Abstract
To assess the clinical spectrum of ataxia and cerebellar oculomotor deficits in the most common spinocerebellar ataxias (SCAs), we analysed the baseline data of the EUROSCA natural history study, a multicentric cohort study of 526 patients with either spinocerebellar ataxia type 1, 2, 3 or 6. To quantify ataxia symptoms, we used the Scale for the Assessment and Rating of Ataxia (SARA). The presence of cerebellar oculomotor signs was assessed using the Inventory of Non-Ataxia Symptoms (INAS). In a subgroup of patients, in which magnetic resonance images (MRIs) were available, we correlated MRI morphometric measures with clinical signs on an exploratory basis. The SARA subscores posture and gait (items 1–3), speech (item 4) and the limb kinetic subscore (items 5–8) did not differ between the genotypes. The scores of SARA item 3 (sitting), 5 (finger chase) and 6 (nose–finger test) differed between the subtypes whereas the scores of the remaining items were not different. In SCA1, ataxia symptoms were correlated with brainstem atrophy and in SCA3 with both brainstem and cerebellar atrophy. Cerebellar oculomotor deficits were most frequent in SCA6 followed by SCA3, whereas these abnormalities were less frequent in SCA1 and SCA2. Our data suggest that vestibulocerebellar, spinocerebellar and pontocerebellar circuits in SCA1, SCA2, SCA3 and SCA6 are functionally impaired to almost the same degree, but at different anatomical levels. The seemingly low prevalence of cerebellar oculomotor deficits in SCA1 and SCA2 is most probably related to the defective saccadic system in these disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The spinocerebellar ataxias (SCAs) are a clinically and genetically heterogeneous group of autosomal dominantly inherited progressive ataxia disorders. Up to now, almost 30 different gene loci have been found. In 19 SCAs the affected genes and causative mutations have been identified. The most common SCAs, which together account for more than half of all affected families, are SCA1, SCA2, SCA3 and SCA6 [1, 2]. Each of these disorders is caused by a translated CAG repeat expansion mutation [3–7]. Expanded CAG repeats are dynamic mutations with variable length that may change during transmission. Patients with longer repeats have an earlier disease onset, a more severe phenotype and faster disease progression than patients with shorter repeats [8].
The clinical phenotype of the common SCAs, SCA1, SCA2, SCA3 and SCA6 is characterized by prominent cerebellar ataxia. Phenotypical differences between these disorders mainly depend on the occurrence of additional non-cerebellar symptoms. Thus, SCA1 patients frequently have pyramidal tract signs, whereas peripheral neuropathy is often present in SCA2 and SCA3. Basal ganglia symptoms are more frequent in SCA2 and SCA3 than in SCA1, while brainstem oculomotor signs including saccadic slowing and ophthalmoparesis are encountered in all three genotypes. However, saccadic slowing is most prominent in SCA2 [9–15]. Other than in SCA1, SCA2 and SCA3, non-cerebellar symptoms in SCA6 are rare and usually mild [16, 17].
It is generally assumed that these disorders are uniform with respect to the type and severity of cerebellar ataxia symptoms. However, a proof for this assumption is lacking. This is mainly due to the fact that validated clinical instruments that allow to separately assess ataxia symptoms in a simple and reliable fashion have only recently been developed [18]. The Scale for the Assessment and Rating of Ataxia (SARA) is a novel clinical rating scale that is based on a semi-quantitative assessment of ataxia on an impairment level. SARA underwent a rigorous validation procedure involving three clinical trials in large groups of SCA, non-SCA ataxia patients and controls [18, 19]. Cerebellar oculomotor symptoms which are part of the spectrum of cerebellar ataxia are not considered in SARA because the first SARA validation trial indicated that the results of oculomotor examination were determined by a different underlying factor than the remaining ataxia symptoms [18]. The Inventory of Non-Ataxia Symptoms (INAS) was devised to assess the presence or absence of clinical signs that may occur in SCAs, but are not covered by SARA. INAS consists of 30 items, among which items 15 (broken up smooth pursuit), 17 (downbeat nystagmus), 18 (gaze-evoked nystagmus on horizontal testing), 23 (hypometric saccades) and 24 (hypermetric saccades) are related to cerebellar oculomotor signs [20].
To define and compare the spectrum of ataxia in the common SCAs, SCA1, SCA2, SCA3 and SCA6, we analysed the results of the individual SARA items, of SARA subscores related to posture and gait, speech and limb kinetic function as well as the INAS items 15, 17, 18, 23 and 24 obtained at the baseline visit of the EUROSCA natural history study. The EUROSCA natural history study is an ongoing multicentric cohort study of 526 patients with either SCA1, SCA2, SCA3 or SCA6 [20]. To better understand the anatomical basis of the ataxia symptoms, we correlated the severity of ataxia symptoms with volumetric measures of the brainstem and cerebellum obtained in a magnetic resonance imaging (MRI) substudy of the EUROSCA natural history study [21].
Patients and Methods
The study was performed at 17 European centres which together form the EUROSCA clinical group. Patients were eligible when they had progressive, otherwise unexplained ataxia and a positive molecular genetic test for either SCA1, SCA2, SCA3 or SCA6. Cases were ascertained with the help of an electronic patient registry that contains data of all SCA patients that have been in contact with one of the study centres. Patients were consecutively recruited within a predetermined period between July 2005 and August 2006. The study population consisted of 526 patients (SCA1 117, SCA2 163, SCA3 139, SCA6 107) [20]. The subgroup which underwent MRI consisted of 82 patients (SCA1 48, SCA3 24, SCA6 10) [21]. Inclusion into the MRI substudy depended on the informed consent and the availability of MRI scanners and scanning time of the participating centres. Demographic, genetic and clinical data of the study population are given in Table 1.
Assessments were done according to a written study protocol. There were maximally three investigators at each centre. All investigators were experienced in the use of the applied scales.
To measure ataxia symptoms, we used the SARA that has been recently developed and validated. SARA has eight items that yield a sum score of 0 (no ataxia) to 40 (most severe ataxia): 1 = gait (score 0–8), 2 = stance (score 0–6), 3 = sitting (score 0–4), 4 = speech disturbance (score 0–6), 5 = finger chase (score 0–4), 6 = nose-finger test (score 0–4), 7 = fast alternating hand movements (score 0–4) and 8 = heel–shin slide (score 0–4). Items 5–8 are rated independently for both sides, and the arithmetic mean of both sides is included in the SARA sum score. In addition, we formed subscores related to posture and gait, speech and limb kinetic function. The posture and gait subscore was formed by adding the scores of items 1–3, the limb kinetic function subscore by adding the scores of items 5–8, while the speech subscore is identical with item 4 [18, 22].
Cerebellar oculomotor signs were assessed with the INAS. INAS consists of 30 items, each of which is related to one of the following 16 symptoms or syndromes: areflexia, hyperreflexia, extensor plantar response, spasticity, paresis, amyotrophy, fasciculations, myoclonus, rigidity, chorea, dystonia, resting tremor, sensory symptoms, oculomotor signs, urinary dysfunction and cognitive impairment. For the purpose of this study, item 15 (broken up smooth pursuit), item 17 (downbeat nystagmus), item 18 (gaze-evoked nystagmus on horizontal testing), item 23 (hypometric saccades) and item 24 (hypermetric saccades) were considered. In these items, only the presence or absence of the respective symptom is determined. Hypometric (item 23) and hypermetric saccades (item 24) were summarized as dysmetric saccades, if one of these symptoms occurred. Reliability of INAS ratings was tested in two large multi-centre trials. For most INAS items, it was found to be acceptable [20] (own unpublished data).
The MRI studies were performed, as previously described [21]. In brief, patients were scanned on 1.5 T scanners (Siemens Sonata, Siemens Vision, Philips, General Electrics) in eight participating imaging centres (Bonn, Brussels, Essen, Milan, Nijmegen, Paris, Santander, Tübingen and Warsaw). The imaging protocol comprised a 3D T1-weighted anatomical dataset measured with a standard head coil. For each scanner model, a custom-tailored imaging protocol was used to make datasets as similar as possible without sacrificing image quality. Image protocols were chosen to optimize image contrast and signal-to-noise ratio for each scanner. According to the different manufacturer-specific conditions, the following settings were used: Siemens: MPRAGE, TRπ 1,200–1,500; Philips: FFE, TRπ 6–15 and General Electrics: TRπ 2,200–3,000. The following settings were identical on all scanners: TE 2–7, TI 450–700, matrix 256 × 256, field of view 230 mm, slice thickness 1 mm and voxel size of 1 × 1 × 1 mm. The resulting datasets were converted to ANALYZE format.
All MRI datasets were analysed by one investigator blinded to the diagnosis. MRI-based volumetry was implemented by using the method previously described and validated for its reproducibility [23]. Volumetry consisted of semiautomatic presegmentation based on predefined anatomical landmarks and automated region-growing segmentation. Brain structures that could not be distinguished by the region-growing algorithm were delineated manually. Calculation of the volumes was achieved by multiplying the number of voxels per regions of interest and the voxel size. The cranial border of the brainstem was defined by a plane through the mammillary body and posterior commissure shifted caudally by 1/3 the distance to the cranial notch of the pons. A parallel plane to the latter intersecting with the posterior rim of the foramen magnum represented the caudal border. The posterior border of the brainstem was defined by a plane through the obex and posterior commissure shifted posteriorly to include the inferior colliculus. Paravermal sagittal planes separated the cerebellar vermis from the hemispheres. The total intracranial volume (TICV) was estimated by tracing the dura mater using a 1-in-10 sampling strategy [24]. To adjust for individual differences of head size, TICV was used for the normalization of localized brain volumes.
Statistical analyses were performed with SAS 8 software (SAS Institute, Cary, NC, USA) and SPSS 17.0 for Windows (SPSS Inc., 2008). Test results were considered significant at the 0.05 level. Patient clinical characteristics were compared among SCA groups with an analysis of variance for continuous variables and with a [chi]2 test for categorical variables. Descriptive results are given as mean ± standard deviation or as median and lower (Q1) and upper quartile (Q3). For comparison of the SARA, we used Kruskal–Wallis one-way analysis of variance with Bonferroni correction. Wilcoxon–Mann–Whitney test on each pair of groups was used to qualify the differences. For the comparison of INAS, we used Pearson’s chi-square test with Bonferroni correction.
To explore the association of regional brain volumes with SARA scores, we calculated Spearman’s rank correlation coefficient. As this part of the study had an exploratory character, we did not perform a Bonferroni correction.
The procedures followed were in accordance with the ethical standards of the ethics committees of the contributing centres and with the Helsinki Declaration 1975, as revised in 2000 (World Medical Association Declaration of Helsinki 2000). Informed and written consent was obtained from all study participants.
Results
Ataxia Symptoms
The four genotypes were comparable in terms of disease duration (Table 1). There were no differences between the SARA sum scores in SCA1 (15.6 ± 9.1), SCA2 (15.8 ± 8.0), SCA3 (15.1 ± 8.6) and SCA6 (15.0 ± 6.7). Similarly, the posture and gait subscore (items 1–3), the speech subscore (item 4) and the limb kinetic subscore (items 5–8) did not differ between the genotypes (Table 2). At the level of the single items, we found differences for item 3 (sitting, p = 0.0050), item 5 (finger chase, p = 0.0025) and item 6 (nose–finger test, p < 0.0001), whereas the scores of the remaining items were not different (Table 3). Further analyses showed that the differences in item 3 (sitting) were due to a better performance of SCA6 patients compared to all other genotypes. In item 5 (finger chase), SCA3 patients performed better than the other genotypes, while item 6 (nose–finger test) was less impaired in SCA3 and SCA6 compared to SCA1 and SCA2. In a subgroup of SCA1, SCA3 and SCA6 patients who underwent MRI, sum scores, subscores and single item scores did not differ between the genotypes with the exception of the speech subscore which was lower in SCA3 compared to SCA1 and SCA6 (Tables 2 and 3).
Cerebellar Oculomotor Deficits
The prevalence of cerebellar oculomotor signs assessed in this study differed between the genotypes (Table 4). Post hoc analyses showed that INAS item 15 (broken up smooth pursuit) was more frequent in SCA3 and SCA6 compared to SCA1 and SCA2. Downbeat nystagmus (item 17) was encountered in 20.6% of all SCA6 patients, but only rarely in the other genotypes. Horizontal gaze-evoked nystagmus (item 18) was frequently found in SCA3 and SCA6. Prevalence was lower in SCA1 and even lower in SCA2. Dysmetric saccades (item 23 and/or 24) were most frequent in SCA6 followed by SCA1 and SCA3, while dysmetric saccades were rare in SCA2.
Correlation of Ataxia Symptoms with Volumetric Measures
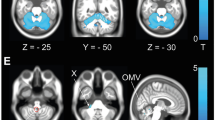
In a subgroup of SCA1, SCA3 and SCA6 patients, we correlated clinical signs with the volumes of the brainstem, cerebellar vermis and cerebellar hemispheres. Representative MRI scans are shown in Fig. 1. SCA1 patients with higher posture and gait subscores had smaller volumes of the brainstem (r = −0.28, p < 0.05). In SCA3, we found inverse relationships between the posture and gait subscore and the brainstem volume (r = −0.60, p < 0.01) as well as the cerebellar hemispheric volume (r = −0.36, p < 0.05). In addition, the limb kinetic subscore was inversely correlated with the brainstem (r = −0.65, p < 0.01) and cerebellar hemispheric volumes (r = −0.35, p < 0.05). There were no significant correlations between SARA subscores and brainstem and cerebellar volumes in SCA6. Correlation of the single item scores with the volumes of the brainstem, cerebellar vermis and cerebellar hemispheres yielded largely congruent results (data not shown). Beyond this, we found an inverse correlation between gait ataxia (item 1) and cerebellar volume in SCA6 (r = −0.56, p < 0.05; Table 5).
Discussion
We have used two novel clinical instruments, SARA and INAS, to assess and compare the spectrum of ataxia and cerebellar oculomotor symptoms in a large cohort of SCA1, SCA2, SCA3 and SCA6 patients. SARA has eight items which are related to gait, stance, trunk stability during sitting, speech and coordination of arm and leg movements [18]. When we used three subscores that were related to posture and gait, speech and limb kinetic function, we failed to find differences between SCA1, SCA2, SCA3 and SCA6. Comparison of the scores of the individual SARA items revealed differences only in item 3 which is related to sitting and items 5 and 6 which are related to control of arm movements. Sitting was more stable in SCA6 than in the other genotypes. In addition, SCA3 and to a lesser degree SCA6 patients had less ataxia of arm movements than SCA1 and SCA2 patients. Similarly, comparison of the scores of the subgroups that underwent MRI did not reveal relevant differences between the genotypes.
INAS was designed to assess non-ataxia and oculomotor symptoms in SCA patients. For the purpose of this study, we selected those INAS items that are related to cerebellar oculomotor functions. In contrast to previous electrooculographic studies, the present study completely relied on clinical observation and lacked quantification. On the other hand, our clinical approach allowed enrolling a large number of SCA patients that by far exceeds that of all earlier eye movement studies in SCAs [25–28]. Whereas the phenotype of SCA1, SCA2, SCA3 and SCA6 patients was quite similar with respect to ataxia of gait, stance and limb movements, there were marked differences of the frequency of cerebellar oculomotor deficits. Pursuit deficits, horizontal gaze-evoked nystagmus and saccadic dysmetria were most frequently encountered in SCA6 followed by SCA3, whereas these abnormalities were less frequent in SCA1 and SCA2. These findings are in good accordance with previous studies that observed pursuit deficits, horizontal gaze-evoked nystagmus and saccadic dysmetria in patients with all four genotypes, but noted a low frequency of horizontal gaze-evoked nystagmus in SCA2 [26, 27, 29]. Downbeat nystagmus was encountered almost exclusively in SCA6, a finding that confirms previous reports on downbeat nystagmus in SCAs. However, the prevalence of downbeat nystagmus in SCA6 was lower than previously reported, a discrepancy that is probably due to lower sensitivity of clinical testing [25, 26, 28].
Although the cerebellar cortex has a remarkably homogeneous microanatomical structure, there is evidence for subdivision of the cerebellar cortex into functionally distinct compartments that emerge as a consequence of specific afferent and efferent connections. Vestibular afferents strongly project to the flocculonodular lobe, spinal afferents to anterior and posterior parts of the vermis and the paravermal hemispheric regions of the anterior lobe and cortical afferents via the pontine nuclei to the cerebellar hemispheres. With respect to efferents, the cerebellar cortex is divided into three sagittal zones: the vermis projecting via the fastigial nucleus to brainstem and spinal targets, an intermediate zone projecting via the interposed nucleus to the red nucleus and thalamus and a lateral zone projecting via the dentate nucleus to the thalamus. In addition, the flocculonodular lobe projects to the vestibular nuclei [30–32]. As a consequence of these anatomical connections, lesions of the flocculonodular lobe and caudal vermis typically result in truncal and gait ataxia, lesions of the rostral vermis in gait ataxia and hemispheric lesions in limb ataxia. Similar symptoms may occur as a result of lesions in different anatomical structures and tracts that project to the cerebellar compartments or are targets of them. For example, ataxia of limb movements may not only occur as the consequence of cerebellar hemispheric damage but also of pontine and thalamic lesions [33–35]. For the understanding of clinical–pathological correlations in degenerative ataxias, it is therefore appropriate to conceive the anatomy of the cerebellum and its connections in terms of vestibulocerebellar, spinocerebellar and pontocerebellar circuits rather than cerebellar cortical divisions [31].
The flocculus and posterior vermis have a key role in the cerebellar control of eye movements. Bilateral lesions of the flocculus result in downbeat nystagmus. The flocculus is also part of the neural integrator network that is the anatomical substrate of the ability to keep eyes in an eccentric position. Consequently, floccular lesions results in a drift towards the primary position which is compensated by corrective saccades towards the eccentric position resulting in gaze-evoked nystagmus. Impaired control of saccadic eye movements by the posterior vermis results in saccadic hypermetria. Cerebellar control of smooth pursuit involves the flocculus and posterior vermis [36].
In the light of this knowledge, the SARA data suggest that vestibulocerebellar, spinocerebellar and pontocerebellar circuits are involved in SCA1, SCA2, SCA3 and SCA6 to almost the same degree. The clinical differences that we found between the genotypes were minor and detected only at the single item level, whereas the more robust analysis using SARA subscores did not confirm any differences. The large concordance of the spectrum of ataxia symptoms between SCA1, SCA2, SCA3 and SCA6 is surprising, as the cerebellum and its connections are differentially affected in SCA1, SCA2, SCA3 and SCA6. In SCA6, there is an almost exclusive degeneration of the cerebellar cortex [37], whereas SCA1, SCA2 and SCA3 are characterized by a more complex pattern of degeneration. SCA1 and SCA2 are prototypes of olivopontocerebellar atrophy with degeneration of the inferior olives, pontine nuclei and cerebellar cortex [38, 39]. In SCA3, there is prominent degeneration of the spinocerebellar tracts, pontine nuclei and dentate nuclei, whereas the cerebellar cortex is relatively spared [12]. The different degeneration patterns occurring in the various SCAs have been reproduced in MRI studies including a recent multicentric morphometric study performed in the present patient cohort [21, 40].
To shed light on the seeming paradox between the uniform spectrum of ataxia and the differential involvement of cerebellar and brainstem structures in the SCAs, we correlated the SARA subscores with brainstem and cerebellar volumes as measured by MRI volumetry in a subset of SCA1, SCA3 and SCA6 patients. This part of our study has an exploratory character because the number of patients, in particular SCA6 patients, for whom MRI data were available, was small. Moreover, the morphometric analysis was restricted to the brainstem, cerebellar vermis and cerebellar hemispheres, whereas functionally important structures such as the flocculonodular lobe or the dentate nuclei were not segmented and measured. MRI data from SCA2 patients were not available, but the degeneration pattern of SCA2 closely matches that of SCA1. Despite these limitations, the results give important clues to the understanding of the pathoanatomical basis of ataxia. Instead of finding a relation between ataxia symptoms and volume loss of certain anatomical structures across all three genotypes, we rather found patterns of correlations that were specific for each disorder. In SCA1, posture and gait abnormalities correlated with brainstem atrophy. In SCA3, posture and gait abnormalities and limb kinetic dysfunction correlated with both, brainstem and cerebellar atrophy. In the SCA6 MRI subgroup that comprised only ten patients, we found weak evidence for a relation between gait ataxia and cerebellar atrophy. A recent study of 17 patients with a cerebellar cortical degeneration, among them 11 with SCA6, reported robust correlations between the SARA posture and gait subscore and atrophy of the medial and intermediate cerebellum and between the SARA limb kinetic subscore and atrophy of the cerebellar hemispheres [22].
The present data together with knowledge from earlier neuropathological and clinical studies support the view that vestibulocerebellar, spinocerebellar and pontocerebellar circuits in SCA1, SCA2, SCA3 and SCA6 are functionally impaired to almost the same degree, but at different anatomical levels. In SCA1 and possibly SCA2, brainstem degeneration appears to be most critical for the development of ataxia, whereas cerebellar degeneration plays the major role in SCA6. The anatomical structures responsible for ataxia in SCA3 appear to be more distributed between brainstem and cerebellum.
Whereas the clinical spectrum of ataxia of gait, stance and limb movements was widely uniform among the four genotypes, examination of cerebellar oculomotor function revealed major differences suggesting that the oculomotor regions of the cerebellum, in particular the flocculus, are most heavily affected in SCA6 followed by SCA3, whereas the oculomotor cerebellum appears to be only mildly affected in SCA1 and SCA2. This assumption appears reasonable to explain the differences between SCA6 and SCA3, since involvement of the cerebellar cortex is much milder in SCA3 than in SCA6. However, cerebellar cortical degeneration is severe in SCA1 and SCA2, and there is no indication that oculomotor parts of the cerebellum are spared in these disorders [29]. The seemingly low prevalence of “cerebellar” oculomotor disturbances in SCA1 and SCA2 is rather due to prominent pontine involvement resulting in saccadic slowing. As a consequence, corrective saccades are also slow and hard to discern in clinical testing [29]. Similarly, saccadic hypermetria is unlikely to occur in patients with slow saccades. Thus, the defective saccadic system occludes cerebellar deficits of pursuit, gaze holding and saccadic accuracy in SCA1 and SCA2.
Conclusion
Our data suggest that vestibulocerebellar, spinocerebellar and pontocerebellar circuits in SCA1, SCA2, SCA3 and SCA6 are functionally impaired to almost the same degree, but at different anatomical levels. The seemingly low prevalence of cerebellar oculomotor deficits in SCA1 and SCA2 is most probably related to the defective saccadic system in these disorders.
References
Durr A. Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond. Lancet Neurol. 2010;9(9):885–94.
Paulson HL. The spinocerebellar ataxias. J Neuroophthalmol. 2009;29(3):227–37.
Orr HT, Chung MY, Banfi S, Kwiatkowski Jr TJ, Servadio A, Beaudet AL, et al. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet. 1993;4(3):221–6.
Pulst SM, Nechiporuk A, Nechiporuk T, Gispert S, Chen XN, Lopes-Cendes I, et al. Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nat Genet. 1996;14(3):269–76.
Imbert G, Saudou F, Yvert G, Devys D, Trottier Y, Garnier JM, et al. Cloning of the gene for spinocerebellar ataxia 2 reveals a locus with high sensitivity to expanded CAG/glutamine repeats. Nat Genet. 1996;14(3):285–91.
Kawaguchi Y, Okamoto T, Taniwaki M, Aizawa M, Inoue M, Katayama S, et al. CAG expansions in a novel gene for Machado–Joseph disease at chromosome 14q32.1. Nat Genet. 1994;8(3):221–8.
Zhuchenko O, Bailey J, Bonnen P, Ashizawa T, Stockton DW, Amos C, et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the a1A-voltage-dependent calcium channel. Nat Genet. 1997;15(1):62–9.
Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci. 2007;30:575–621.
Dubourg O, Dürr A, Cancel G, Stevanin G, Chneiweiss H, Penet C, et al. Analysis of the SCA1 CAG repeat in a large number of families with dominant ataxia: clinical and molecular correlations. Ann Neurol. 1995;37:176–80.
Orozco-Diaz G, Nodarse-Fleites A, Cordoves-Sagaz R, Auburger G. Autosomal dominant cerebellar ataxia: clinical analysis of 263 patients from a homogeneous population in Holguin, Cuba. Neurology. 1990;40:1369–75.
Schöls L, Gispert S, Vorgerd M, Menezes Vieira-Saecker AM, Blanke P, Auburger G, et al. Spinocerebellar ataxia type 2—genotype and phenotype in German kindreds. Arch Neurol. 1997;54(9):1073–80.
Dürr A, Stevanin G, Cancel G, Duyckaerts C, Abbas N, Didierjean O, et al. Spinocerebellar ataxia 3 and Machado–Joseph disease: clinical, molecular, and neuropathological features. Ann Neurol. 1996;39:490–9.
Bürk K, Abele M, Fetter M, Dichgans J, Skalej M, Laccone F, et al. Autosomal dominant cerebellar ataxia type I—clinical features and MRI in families with SCA1, SCA2 and SCA3. Brain. 1996;119(5):1497–505.
Filla A, De Michele G, Campanella G, Perretti A, Santoro L, Serlenga L, et al. Autosomal dominant cerebellar ataxia type I. Clinical and molecular study in 36 Italian families including a comparison between SCA1 and SCA2 phenotypes. J Neurol Sci. 1996;142(1–2):140–7.
Schöls L, Amoiridis G, Büttner T, Przuntek H, Epplen JT, Riess O. Autosomal dominant cerebellar ataxia: phenotypic differences in genetically defined subtypes? Ann Neurol. 1997;42(6):924–32.
Schöls L, Krüger R, Amoiridis G, Przuntek H, Epplen JT, Riess O. Spinocerebellar ataxia type 6: genotype and phenotype in German kindreds. J Neurol Neurosurg Psychiatry. 1998;64(1):67–73.
Matsumura R, Futamura N, Fujimoto Y, Yanagimoto S, Horikawa H, Suzumura A, et al. Spinocerebellar ataxia type 6—molecular and clinical features of 35 Japanese patients including one homozygous for the CAG repeat expansion. Neurology. 1997;49(5):1238–43.
Schmitz-Hubsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66(11):1717–20.
Weyer A, Abele M, Schmitz-Hubsch T, Schoch B, Frings M, Timmann D, et al. Reliability and validity of the scale for the assessment and rating of ataxia: a study in 64 ataxia patients. Mov Disord. 2007;22:1633–7.
Schmitz-Hübsch T, Coudert M, Bauer P, Giunti P, Globas C, Baliko L, et al. Spinocerebellar ataxia type 1, 2, 3, and 6. Disease severity and nonataxia symptoms. Neurology. 2008;71:982–9.
Schulz JB, Borkert J, Wolf S, Schmitz-Hübsch T, Rakowicz M, Mariotti C, et al. Visualization, quantification and correlation of brain atrophy with clinical symptoms in spinocerebellar ataxia types 1, 3 and 6. Neuroimage. 2010;49(1):158–68.
Brandauer B, Hermsdorfer J, Beck A, Aurich V, Gizewski ER, Marquardt C, et al. Impairments of prehension kinematics and grasping forces in patients with cerebellar degeneration and the relationship to cerebellar atrophy. Clin Neurophysiol. 2008;119(11):2528–37.
Luft AR, Skalej M, Welte D, Kolb R, Bürk K, Schulz JB, et al. A new semiautomated, three-dimensional technique allowing precise quantification of total and regional cerebellar volume using MRI. Magn Reson Med. 1998;40(1):143–51.
Eritaia J, Wood SJ, Stuart GW, Bridle N, Dudgeon P, Maruff P, et al. An optimized method for estimating intracranial volume from magnetic resonance images. Magn Reson Med. 2000;44(6):973–7.
Gomez CM, Thompson RM, Gammack JT, Perlman SL, Dobyns WB, Truwit CL, et al. Spinocerebellar ataxia type 6: gaze-evoked and vertical nystagmus, Purkinje cell degeneration, and variable age of onset. Ann Neurol. 1997;42(6):933–50.
Buttner N, Geschwind D, Jen JC, Perlman S, Pulst SM, Baloh RW. Oculomotor phenotypes in autosomal dominant ataxias. Arch Neurol. 1998;55(10):1353–7.
Burk K, Fetter M, Abele M, Laccone F, Brice A, Dichgans J, et al. Autosomal dominant cerebellar ataxia type I: oculomotor abnormalities in families with SCA1, SCA2, and SCA3. J Neurol. 1999;246(9):789–97.
Yabe I, Sasaki H, Takeichi N, Takei A, Hamada T, Fukushima K, et al. Positional vertigo and macroscopic downbeat positioning nystagmus in spinocerebellar ataxia type 6 (SCA6). J Neurol. 2003;250(4):440–3.
Ying SH, Choi SI, Lee M, Perlman SL, Baloh RW, Toga AW, et al. Relative atrophy of the flocculus and ocular motor dysfunction in SCA2 and SCA6. Ann N Y Acad Sci. 2005;1039:430–5.
Dichgans J, Fetter M. Compartmentalized cerebellar functions upon the stabilization of body posture. Rev Neurol (Paris). 1993;149(11):654–64.
Timmann D, Diener HC. Coordination and ataxia. In: Goetz CG, Pappert EJ, editors. Textbook of clinical neurology. 3rd ed. Philadelphia: Saunders Elsevier; 2007. p. 307–25.
Dietrichs E. Clinical manifestation of focal cerebellar disease as related to the organization of neural pathways. Acta Neurol Scand Suppl. 2008;188:6–11.
Solomon DH, Barohn RJ, Bazan C, Grissom J. The thalamic ataxia syndrome. Neurology. 1994;44(5):810–4.
Mitoma H, Hayashi R, Yanagisawa N, Tsukagoshi H. Gait disturbances in patients with pontine medial tegmental lesions: clinical characteristics and gait analysis. Arch Neurol. 2000;57(7):1048–57.
Marx JJ, Iannetti GD, Thömke F, Fitzek S, Galeotti F, Truini A, et al. Topodiagnostic implications of hemiataxia: an MRI-based brainstem mapping analysis. Neuroimage. 2008;39(4):1625–32.
Voogd J, Schraa-Tam CK, van der Geest JN, de Zeeuw CI. Visuomotor cerebellum in human and nonhuman primates. Cerebellum 2010. doi:10.1007/s12311-010-0204-7.
Wang X, Wang H, Xia Y, Jiang H, Shen L, Wang S et al. Spinocerebellar ataxia type 6: systematic patho-anatomical study reveals different phylogenetically defined regions of the cerebellum and neural pathways undergo different evolutions of the degenerative process. Neuropathology 2010. doi:10.1111/j.1440-1789.2009.01094.x.
Genis D, Matilla T, Volpini V, Rosell J, Dávalos A, Ferrer I, et al. Clinical, neuropathologic, and genetic studies of a large spinocerebellar ataxia type 1 (SCA1) kindred: (CAG)n expansion and early premonitory signs and symptoms. Neurology. 1995;45:24–30.
Orozco G, Estrada R, Perry TL, Araña J, Fernandez R, Gonzalez-Quevedo A, et al. Dominantly inherited olivopontocerebellar atrophy from eastern Cuba. Clinical, neuropathological, and biochemical findings. J Neurol Sci. 1989;93:37–50.
Klockgether T, Skalej M, Wedekind D, Luft AR, Welte D, Schulz JB, et al. Autosomal dominant cerebellar ataxia type I. MRI-based volumetry of posterior fossa structures and basal ganglia in spinocerebellar ataxia types 1, 2 and 3. Brain. 1998;121(Pt 9):1687–93.
Acknowledgments
We are grateful to Drs. Klopstock and Petersen (Department of Neurology, University of Munich, Munich, Germany), Dr. Abdo (Department of Neurology, Radboud University Nijmegen Medical Center, Nijmegen, the Netherlands) and Dr. Verstappen (Canisius Wilhelina Hospital, Nijmegen, the Netherlands) for contribution of patients and help in patient assessment. We thank all the patients for participating in the study. This study was supported by grant EUROSCA/LSHM-CT-2004-503304 from the European Union, GeneMove/01 GM 0503 from the German Ministry of Education and Research and grant 3 PO5B 019 24 from the Polish Ministry of Scientific Research and Information Technology.
Conflicts of Interest
The authors report no conflicts of interest.
Full Financial Disclosures
H. Jacobi, T.-K. Hauser, P. Giunti, C. Globas, T. Schmitz-Hübsch, L. Baliko, A. Filla, C. Mariotti, P. Charles, P. Ribai, S. Szymanski, J. Infante, A. Dürr, D. Timmann, R. Fancellu, R. Rola, E. Zdzienicka, J.-S. Kang, S. Ratzka, D.A. Stephenson, S. Tezenas du Montcel and J. Borkert have declared no financial disclosure in stock ownership in medically related fields, consultancies, advisory boards, partnerships, honoraria, intellectual property rights, expert testimony, contracts, royalties and others. H. Jacobi, T.-K. Hauser, C. Globas, L. Baliko, P. Charles, S. Szymanski, R. Fancellu, R. Rola, J.-S. Kang, S. Ratzka, D.A. Stephenson and J. Borkert have declared no grants. P. Giunti was supported by grants from EUROSCA FP7 and Ataxia UK; T. Schmitz-Hübsch by grants from EUROSCA; A. Filla by grants from Ministry of Health: RF-SDN-2007-666932 and MIUR (Ministry of Research): E61J10000020001; C. Mariotti by grants from E-Rare; EFACTS, FP7 no: 242193; Study No.: 12631A; EudraCT No.: 2008-003662-25; Sponsor: International Clinical Research; H. Lundbeck A/S 2500 Valby (Copenhagen), Denmark; P. Ribai by grants from Société Française de Neurologie (SFN) and Association Française contre l’ataxie de Friedreich (AFAF); J. Infante by grants from Fondo de Investigación Sanitaria (PI 070014); A. Dürr by grants from PHRC National 2003 (agency: Assistance Publique-Hôpitaux de Paris (2005–2012)), Eurosca (agency: European Union (2004–2009)) and Spax (agency: French Agency for Research (2010–2012)); D. Timmann by grants from German Research Foundation (DFG TI 239/9-1), EU (Partner Marie Curie Initial Training Network) and Bernd Fink Foundation; E. Zdzienicka by grants from EUROSCA grant LSHM-CT-2004-503304 PO5E 019 29-Myotonic Dystrophy type 1 and 2 by the Polish State Committee of Scientific Research and RISCA 511-006-10001 from the Polish Ministry of Science and Higher Education and S. Tezenas du Montcel by grants from the European Union (grant EUROSCA (LSHM-CT-2004-503304)), the Assistance Publique-Hôpitaux de Paris (grant PHRC AOM 03059) and from the European Union C08A043 (funded by the French National Agency for Research). H. Jacobi is employed by the University of Bonn while T.-K. Hauser is by the University of Tübingen; P. Giunti by the UCL/UCLH; T. Schmitz-Hübsch by the University of Bonn; L. Baliko by the County Hospital, Veszprém, Hungary; A. Filla by the Federico II University, Napoli, Italy; C. Mariotti by the Fondazione IRCCS Neurological Institute Carlo Besta, Milan; P. Charles by the Hôpital de la Salpêtrière, Département de Génétique, Paris, France; P. Ribai by the Hôpital de la Salpêtrière, Département de Génétique, Paris, France; S. Szymanski by the St. Josef Hospital, University Hospital of Bochum; J. Infante by the University of Cantabria, Santander, Spain; A. Dürr by the Hôpital de la Salpêtrière, Département de Génétique, Paris, France; D. Timmann by the University of Duisburg-Essen; R. Fancellu by the Ospedale Villa Scassi, ASL3 Genovese, Genoa, Italy; R. Rola by the Institute of Psychiatry and Neurology and The Medical University of Warsaw, Poland; E. Zdzienicka by the Institute of Psychiatry and Neurology, Warsaw, Poland; J.-S. Kang by the University of Frankfurt, Frankfurt am Main, Germany; S. Ratzka by the Klinikum Augsburg, Department of Neurology, Germany; D.A. Stephenson by the UCL Medical Institute, USA; S. Tezenas du Montcel by the Department of Biostatistics and Medical Informatics and by the University Pierre et Marie Curie, Paris, France and J. Borkert by the University of Göttingen. C. Globas was employed by the Clinic of Psychiatry, University Tübingen from April 2009 to March 2010 and is currently employed by the Clinic of Neurology, University, Zürich since May 2010.
P. Bauer has declared no financial disclosure in stock ownership in medically related fields, advisory boards, partnerships, intellectual property rights, expert testimony, contracts, royalties and other; is a consultant at CENTOGENE (Rostock, Germany) and was supported by grants from Research Council (BMBF) to GeNeMove (01GM0603), EUROSPA (01GM0807) and RISCA (09GM0820) as well as from the EU for EUROSCA (LSHM-CT-2004-503304), MarkMD (FP7-People PIAP-2008-230596) and TECHGENE (FP7-Health 2007-B 223143). A further project received funding from the HSP-Selbsthilfegruppe Deutschland e.V. Honoraria was received from Roche Diagnostics (Mannheim, Germany) and Actelion Pharmaceuticals (Basel, Switzerland). He is employed by the University of Tübingen.
M. Rakowicz has declared no financial disclosure in stock ownership in medically related fields, advisory boards, honoraria, intellectual property rights, expert testimony, contracts, royalties and other; is a consultant at the Private Neurological Office, Warsaw, Poland; is partner of Ma-Je-R Ltd, Warsaw, Poland; was supported by grants from EUROSCA grant LSHM-CT-2004-503304, PO5E 019 29 by the Polish State Committee of Scientific Research and RISCA 511-006-10001 from the Polish Ministry of Science and Higher Education and is employed by the Institute of Psychiatry and Neurology, Warsaw.
B.P.C. van de Warrenburg has declared no financial disclosure in stock ownership in medically related fields, consultancies, partnerships, intellectual property rights, expert testimony, contracts, royalties and other; is a medical advisor for the Dutch patient organizations for ADCA, dystonia and hereditary spastic paraplegia; was supported by grants from Prinses Beatrix Fonds, the Brain Foundation, Department of Neurology of the Radboud University Nijmegen Medical Centre, Ipsen Pharmaceuticals (all in the Netherlands) and from the European Union (EUROSCA/LSHM-CT-2004-503304); has received honoraria from editorial work for the Dutch Health Direct Neurology journal (Reed Elsevier) and is employed by the Radboud University Nijmegen Medical Centre, Nijmegen, the Netherlands.
S. Boesch has declared no financial disclosure in stock ownership in medically related fields, partnerships, grants, intellectual property rights, expert testimony, contracts, royalties and other; is a consultant at Lundbeck; has been a member of the advisory board of Takeda, Novartis and Glaxo; has received honoraria from Takeda, Glaxo and Novartis and is employed by the University of Innsbruck, Austria.
C. Depondt has declared no financial disclosure in stock ownership in medically related fields, consultancies, advisory boards, partnerships, honoraria, grants, intellectual property rights, expert testimony, contracts and royalties but with financial disclosure in payment of congress expenses by UCB Pharma and is employed by the Université Libre de Bruxelles, Belgium.
L. Schöls has declared no financial disclosure in stock ownership in medically related fields, consultancies, advisory boards, partnerships, intellectual property rights, expert testimony, contracts and royalties; has received support from the HSP-Selbsthilfegruppe Deutschland e.V. for research in spastic paraplegia; has received study fees for the MICONOS trial from Santhera Pharmaceuticals; was supported by grants of the German Research Council (BMBF) to Leukonet (01GM0644) and mitoNET (01GM0864) and E-RARE grants of the EU to EUROSPA (01GM0807) and RISCA (01GM0820) and is employed by the University of Tübingen.
B. Kremer has declared no financial disclosure in stock ownership in medically related fields, consultancies, partnerships, intellectual property rights, contracts, royalties and other; has been a member of the advisory board of Hersenstichting Nederland, Prinses Beatrix Foundation and Nederlandse Transplantatiestichting; has received honoraria from the Netherlands and Actelion, the Netherlands; was supported by grants from UMC Nijmegen, UMC Groningen and MS Anders; has various testimonies in legal cases and medical insurance cases and is employed by the University Medical Centre Groningen, Groningen, the Netherlands.
B. Melegh has declared no financial disclosure in stock ownership in medically related fields, honoraria, intellectual property rights, expert testimony, contracts, royalties and other; has been a consultant for national grant funding opportunities; has been a member of the advisory board of Biobanking and Biomolecular Resources (BBMRI); is partner of the WestTeam Biotech Ltd; was supported by grants from ETT (Grant from Ministry of Health) and OTKA (Hungarian Science Foundation) E-Rare-2, EU7 supported project and is employed by the University of Pécs.
M. Pandolfo has declared no financial disclosure in stock ownership in medically related fields, consultancies, partnerships, expert testimony, contracts and other; has been a member of the advisory board for Apopharma; has received honoraria from Santhera AG; was supported by grants from Repligen Corporation; has intellectual property rights: “Direct molecular diagnosis of Friedreich ataxia” M. Pandolfo, L. Montermini, M.D. Molto’, M. Koenig, V. Campuzano, M. Cossee’. US patent (2000) 6,150,091, WO patent (1997) 9705234; is employed by the University of Brussles and receives royalties from Athena Diagnostics.
J. Schulz has declared no financial disclosure in stock ownership in medically related fields, partnerships, intellectual property rights, expert testimony, contracts, royalties and other; acted as a consultant of Santhera; has been a member of the advisory board of Santhera; has received honoraria from Merz, Pfizer and GSK; was supported by grants from BMBF, EU and is employed by the University of Aachen.
T. Klockgether has declared no financial disclosure in stock ownership in medically related fields, consultancies, partnerships, intellectual property rights, expert testimony, contracts, royalties and other; has been a member of the advisory board of the The Cerebellum, Parkinsonism & Related Disorder, ataxia lay organizations; has received lecture honorarium from Lundbeck; was supported by grants from Deutsche Forschungsgemeinschaft (DFG), KFO177 Clinical Research Group (Innate Immunity in Chronic Neurodegeneration) and from the German Ministry of Education and Research (BMBF), E-Rare 01GM0819 RISCA and is employed by the University of Bonn.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jacobi, H., Hauser, TK., Giunti, P. et al. Spinocerebellar Ataxia Types 1, 2, 3 and 6: the Clinical Spectrum of Ataxia and Morphometric Brainstem and Cerebellar Findings. Cerebellum 11, 155–166 (2012). https://doi.org/10.1007/s12311-011-0292-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-011-0292-z