Abstract
Empirical evidence indicates that cognitive consequences of cerebellar lesions tend to be mild and less important than the symptoms due to lesions to cerebral areas. By contrast, imaging studies consistently report strong cerebellar activity during tasks of action observation and action understanding. This has been interpreted as part of the automatic motor simulation process that takes place in the context of action observation. The function of the cerebellum as a sequencer during executed movements makes it a good candidate, within the framework of embodied cognition, for a pivotal role in understanding the timing of action sequences. Here, we investigated a cohort of eight patients with chronic, first-ever, isolated, ischemic lesions of the cerebellum. The experimental task consisted in identifying a plausible sequence of pictures from a randomly ordered group of still frames extracted from (a) a complex action performed by a human actor (“biological action” test) or (b) a complex physical event occurring to an inanimate object (“folk physics” test). A group of 16 healthy participants was used as control. The main result showed that cerebellar patients performed significantly worse than controls in both sequencing tasks, but performed much worse in the “biological action” test than in the “folk physics” test. The dissociation described here suggests that observed sequences of simple motor acts seem to be represented differentially from other sequences in the cerebellum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The motor system of humans is involved in a wide series of cognitive functions, such as action observation [1, 2], motor imagery [3], space perception [4], numerical cognition [5, 6] and language [7]. In the specific domain of social cognition, it has been shown that the human motor system is recruited during the observation of other individuals performing intentional actions. Whole-brain measures of brain function have shown that a wide range of regions that contribute to motor behaviour are also active during motor observation. These include the frontal motor areas, the posterior parietal cortex and the cerebellum (among others, authors who reported cerebellar activations to action observation, see [8–10]). The action observation/execution system is therefore not considered a single “organ” but rather a property of many portions of the motor system that allows them to access directly visual information on others’ behaviour. Consequently, the function of the visuo-motor matching (mirror) mechanism in the different modular brain structures that contribute to movement is not unique, but rather depends on the motor properties of the cortical module that it is endowed in [1].
For example, motor neurons in the ventral premotor cortex code the movements and postures of the distal part of the upper limb within a frame of reference that is mostly independent of the location in space of the target in monkeys (for a review see [11]) and humans [12–14]. Accordingly, in humans, causal evidence on the role of the ventral premotor cortex in action understanding based on transcranial magnetic stimulation (TMS) points at a coding of predominantly distal acts, irrespective of intrinsic motor features and of effectors [15–19]. On the contrary, the dorsal premotor cortex and superior parietal lobule in humans is involved in representing the peripersonal space and the reaching motor acts aiming at particular sectors of such space [20]. Accordingly, its mirror counterpart is dedicated to coding others’ reaching movements [21, 22].
According to this model, the mirror activity in the cerebellum probably reflects its motor functions. One main motor role of the cerebellum is that of sequencing, in a smooth way, motor acts in action chains. We devised a task that required the subject to choose between several possible sequences of motor acts the one that was more biomechanically economic and smooth. According to our simulation theory, the cerebellum is a good candidate for this strategy in the framework of embodied cognition. Interestingly, the role in assembling individual motor acts and in dealing with complex motor hierarchical structures has also being described for the cerebellum [23] and a general framework theory of cognitive cerebellar functions attributes to it the role of sequencer of orderly elements. The idea that the cerebellum is somehow involved in social cognition, in particular in response to dynamic stimuli, is now suggested by a wide series of imaging experiments. The cerebellum was originally found to be selectively active in response to point-light displays of biological motion [24, 25]. In parallel, studies in healthy subjects using fully displayed moving body engaged in simple activities have reported cerebellar activation [8–10, 26, 27]. Cerebellar activation was recorded during perception of dynamic emotional expressions of the whole body as compared to static stimuli [28]. Lesional studies are somewhat more controversial, with one study [29] showing that patients with chronic unilateral cerebellar lesions are impaired in non-biological rather than biological motion detection. That result could be due to the majority of medial lesions in that population (4/7), since only lateral lesions have been shown to cause deficits in whole-body motion perception [30].
Here, we investigate the role of the cerebellum in action understanding in a population of stroke patients with cerebellar lesions, by studying whether a specific lesion of the cerebellum could impair the capability to assemble individual observed motor acts in a biomechanically plausible sequence. We adopted a paradigm that is common in many studies on action observation, which consists in comparing goal-directed actions with inanimate objects. This approach has been paradigmatic since the very first studies on action observation [2] up to the most recent ones [31, 32].
Methods
Patients and Controls
We evaluated a cohort of patients with chronic ischemic lesions and a comparable population of neurologically healthy volunteers. All participants gave informed consent to the procedure, which was conducted according to international principles of ethics in human research [33]. The study was approved by the local ethical committee of the “Azienda Provinciale per i Servizi Sanitari” in the context of a more general evaluation of cognitive abilities in patients with posterior circulation ischemic lesions.
Patients were evaluated among those with ischemic cerebellar lesions who had been treated at the Intensive Stroke Unit of the Trento Hospital up to 24 months before the study and identified a total f 12 patients. Exclusion criteria consisted in concomitance of any medical condition that could limit the evaluation, of other intracranial disorders, and of previous cortical or sub-cortical stroke. For this purpose, we evaluated retrospectively all patients’ MRI scans of the brain performed in the acute phase between 4 and 12 days after onset with T2-weighted and fluid-attenuated inversion recovery sequences. For these reasons four patients were excluded.
We also assessed, as exclusion criteria, the presence of limb apraxia, evaluated by the items “intransitive gesture production on command”, “actual use of objects”, “mimes production on command” and “imitation of meaningless gestures” of the Limb Apraxia Battery [34]; the presence of moderate to severe dysarthria, assessed clinically and the presence of depression as defined by a score >10 on the Hamilton Rating Scale for Depression [35]. No patient was excluded for these criteria. The general cognitive functions were tested in the eight patients by means of standardized tests administered by the same clinician. Selective attention, assessed by the Attentional Matrix Test [36] was normal in all (patients’ corrected scores, 41.6–54.75; normal values, >30). Executive functions assessed by the Stroop Color Word Test [37, 38] were normal in all (patients’ corrected errors, 0–3.5; normal values, <4.24; patients’ reaction times, between 11.9 and 30.0; normal values, <36.92). Logical functions tested by the Raven Matrices Test (SPM 38) [39] were normal in all but one who had marginally abnormal values (patient no. 5 scored 19.75, all others scored between 30 and 46; normal values, >20.72).
A summary of the demographic and clinical characteristics of the eight included patients is presented in Table 1. An illustration of their individual ischemic lesions is shown in Fig. 1. A group of 16 volunteer participants matched for age (31–70 years), gender (eight females) and education (5–13 years) were tested as control group. A t test for unpaired data showed no difference (p = 0.33) between the age of controls (mean = 42.8 years, SD = 10.5 years) and that of patients (mean = 48.5 years, SD = 13.9). Also the education level expressed in years was not significantly different (p = 0.13) between the 16 controls (mean = 12.9 years, SD = 3.4 years) and the eight patients (mean = 10.8 years, SD = 2.9 years).
No extra-cerebellar lesions were present in the MRI of all subjects. At the time of data analysis, the patients’ cerebellar symptoms were quantified retrospectively on the basis of detailed physical and neurological examinations performed at the time of admission in hospital (i.e. shortly after symptom onset) and at the time of neuropsychological examination (ranging from 3 to 24 months). Such symptoms were graded according to the International Cooperative Ataxia Rating Scale (ICARS) [40]. In particular, we reconstructed on the basis of anamnestic information the scores of items 1–7 (posture and gait), of items 8–13 (kinetic functions) and of items 15–16 (speech). Item 14 (drawing of Archimedes’ spiral) and all the items of oculomotor function (items 17–19) could not be reconstructed reliably on the basis of the written records.
Procedure and Stimuli
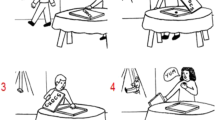
Participants sat comfortably in front of a PC monitor. They were informed of the experimental procedure and were asked to perform a few practice trials under the experimenter’s guidance. Each trial consisted in the simultaneous presentation on the monitor of four pictures each positioned in one quadrant of the slide. Three of the pictures were frames extracted from a single movie, taped on the same day. The fourth picture was not extracted from the same movie but had visual characteristics similar to the three congruent pictures. The spatial distribution of the four pictures was random in every trial, i.e. the three congruent pictures were not necessarily in the correct order and the intruder picture could be in any of the four quadrants. The participants’ task was to indicate the intruder picture. Two different sets of 16 trials (each trial being composed of four pictures) were presented to each participant. One set of stimuli depicted a human being performing actions and was named “biological action” test; the other depicted physical events occurring to inanimate objects and was named “folk physics” (Fig. 2). The two sets of 16 trials of both tasks are shown in Electronic Supplementary Figs. 1 and 2. The single “biological action” trials were classified according to the typology of the intruder picture in the following categories: (1) unusual or context-inappropriate hand–object interaction, (2) biomechanically inefficient limb or body movement and (3) change of effector. Also the single “folk physics” trials were categorized according to the intruder type which could be either: (1) an impossible static endpoint of a movement sequence, (2) an impossible intermediate point in a trajectory of a moving object and (3) change of object identity or physical characteristics. Electronic Supplementary Table 1 lists the category to which the single trials belong.
Example of the two experimental tasks. In the left panel is represented the “biological action” task. In the specific example, the action is to open a bottle and the intruder picture is no. 3. In the right panel is represented an example of the “folk physics” task showing a broom falling on the floor and the intruder picture is no. 3
Participants received the following instructions. They were told that three of the four pictures were extracted from a real sequence and that a fourth one was not. Their task was to identify the intruder picture. They were told to take their time in choosing but that they were required to give a response at every trial. It was also specified that the intruder pictures ranged from obviously incongruent to minimally incongruent. The two tasks were presented in blocks and their order was balanced between subjects.
Preliminary Assessment of the Stimuli in a Cohort of Healthy Participants
A cohort of 30 healthy participants was tested on both tasks in order to evaluate the scores in a healthy population. Participants were 13 male and 17 female, aged on average 38 years (SD, 13 years; range, 22–68 years) and with an average of 14 .3 years of education (SD, 3.5 years; range, 8–18 years). The demographic information on single participants is shown in Electronic Supplementary Table 2. The average accuracy in the biological action test was of 91.9% (SD, 6.8) and in the folk physics test it was of 89.4% (SD, 7.2). The difference between the scores in the two tests was assessed by means of a paired-sample t tests that yielded a significant result [t(DF = 29) = 2.35, p = 0.026]. Individual scores of the 30 participants in single tests are reported in Electronic Supplementary Tables 2 and 3.
Statistical Analysis
Performances in the two tasks were measured as ratio between the number of correct responses and the total number of responses. This “correct response rate” consisted therefore in a continuous variable ranging between 0 and 1. The correct response rate was used as dependent variable in an ANOVA with one between-subjects factor, “group” (two levels: patients or controls) and one within-subjects factor, “task” (two levels: “biological action” or “folk physics”) as independent variables. Post-hoc comparisons were made with two t tests for paired data for comparing the response rate in the two tasks and within each group of participants and with two t tests for independent samples for comparing the response rates between the two groups and within the same task. Finally, a score was calculated for each subject as the ratio between the accuracy in the biological action task and the accuracy in the folk physics test. This score was compared between the two groups by means of a t test for unpaired data.
Results
Neurological examination at the time of testing showed almost complete recovery of all symptoms. In particular all patients had recovered from dizziness and vertigo and were left with minimal ataxia of stance and gait. Arm dysmetria was minimal in all patients. A quantification of motor symptoms at the time of neuropsychological testing in the form of ICARS subscores and the interval from onset and the tests is reported in Table 1. Patients or controls participated in the task with sufficient attention and compliance. The mean performance in the two tests is reported in Table 2. Patients’ individual responses to each trial are reported in detail in Electronic Supplementary Tables 4 and 5. The ANOVA showed a main effect of the “group” factor (F(1, 22) = 15.609, p = 0.0007), with patients performing overall worse than controls (average correct response rates, 0.75 vs 0.87). A significant effect of the “task” factor was also recorded (F(1, 22) = 15.183, p = 0.0008), with an overall worse performance in the “biological action” task than in the “folk physics” task (average correct response rates, 0.82 vs 0.84). The most interesting finding however is that of a significant two-way interaction between “group” and “task” (F(1, 22) = 33.086, p = 0.00001) that is illustrated in Fig. 3, together with all the individual values of correct response rates. Mean values of the interaction are listed in Table 3. Post-hoc t tests for independent samples revealed a significant difference between correct response rates of controls and patients for both the “biological action” and the “folk physics” tasks (p values are provided in Fig. 3). Interestingly, the post-hoc paired-samples t tests between the two tasks clearly showed that patients performed dramatically worse in the “biological action” task, but controls did not show any significant difference between the two tasks, with a trend towards a worse performance in the ‘folk physics’ task (p values of the t tests are shown in Table 3).
Accordingly, the ratios of accuracy (biological action)/accuracy (folk physics) were all below zero (average, 0.87; SD, 0.09) for the eight patients but were equal to or above zero in 15 of the 16 healthy controls (average, 1.02; SD, 0.04). The t test showed a significantly different distribution of the ratios in the two groups [t(DF = 22) = 5.64, p = 0.030] thus further confirming the differential behaviour in the two tasks of patients and controls. The individual data of the scores are represented in Table 3.
Discussion
Our results show a clear impairment in patients when performing both the “folk physics” and the “biological action” tasks. This finding is in line with the theory of the cerebellum as a “general purpose” sequencer of orderly elements, which gets involved in tasks even when these are not related to the motor domain [41]. According to this theory, the main role of the cerebellum in different cognitive processes is that of a sequence detector, inheriting its role of feed-forward control from more simple motor-related behaviours. With respect to our data, also the strategy used to solve our tasks task is that of sequence detection. A necessary step to correctly detect the intruder picture is that of mentally reconstructing the most plausible chronological sequence of the depicted event that could account for three out of four pictures, building and comparing most of the possible permutations of three of the four pictorial elements. Other experiments in literature have used similar protocols [31, 42] as a way to test the capacity in reconstructing and controlling complex sequences. In these works the experimental paradigm is based on the idea that the subjects had to gain access to “how” a given action was composed in terms of simple units, and harmonically (and pragmatically) restructure it through an embodiment process. Conversely, in the case of physical events, such an implicit and embodied motor representation was unnecessary to solve the task (as in [31]).
The most interesting of our results is the fact that a dissociation is found in the patients’ performance in the two tasks. Despite the fact that patients perform worse than controls in both the “biological action” and the “folk physics” task, they performed significantly worse when the intruder-seeking task is applied to pictures depicting biological actions (Fig. 3). This finding demonstrates that the cerebellum contains a representation of sequences of biological actions that is separated and can therefore be dissociated from the sequences of non-biological events. Given the nature of the experiment, both tasks require the subject to mentally reconstruct the whole event in order to detect the intruder picture, but different laws specify how the mental reconstruction of the event occurs. While the “folk physic” task requires the knowledge of simple physical laws (for instance, the law of gravity), the “biological action” task requires the detection of implausible ways to accomplish the action, such as changing the effectors, adopting an unusual kinematics or employing a useless movements to accomplish an action. It is worth noting that an unusual kinematics does not prevent to accomplish the action; rather, it is an uncommon strategy according to how we normally behave. In other words, in the “biological action” task the criteria to reconstruct the sequence are embodied motor laws that are rooted in one’s own motor experience, and not gained by logical reasoning. The patients’ differential performance in the two tasks is not likely to be attributed to differences in the cognitive load or difficulty of the task since healthy controls performed with an equal amount of errors in the two tasks. If anything, the “folk physics” task tended to be more difficult than the “biological action” task to healthy controls (Fig. 3 and Tables 2 and 3) and in the validation group a clear difference was found in this direction (see Methods). It should be also noted that previous reports have demonstrated asymmetries and lateralization of the sequencing properties of the cerebellum [30, 42]. The small size of our cohort did not allow an analysis in this direction.
The neural mechanism by which the cerebellum is specialized in encoding sequences of biological actions can only be speculated upon. According to our original embodied prediction, the different modular brain structures involved in movements are used for decoding different features of observed movements depending on the motor properties of the same cortical modules. In our specific case we can then speculate that the cerebellum’s role of sequencer in the motor domain is exploited also in the action observation domain.
On the other hand it could be argued that the impairment observed in patients is due to a purely cognitive deficit and not to a motor one. An increasing number of data supports the view that specific sectors of the cerebellum plays a specific role in the cognitive, social and affective domains; a lesion of this sectors could produce cognitive deficits while the motor functions of the cerebellum are spared. According to Schmahmann [43], there appeared to be an anatomically identifiable motor–non-motor dichotomy in the cerebellum, with a sensorimotor part in the cerebellar anterior lobe and a cognitive and limbic cerebellum is in the posterior lobe. Patients with the cerebellar cognitive and affective syndrome, a cerebellar syndrome characterized by cognitive deficit such as personality change, impairments in working memory and lack of mental flexibility, show lesions of the posterior lobe, without showing motor deficit. In contrast, cerebellar motor syndrome resulted from strokes involving the anterior lobe [44]. For this reason, we cannot rule out the possibility that the cognitive deficit observed in our cohort is a purely non-motor deficit.
In contrast, also the view of an intrinsic motor nature of the observed deficit could be supported. The patients of our series at the time of evaluation had recovered completely from motor symptoms, and the cognitive deficit that we observed was mild and mainly subclinical (none of the patients had actually complained of such inability). However, if we consider the symptoms at onset (Table 1) we can see a wide prevalence of ataxia and dysmetria, which we believe to be the motor execution counterpart of the deficits in motor observation that we recorded. From this point of view our report represent a demonstration of an association between motor execution and motor observation deficits with lesions in one single portion of the central nervous system, therefore providing neuropsychological evidence for a mirror mechanism. Such evidence was up to now limited to the posterior parietal cortex and to the premotor cortex [45, 46].
The present results support the view that the cerebellum plays a specific role in action understanding and accounts for the numerous and up to now unexplained reports of cerebellar metabolic activation specific to action observation in neuroimaging studies. We suggest that the deficit observed represents the failure of an embodied, possibly mirror, mechanism, and therefore could be a primitively visuo-motor deficit rather than a purely cognitive deficit. We cannot however state whether the role of the cerebellum in this context is that of directly coding visual responses or if we are observing remote effects of cerebellar dysfunction on remote cerebral regions. Electrical imaging of cortical activity in cerebellar patients has demonstrated that their visual motion detection deficits is strongly correlated with altered activity in the more “canonical” visual motion processing cortical areas in the parietal and temporal lobes [47].
Our interpretation partly bridges the gap between the view that the cerebellum is involved in monitoring sequences of internally generated or external events [41] and the view that cerebellum plays a role in the social and affective domain [43, 48].
References
Cattaneo L, Rizzolatti G. The mirror neuron system. Arch Neurol. 2009;66:557–60.
Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: a magnetic stimulation study. J Neurophysiol. 1995;73:2608–11.
Jeannerod M. Neural simulation of action: a unifying mechanism for motor cognition. NeuroImage. 2001;14:S103–9.
Rizzolatti G, Fadiga L, Fogassi L, Gallese V. The space around us. Science. 1997;277:190–1.
Andres M, Seron X, Olivier E. Contribution of hand motor circuits to counting. J Cogn Neurosci. 2007;19:563–76.
Sato M, Cattaneo L, Rizzolatti G, Gallese V. Numbers within our hands: modulation of corticospinal excitability of hand muscles during numerical judgment. J Cogn Neurosci. 2007;19:684–93.
Glenberg AM, Sato M, Cattaneo L. Use-induced motor plasticity affects the processing of abstract and concrete language. Curr Biol. 2008;18:R290–1.
Calvo-Merino B, Grezes J, Glaser DE, Passingham RE, Haggard P. Seeing or doing? Influence of visual and motor familiarity in action observation. Curr Biol. 2006;16:1905–10.
Gazzola V, Keysers C. The observation and execution of actions share motor and somatosensory voxels in all tested subjects: single-subject analyses of unsmoothed fmri data. Cereb Cortex. 2008;19:1239–55.
Hamilton AF, Wolpert DM, Frith U, Grafton ST. Where does your own action influence your perception of another person's action in the brain? NeuroImage. 2006;29:524–35.
Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol. 1998;106:283–96.
Davare M, Montague K, Olivier E, Rothwell JC, Lemon RN. Ventral premotor to primary motor cortical interactions during object-driven grasp in humans. Cortex. 2009;45:1050–7.
Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson RS, Forssberg H. Cortical activity in precision- versus power-grip tasks: an fmri study. J Neurophysiol. 2000;83:528–36.
Grafton ST, Arbib MA, Fadiga L, Rizzolatti G. Localization of grasp representations in humans by positron emission tomography. 2. Observation compared with imagination. Exp Brain Res. 1996;112:103–11.
Avenanti A, Bolognini N, Maravita A, Aglioti SM. Somatic and motor components of action simulation. Curr Biol. 2007;17:2129–35.
Cattaneo L. Tuning of ventral premotor cortex neurons to distinct observed grasp types: a tms-priming study. Exp Brain Res. 2010;207:165–72.
Cattaneo L, Barchiesi G, Tabarelli D, Arfeller C, Sato M, Glenberg AM. One's motor performance predictably modulates the understanding of others' actions through adaptation of premotor visuo-motor neurons. Soc Cogn Affect Neurosci. 2010;6:301–10.
Cattaneo L, Sandrini M, Schwarzbach J. State-dependent tms reveals a hierarchical representation of observed acts in the temporal, parietal, and premotor cortices. Cereb Cortex. 2010;20:2252–8.
Pobric G, Hamilton AF. Action understanding requires the left inferior frontal cortex. Curr Biol. 2006;16:524–9.
Clavagnier S, Prado J, Kennedy H, Perenin MT. How humans reach: distinct cortical systems for central and peripheral vision. Neuroscientist. 2007;13:22–7.
Filimon F, Nelson JD, Hagler DJ, Sereno MI. Human cortical representations for reaching: mirror neurons for execution, observation, and imagery. NeuroImage. 2007;37:1315–28.
Malfait N, Valyear KF, Culham JC, Anton JL, Brown LE, Gribble PL. Fmri activation during observation of others' reach errors. J Cogn Neurosci. 2010;22:1493–503.
Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–34.
Grossman E, Donnelly M, Price R, Pickens D, Morgan V, Neighbor G, et al. Brain areas involved in perception of biological motion. J Cogn Neurosci. 2000;12:711–20.
Vaina LM, Solomon J, Chowdhury S, Sinha P, Belliveau JW. Functional neuroanatomy of biological motion perception in humans. Proc Natl Acad Sci U S A. 2001;98:11656–61.
Frey SH, Gerry VE. Modulation of neural activity during observational learning of actions and their sequential orders. J Neurosci. 2006;26:13194–201.
Gallagher HL, Frith CD. Dissociable neural pathways for the perception and recognition of expressive and instrumental gestures. Neuropsychologia. 2004;42:1725–36.
Grezes J, Pichon S, de Gelder B. Perceiving fear in dynamic body expressions. NeuroImage. 2007;35:959–67.
Jokisch D, Troje NF, Koch B, Schwarz M, Daum I. Differential involvement of the cerebellum in biological and coherent motion perception. Eur J Neurosci. 2005;21:3439–46.
Sokolov AA, Gharabaghi A, Tatagiba MS, Pavlova M. Cerebellar engagement in an action observation network. Cereb Cortex. 2010;20:486–91.
Fazio P, Cantagallo A, Craighero L, D'Ausilio A, Roy AC, Pozzo T, et al. Encoding of human action in broca's area. Brain. 2009;132:1980–8.
Jastorff J, Begliomini C, Fabbri-Destro M, Rizzolatti G, Orban GA. Coding observed motor acts: different organizational principles in the parietal and premotor cortex of humans. J Neurophysiol. 2010;104:128–40.
World Medical Association General Assembly. Declaration of Helsinki. Ethical principles for medical research involving human subjects. World Med J. 2008;54:122–5.
Bartolo A, Cubelli R, Della Sala S. Cognitive approach to the assessment of limb apraxia. Clin Neuropsychol. 2008;22:27–45.
Hamilton M. Rating depressive patients. J Clin Psychiatry. 1980;41:21–4.
Spinnler H, Tognoni G. Standardizzazione e taratura italiana di test neuropsicologici. Ital J Neurol Sci. 1987;6:1–120.
Venturini R, Lombardo Radice M, Imperiali MG. Il ‘colour-word test’ o test di Stroop. Firenze: Giunti Organizzazioni Speciali; 1983.
Golden CJ (1978) Stroop color and word test: a manual for clinical and experimental uses Chicago: Skoelting.
Raven J, Raven JC, Court JH. Manual for Raven's progressive matrices and vocabulary scales. San Antonio: Harcourt Assessment; 2003.
Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, et al. International cooperative ataxia rating scale for pharmacological assessment of the cerebellar syndrome. The ataxia neuropharmacology committee of the world federation of neurology. J Neurol Sci. 1997;145:205–11.
Molinari M, Chiricozzi FR, Clausi S, Tedesco AM, De Lisa M, Leggio MG. Cerebellum and detection of sequences, from perception to cognition. Cerebellum. 2008;7:611–5.
Leggio MG, Tedesco AM, Chiricozzi FR, Clausi S, Orsini A, Molinari M. Cognitive sequencing impairment in patients with focal or atrophic cerebellar damage. Brain. 2008;131:1332–43.
Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev. 2010;20:236–60.
Schmahmann JD, Macmore J, Vangel M. Cerebellar stroke without motor deficit: clinical evidence for motor and non-motor domains within the human cerebellum. Neuroscience. 2009;162:852–61.
Leiguarda RC, Marsden CD, Limb apraxias. Higher-order disorders of sensorimotor integration. Brain. 2000;123(Pt 5):860–79.
Pazzaglia M, Pizzamiglio L, Pes E, Aglioti SM. The sound of actions in apraxia. Curr Biol. 2008;18:1766–72.
Handel B, Thier P, Haarmeier T. Visual motion perception deficits due to cerebellar lesions are paralleled by specific changes in cerebro-cortical activity. J Neurosci. 2009;29:15126–33.
Timmann D, Daum I. How consistent are cognitive impairments in patients with cerebellar disorders? Behav Neurol. 2010;23:81–100.
Acknowledgements
We thank Dr. M. Leone for most valuable comments on the manuscript. This work has been realized through the financial support from the Provincia autonoma di Trento and the Fondazione Cassa di Risparmio di Trento e Rovereto.
Conflict of Interest Notification Page
None of the authors report any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.

Supplementary Fig. 1
(JPEG 3795 kb)

Supplementary Fig. 2
(JPEG 6783 kb)
Supplementary Table 1
(DOC 55 kb)
Supplementary Table 2
(DOC 109 kb)
Supplementary Table 3
(DOC 95 kb)
Supplementary Table 4
(DOC 43 kb)
Supplementary Table 5
(DOC 44 kb)
Rights and permissions
About this article
Cite this article
Cattaneo, L., Fasanelli, M., Andreatta, O. et al. Your Actions in My Cerebellum: Subclinical Deficits in Action Observation in Patients with Unilateral Chronic Cerebellar Stroke. Cerebellum 11, 264–271 (2012). https://doi.org/10.1007/s12311-011-0307-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-011-0307-9