation. London: Harwood; 1994.
Feder KP, Majnemer A. Children's handwriting evaluation tools and their psychometric properties. Phys Occup Ther Pediatr. 2003;23:65–84.
Gubbay SS, de Klerk NH. A study and review of developmental dysgraphia in relation to acquired agraphia. Brain Dev. 1995;17:1–8.
Schmahmann JD, Gardner R, MacMore J, Vangel MG. Development of a Brief Ataxia Rating Scale (BARS) based on a modified form of the ICARS. Mov Disord. 2009;24:1820–8.
Kiphard BJ, Schilling F. Körpercoordinationstest für Kinder. Beltz Test GmbH: Weinheim; 1974.
Kort W, Schittekatte M, Compaan EL, Bosmans M, Bleichrodt N, Vermeir G, et al. WISC-III NL. Handleiding. London: The Psychological Corporation; 2002.
Vos PG. Bourdon Vos Test. 3de herziene uitgave. Lisse: Swets & Zeitlinger B.V. 1998.
Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtis G. Wisconsin Card Sorting Test (WCST) Manual Revised and Expanded. Odessa: Psychological Assessment Resources; 1993.
Golden JC. Stroop color and word test. Chicago: Stoelting; 1978.
Reitan RM. Validity of the Trail Making test as an indicator of organic brain damage. Percept Motor Skill. 1958;8:271–6.
Osterrieth PA. Le Test de Copie d’une Figure Complexe. Neuchatel: Delachaux & Niestle; 1944.
Cole M, Dastoor D. A new hierarchic approach to the measurement of dementia. Psychosom. 1987;28:298–305.
Beery KE. The developmental test of visual–motor integration: administration, scoring, and teaching manual. 3rd ed. Cleveland: Modern Curriculum; 1989.
Martin NA. Test of visual perceptual skills: third edition. Novato: Academic Therapy; 2006.
Mariën P, Mampaey E, Vervaet A, Saerens J, De Deyn PP. Normative data for the Boston Naming Test in native Dutch-speaking Belgian elderly. Brain Lang. 1998;65:447–67.
Graetz P, De Bleser R, Willmes K. De Akense Afasie Test. Lisse: Swets & Zeitlinger; 1986.
Dudal P. CSBO zinnen en woorddictee. Leuven: CSBO & Garant; 1998.
Dudal P. Toetsen dictee, 2 genormeerde dictees, einde basisonderwijs, begin secundair onderwijs, VCLB Service cvba;2004.
Bastiaanse R, Bosje M, Visch-Brink E. Psycholinguistic assessment of language processing in aphasia. Hove: Erlbaum; 1995.
Oldfield R. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113.
Smits-Engelsman B, Stevens M, Vrenken I, van Hagen A. Systematische Opsporing Schrijfproblemen (SOS): een hulpmiddel voor leerkrachten bij het signaleren van motorische schrijfproblemen van leerlingen in het Basis en Speciaal Onderwijs. Kinderfysiotherapie 2005;16–20.
Watson RT, Heilman KM. Callosal apraxia. Brain. 1983;106(106):391–403.
Croisile B, Laurent B, Michel D, Trillet M. Pure agraphia after deep left hemisphere haematoma. JNNP. 1990;53:263–5.
Nagaratnam N, Plew J, Cooper S. Pure agraphia following periendarterectomy stroke. Int J Clin Pract. 1998;52:203–4.
Ohno T, Bando M, Nagura H, Ishii K, Yamanouchi H. Apraxic agraphia due to thalamic infarction. Neurol. 2000;54:2336–9.
Mariën P, Verhoeven J, Brouns R, De Witte L, Dobbeleir A, De Deyn PP. Apraxic agraphia following a right cerebellar hemorrhage. Neurol. 2007;69:926–9.
Mariën P, Verhoeven J, Engelborghs S, Rooker S, Pickut BA, De Deyn PP. A role for the cerebellum in motor speech planning: evidence from foreign accent syndrome. Clin Neurol Neurosurg. 2006;108:518–22.
Mariën P, Verhoeven J. Cerebellar involvement in motor speech planning: some further evidence from foreign accent syndrome. Folia Phoniatr Log. 2007;59:210–7.
Longcamp M, Anton J-L, Roth M, Velay J-L. Visual presentation of single letters activates a premotor area involved in writing. NeuroImage. 2003;19:1492–500.
Katanoda K, Yoshikawa K, Sugishita M. A functional MRI study on the neural substrates for writing. Hum Brain Mapp. 2001;13:34–42.
Silveri MC, Misciagna S, Leggio MG, Molinari M. Spatial dysgraphia and cerebellar lesion: a case report. Neurol. 1997;48:1529–32.
Silveri MC, Misciagna S, Leggio MG, Molinari M. Cerebellar spatial dysgraphia: further evidence. J Neurol. 1999;246:312–3.
del Castillo MCF, Maldonato Belmonte MJ, Ruiz-Falco Rojas ML, Lopez Pino MA, Barnabeu Verdu J, Suarez Rodriguez JM. Cerebellum atrophy and development of a peripheral dysgraphia: a paediatric case. Cerebellum. 2010;9(4):530–6.
Frings M, Gaertner K, Buderath P, Christiansen H, Gerwig H, Hein-Kropp C, et al. Megalographia in children with cerebellar lesions and in children with attention-deficit/hyperactivity disorder. Cerebellum. 2010;9(3):429–32.
Haggard P, Jenner J, Wing A. Coordination of aimed movements in a case with unilateral cerebellar damage. Neuropsychologia. 1994;32:827–46.
Fabbro F, Moretti R, Bava A. Language impairments in patients with cerebellar lesions. J Neuroling. 2000;13:173–88.
Baron JC, Bousser MG, Comar D, Soussaline F, Castaingne P. Noninvasive tomographic study of cerebral blood flow and oxygen metabolism in vivo. Potentials, limitations and clinical applications in cerebral ischemic disorders. Eur Neurol. 1981;20:273–84.
Mariën P, Engelborghs S, Fabbro F, De Deyn PP. The lateralized linguistic cerebellum: a review and new hypothesis. Brain Lang. 2001;79:580–600.
Mariën P, Wackenier P, De Surgeloose D, De Deyn PP, Verhoeven J. Developmental coordination disorder: disruption of the cerebello-cerebral network evidenced by SPECT. Cerebellum. 2010;9:405–10.
Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Ann Rev Neurosci. 1992;15:403–42.
Acknowledgments
The authors thank Mrs Lut Porto-Carrero for skillfully conducting the KTK and Bob and Bert for their continuous support.
Conflict of Interest
All authors of the manuscript (Peter Mariën, Eric de Smet, Hyo Jung De Smet, Peggy Wackenier, Andre Dobbeleir and Jo Verhoeven) explicitly disclosed no conflicts of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mariën, P., de Smet, E., de Smet, H.J. et al. “Apraxic dysgraphia” in a 15-Year-Old Left-Handed Patient: Disruption of the Cerebello-Cerebral Network Involved in the Planning and Execution of Graphomotor Movements. Cerebellum 12, 131–139 (2013). https://doi.org/10.1007/s12311-012-0395-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-012-0395-1